Winged insects, including butterflies, wasps and beetles, are some of the most successful animals on the planet, in terms of numbers of species and of individuals. Part of this success comes from their ability to fly and from the evolution of wings, which have evolved as a new type of appendage, independently of limbs. The wing is connected to the insect body through an exquisite hinge. Although the wing hinge is an important joint, its small size, its fast movement and researchers’ inability to directly observe it have made understanding how it works difficult. Writing in Nature, Melis et al.1 go a long way to solving this riddle.
Insects such as flies and bees flap their wings hundreds of times a second to perform extremely rapid, yet controlled, flight manoeuvres. These animals have evolved specialized muscles and body appendages that enable such high-frequency wing movements2. Wing motion is powered by a set of muscles called the indirect flight muscles, which do not attach directly to the wings, but instead attach to and deform the insect’s exterior surface — its exoskeleton. These deformations are transmitted to the wing by the hinge, a complex joint that consists of a series of tiny, hardened structures known as sclerites (Fig. 1). Each sclerite transmits force to its neighbour — in a way reminiscent of a series of gears — thereby transforming tiny exoskeletal deformations into large back-and-forth wing movements.

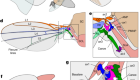
Figure 1 | The wing hinge of the fruit fly Drosophila melanogaster. The fruit fly wing is connected to the insect’s body (a region termed the thorax) by a hinge that contains tiny structures called sclerites. These sclerites (named the basalare, ax1, ax2, ax3, ax4 and the canoe) transmit forces from one to the other, resulting in the back and forth motion of the wing. Melis et al.1 investigated how this wing hinge controls wing movements by using imaging to track muscle activation and the associated simultaneous wing movements, and revealed the relationships between the muscles and the wing movements using machine-learning methods. Some of these sclerites (the basalar, ax1, ax3 and ax4) are connected to steering muscles, and the sclerites and muscles together control wing movements. (Adapted from Fig. 1 of ref. 1.)
Small steering muscles, also called direct flight muscles, attach to sclerites and apply force directly to them to fine-tune the wing movements on a stroke-by-stroke basis. Therefore, the hinge functions not only as a flexible joint between the wing and the body wall of the thorax, but also as an ‘organ’ with several independent elements (the sclerites). Of these, four, studied by Melis et al. in the fruit fly Drosophila melanogaster, are connected to a dozen direct flight muscles that together drive the varied wing movements.
Understanding how the joint functions is difficult, because the hinge and its associated muscles are internal structures that can’t be observed directly in an insect with flapping wings, and the high frequency of wing beats further complicates matters. As a result, the key questions of how muscle activity generates sclerite movement and, as a consequence, causes changes in wing motion have been challenging to address. Melis and colleagues used an innovative approach to examine the neuroanatomical basis of how the wing hinge functions. The authors recorded the calcium activity (a readout of the cellular activity) of the 12 muscles associated with 4 sclerites and mapped this information onto the fly’s wing movements, using machine learning. Their strategy thus provides a glimpse of the potential contribution of individual sclerite–muscle groups to wing motion.
Read the paper: Machine learning reveals the control mechanics of an insect wing hinge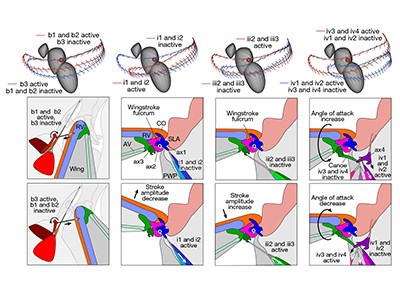
The fruit fly wing hinge has conventionally been studied in dissected tissue in which physical force is applied to each observable muscle and the subsequent effect on wing movement is recorded3. Such experiments are, by design, limited to providing results consisting of static interpretations. Although some researchers have recorded muscle activity in live insects, such as blowflies3,4, and have provided quantitative insights4,5 into the function of individual muscles, the effect of the collective action of all wing muscles remains unknown.
Simultaneous calcium imaging of all steering muscles has shown that each hinge unit (the sclerite and its associated group of muscles) has two functional set of muscles — a constantly active set and a transiently active one, which together drive wing motion6. A technