Article
|
Open Access
Featured
-
-
Article |
 Structure and thiazide inhibition mechanism of the human Na–Cl cotransporter
Structure and thiazide inhibition mechanism of the human Na–Cl cotransporter
Using cryo-electron microscopy, the structures of human Na–Cl cotransporter are determined alone and in complex with a thiazide diuretic.
- Minrui Fan
- , Jianxiu Zhang
- & Liang Feng
-
Article |
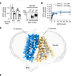 Methotrexate recognition by the human reduced folate carrier SLC19A1
Methotrexate recognition by the human reduced folate carrier SLC19A1
Cryo-EM structures provide insight into how the antifolate methotrexate, a chemotherapy drug, is recognized by the reduced folate carrier.
- Nicholas J. Wright
- , Justin G. Fedor
- & Seok-Yong Lee
-
Article |
 Structure and mechanism of the SGLT family of glucose transporters
Structure and mechanism of the SGLT family of glucose transporters
Cryo-electron microscopy structures of the sodium–glucose cotransporter SGLT1 and a related transporter SMCT1 define the architecture of this protein family and provide insights into substrate binding and transport function.
- Lei Han
- , Qianhui Qu
- & Liang Feng
-
Article |
 Structure and mechanism of blood–brain-barrier lipid transporter MFSD2A
Structure and mechanism of blood–brain-barrier lipid transporter MFSD2A
The cryo-electron microscopy structure of mouse MFSD2A sheds light on the mechanism that underlies its lipid transport functions, which have a pivotal role in regulating the blood–brain barrier.
- Chase A. P. Wood
- , Jinru Zhang
- & Liang Feng
-
Article |
 Lysosome-targeting chimaeras for degradation of extracellular proteins
Lysosome-targeting chimaeras for degradation of extracellular proteins
Lysosome-targeting chimaeras—in which a small molecule or antibody is connected to a glycopeptide ligand to form a conjugate that can bind a cell-surface lysosome-shuttling receptor and a protein target—are used to achieve the targeted degradation of extracellular and membrane proteins.
- Steven M. Banik
- , Kayvon Pedram
- & Carolyn R. Bertozzi
-
Article |
 Structure and mechanism of the cation–chloride cotransporter NKCC1
Structure and mechanism of the cation–chloride cotransporter NKCC1
The cryo-EM structure of the zebrafish cation–chloride cotransporter NKCC1 reveals the domain organization, ion translocation pathway, ion-binding sites and key residues for binding activity, providing insights into the activity of this family of transporter proteins with key roles in physiology.
- Thomas A. Chew
- , Benjamin J. Orlando
- & Liang Feng
-
Article |
 Visualizing multistep elevator-like transitions of a nucleoside transporter
Visualizing multistep elevator-like transitions of a nucleoside transporter
Multiple crystallographic structures of a concentrative nucleoside transporter show how it uses an ‘elevator’ mechanism to move its transport domain across the membrane.
- Marscha Hirschi
- , Zachary Lee Johnson
- & Seok-Yong Lee
-
Letter |
 Structural basis for amino acid export by DMT superfamily transporter YddG
Structural basis for amino acid export by DMT superfamily transporter YddG
The X-ray structure of the drug/metabolite transporter (DMT) protein YddG from Starkeya novella reveals a new membrane transport topology, with ten transmembrane segments in an outward-facing state and two pseudo-symmetric inverted structural repeats.
- Hirotoshi Tsuchiya
- , Shintaro Doki
- & Osamu Nureki
-
Letter |
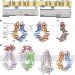 Crystal structure of the human sterol transporter ABCG5/ABCG8
Crystal structure of the human sterol transporter ABCG5/ABCG8
The X-ray structure of human ABCG5/ABCG8 heterodimer in a nucleotide-free state, being the first atomic model of an ABC sterol transporter.
- Jyh-Yeuan Lee
- , Lisa N. Kinch
- & Daniel M. Rosenbaum
-
Letter |
 Structure of a eukaryotic SWEET transporter in a homotrimeric complex
Structure of a eukaryotic SWEET transporter in a homotrimeric complex
The X-ray crystal structure is presented of a seven-transmembrane eukaryotic SWEET glucose transporter, revealing the link between seven-transmembrane eukaryotic SWEETs and their three-transmembrane bacterial homologues and providing insight into eukaryotic sugar transport mechanisms.
- Yuyong Tao
- , Lily S. Cheung
- & Liang Feng
-
Article |
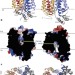 Structure and mechanism of the mammalian fructose transporter GLUT5
Structure and mechanism of the mammalian fructose transporter GLUT5
This study has determined the X-ray crystal structures of GLUT5 from Rattus norvegicus in an open, outward-facing conformation and GLUT5 from Bos taurus in an open, inward-facing conformation; comparison of these structures with previously published structures of the related Escherichia coli d-xylose:H+ symporter XylE suggests that transport in GLUT5 is controlled by both a global ‘rocker-switch’-type motion and a local ‘gated-pore’-type transport mechanism.
- Norimichi Nomura
- , Grégory Verdon
- & David Drew
-
Article |
 Crystal structures of a polypeptide processing and secretion transporter
Crystal structures of a polypeptide processing and secretion transporter
Gram-positive bacteria use peptidase-containing ATP-binding cassette transporters (PCATs) to export quorum-sensing and antimicrobial polypeptides; here, the X-ray crystal structures of PCAT1 from Clostridium thermocellum in the absence and presence of ATP are reported.
- David Yin-wei Lin
- , Shuo Huang
- & Jue Chen
-
Article |
 Molecular basis of ligand recognition and transport by glucose transporters
Molecular basis of ligand recognition and transport by glucose transporters
The SLC2 family glucose transporters facilitate the transport of glucose and other monosaccharides across biological membranes; the X-ray crystal structure of human GLUT3 has been solved in outward-open and outward-occluded conformations and a model for how the membrane protein rearranges itself during a complete transport cycle has been proposed.
- Dong Deng
- , Pengcheng Sun
- & Nieng Yan
-
Article |
 Neurotransmitter and psychostimulant recognition by the dopamine transporter
Neurotransmitter and psychostimulant recognition by the dopamine transporter
Here the X-ray crystal structures of the Drosophila dopamine transporter bound to dopamine, D-amphetamine, methamphetamine and cocaine are solved; these structures show how a neurotransmitter, small molecule stimulants and cocaine bind to a biogenic amine transporter, and are examples of how the ligand binding site of a neurotransmitter transporter can remodel itself to accommodate structurally unrelated small molecules that are different in shape, size and polarity or charge.
- Kevin H. Wang
- , Aravind Penmatsa
- & Eric Gouaux
-
Letter |
 Subnanometre-resolution electron cryomicroscopy structure of a heterodimeric ABC exporter
Subnanometre-resolution electron cryomicroscopy structure of a heterodimeric ABC exporter
The subnanometre-resolution electron cryomicroscopy structure of TmrAB, a heterodimeric ABC transport protein, in a nucleotide-free, inward-facing conformation, is determined.
- JungMin Kim
- , Shenping Wu
- & Yifan Cheng
-
Letter |
 Structures of bacterial homologues of SWEET transporters in two distinct conformations
Structures of bacterial homologues of SWEET transporters in two distinct conformations
The X-ray crystal structures of two bacterial homologues of the SWEET sugar transporters are solved in two conformational states, and comparison of these states suggests that transport occurs via a ‘rocker-switch’ mechanism.
- Yan Xu
- , Yuyong Tao
- & Liang Feng
-
Letter |
 Visualizing the kinetic power stroke that drives proton-coupled zinc(ii) transport
Visualizing the kinetic power stroke that drives proton-coupled zinc(ii) transport
In the transport cycle of Yiip, zinc(ii) binding triggers a highly localized, all-or-nothing change of water accessibility to the transport site and an adjacent hydrophobic gate.
- Sayan Gupta
- , Jin Chai
- & Dax Fu
-
Letter |
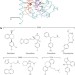 Structural basis for action by diverse antidepressants on biogenic amine transporters
Structural basis for action by diverse antidepressants on biogenic amine transporters
LeuT, a bacterial homologue of eukaryotic biogenic amine transporters (BATs), is engineered to harbour human BAT-like pharmacology by the mutation of key residues around the primary binding pocket; this mutant is able to bind several classes of antidepressant drug with high affinity, helping to define their common mechanisms of action.
- Hui Wang
- , April Goehring
- & Eric Gouaux
-
Letter |
 Unsynchronised subunit motion in single trimeric sodium-coupled aspartate transporters
Unsynchronised subunit motion in single trimeric sodium-coupled aspartate transporters
Glutamate transporters are integral membrane proteins that facilitate neurotransmitter uptake from the synaptic cleft into the cytoplasm of glial cells and neurons, the mechanism of transport involves transitions between extracellular- and intracellular-facing conformations; here the authors used single-molecule fluorescence resonance energy transfer imaging to directly observe conformational dynamics in trimers of a bacterial homologue of glutamate transporters that was embedded in the membrane.
- Guus B. Erkens
- , Inga Hänelt
- & Antoine M. van Oijen
-
Article |
 X-ray structure of dopamine transporter elucidates antidepressant mechanism
X-ray structure of dopamine transporter elucidates antidepressant mechanism
The X-ray crystal structure of the Drosophila dopamine transporter bound to the antidepressant drug nortriptyline is presented, providing the first crystal structure of a eukaryotic neurotransmitter sodium symporter.
- Aravind Penmatsa
- , Kevin H. Wang
- & Eric Gouaux
-
Letter |
 Structural basis for the inhibition of bacterial multidrug exporters
Structural basis for the inhibition of bacterial multidrug exporters
The first inhibitor-bound X-ray crystal structures of the bacterial multidrug efflux transporter AcrB and its homologue MexB are presented, with the inhibitor shown to bind the transporter through a narrow hydrophobic pit, thereby preventing rotation of AcrB and MexB monomers.
- Ryosuke Nakashima
- , Keisuke Sakurai
- & Akihito Yamaguchi
-
Letter |
 Transport dynamics in a glutamate transporter homologue
Transport dynamics in a glutamate transporter homologue
Single-molecule fluorescence resonance energy transfer imaging of a bacterial glutamate transporter reveals how the transport domains move.
- Nurunisa Akyuz
- , Roger B. Altman
- & Olga Boudker
-
Letter |
 Carbon catabolite repression of the maltose transporter revealed by X-ray crystallography
Carbon catabolite repression of the maltose transporter revealed by X-ray crystallography
The X-ray crystal structure of a member of the glucose-specific phosphotransferase system (EIIAGlc) bound to the MalFGK2 maltose transporter is presented, revealing that two EIIAGlcproteins bind to the cytoplasmic ATPase subunits of the maltose transporter to stabilize it in an inward-facing conformation that prevents ATP hydrolysis.
- Shanshuang Chen
- , Michael L. Oldham
- & Jue Chen
-
Letter |
 Structural basis for alternating access of a eukaryotic calcium/proton exchanger
Structural basis for alternating access of a eukaryotic calcium/proton exchanger
The X-ray crystal structure of a member of the Ca2+/H+ (CAX) antiporter family from Saccharomyces cerevisiae in a cytosol-facing, substrate-bound conformation is solved; using the structure, a mechanism by which members of the Ca2+:cation (CaCA) superfamily facilitate Ca2+ transport across cellular membranes is proposed.
- Andrew B. Waight
- , Bjørn Panyella Pedersen
- & Robert M. Stroud
-
Letter |
 Crystal structure of a nitrate/nitrite exchanger
Crystal structure of a nitrate/nitrite exchanger
Cellular nitrite is rapidly removed from the cell to prevent formation of the cytotoxic nitric oxide; here the X-ray crystal structure of NarK, a bacterial nitrate/nitrite transport protein, is determined with and without substrate.
- Hongjin Zheng
- , Goragot Wisedchaisri
- & Tamir Gonen
-
Letter |
 Crystal structure of a folate energy-coupling factor transporter from Lactobacillus brevis
Crystal structure of a folate energy-coupling factor transporter from Lactobacillus brevis
The crystal structure of an inward-facing, nucleotide-free folate energy-coupling factor transporter from Lactobacillus brevis at a resolution of 3 Å suggests a transport model that involves a substantial conformational change of the substrate-specific binding protein, FolT.
- Ke Xu
- , Minhua Zhang
- & Peng Zhang
-
Letter |
 Structural basis for the drug extrusion mechanism by a MATE multidrug transporter
Structural basis for the drug extrusion mechanism by a MATE multidrug transporter
Several X-ray crystal structures of an H+-driven multidrug and toxic compound extrusion (MATE) transporter from Pyrococcus furiosus are presented, whose complex structure with macrocyclic peptides may help facilitate the discovery of efficient inhibitors of MATE transporters.
- Yoshiki Tanaka
- , Christopher J. Hipolito
- & Osamu Nureki
-
Letter |
 Structure and mechanism of a bacterial sodium-dependent dicarboxylate transporter
Structure and mechanism of a bacterial sodium-dependent dicarboxylate transporter
The cytosolic concentration of citrate partially depends on its direct import across the plasma membrane by the Na+-dependent citrate transporter (NaCT); here the X-ray crystal structure of a bacterial homologue of NaCT is reported, which, along with transport-activity studies, suggests how specific conformational changes facilitate substrate translocation across the cellular membrane.
- Romina Mancusso
- , G. Glenn Gregorio
- & Da-Neng Wang
-
Article |
 Crystal structure of a bacterial homologue of glucose transporters GLUT1–4
Crystal structure of a bacterial homologue of glucose transporters GLUT1–4
A study of X-ray crystal structures of the Escherichia coli xylose transporter XylE, which is a bacterial homologue of the human glucose transporters GLUT1–4, complexed with glucose and its analogues yields a framework for understanding the molecular mechanism by which membrane proteins transport glucose and other sugars across cell membranes.
- Linfeng Sun
- , Xin Zeng
- & Nieng Yan
-
Letter |
 The molecular basis of phosphate discrimination in arsenate-rich environments
The molecular basis of phosphate discrimination in arsenate-rich environments
Ultrahigh-resolution X-ray crystallography study of a phosphate-binding protein from Pseudomonas fluorescens yields insight into how phosphate ions essential for life are discriminated from the arsenate ions inimical to it, even in arsenate-rich environments.
- Mikael Elias
- , Alon Wellner
- & Dan S. Tawfik
-
Article |
 Structure of AMP-PNP-bound vitamin B12 transporter BtuCD–F
Structure of AMP-PNP-bound vitamin B12 transporter BtuCD–F
The X-ray crystal structure of the transporter-binding protein complex BtuCD–F, involved in the uptake of vitamin B12 across the inner membrane of Escherichia coli, is determined in an ATP analogue-bound state; the membrane-spanning BtuC subunits adopt a previously unseen conformation in which the central translocation pathway is sealed by an additional gate, and membrane transport is seen to occur through an unexpected peristaltic transport mechanism, distinct from what has been observed for other ABC transporters.
- Vladimir M. Korkhov
- , Samantha A. Mireku
- & Kaspar P. Locher
-
Article |
 Antiparallel EmrE exports drugs by exchanging between asymmetric structures
Antiparallel EmrE exports drugs by exchanging between asymmetric structures
NMR and single molecule FRET experiments show that antiparallel EmrE dimers interconvert between two identical but oppositely oriented conformations that are each open only to one side of the membrane.
- Emma A. Morrison
- , Gregory T. DeKoster
- & Katherine A. Henzler-Wildman
-
Letter |
 Structures of the multidrug exporter AcrB reveal a proximal multisite drug-binding pocket
Structures of the multidrug exporter AcrB reveal a proximal multisite drug-binding pocket
Crystallographic studies show that high-molecular-mass drugs bind to the bacterial multidrug transporter AcrB at a previously unseen ‘proximal’ binding pocket before peristaltic transfer to the known ‘distal’ pocket, whereas low-molecular-mass drugs bind directly to the distal pocket.
- Ryosuke Nakashima
- , Keisuke Sakurai
- & Akihito Yamaguchi
-
Article |
 Sugar transporters for intercellular exchange and nutrition of pathogens
Sugar transporters for intercellular exchange and nutrition of pathogens
Sugar efflux transporters are essential for diverse processes such as nectar production and seed and pollen development, as well for the maintenance of blood glucose levels in animals. These authors identify and characterize a novel sugar transporter family, SWEET, and show that several Arabidopsis, rice and metazoan homologues mediate glucose transport. In addition, some of these transporters are exploited by plant pathogens for nutritional gain and virulence.
- Li-Qing Chen
- , Bi-Huei Hou
- & Wolf B. Frommer
-
Letter |
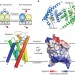 Structure and mechanism of the S component of a bacterial ECF transporter
Structure and mechanism of the S component of a bacterial ECF transporter
The energy-coupling factor transporters are responsible for vitamin uptake in prokaryotes. Here, the X-ray crystal structure of the membrane-embedded, substrate-binding domain of a riboflavin transporter from Staphylococcus aureus is reported. The transporter adopts a previously unreported fold and contains a riboflavin molecule bound to a loop and the periplasmic portion of several transmembrane segments.
- Peng Zhang
- , Jiawei Wang
- & Yigong Shi
-
News & Views |
 Last of the multidrug transporters
Last of the multidrug transporters
Proteins that pump a wide range of toxic compounds out of cells are ubiquitous in nature, but crystal structures for one family of these transporters have remained elusive. Until now. See Letter p.991
- Hendrik W. van Veen
-
Letter |
 Structure of a fucose transporter in an outward-open conformation
Structure of a fucose transporter in an outward-open conformation
In Escherichia coli, the uptake of L-fucose, an important source of carbon for microorganisms, is mediated by a proton symporter from the major facilitator superfamily (MFS). These authors report the first X-ray crystal structure of the outward-open conformation of an MFS proton transporter, FucP. Building on previous work, they develop a working model for how the substrate is recognized by the transporter and how the protein mediates L-fucose/proton symport.
- Shangyu Dang
- , Linfeng Sun
- & Nieng Yan
-
Letter |
 Structure of a cation-bound multidrug and toxic compound extrusion transporter
Structure of a cation-bound multidrug and toxic compound extrusion transporter
Transporter proteins from the MATE (multidrug and toxic compound extrusion) family are involved in metabolite transport in plants, and in multiple-drug resistance in bacteria and mammals. Here, the X-ray crystal structure of a MATE transporter from Vibrio cholerae is reported. The structure is in an outward-facing conformation, and reveals a cation-binding site near to residues previously deemed essential for transport.
- Xiao He
- , Paul Szewczyk
- & Geoffrey Chang
-
Letter |
 Mechanism of substrate recognition and transport by an amino acid antiporter
Mechanism of substrate recognition and transport by an amino acid antiporter
The amino acid antiporter AdiC is important for the survival of enteric bacteria such as Escherichia coli in extremely acid environments. Although the structure of substrate-free AdiC is known, how the substrate (arginine or agmatine) is recognized and transported by AdiC remains unclear. The crystal structure of an E. coli AdiC variant bound to arginine is now reported and analysed.
- Xiang Gao
- , Lijun Zhou
- & Yigong Shi

 A new antibiotic traps lipopolysaccharide in its intermembrane transporter
A new antibiotic traps lipopolysaccharide in its intermembrane transporter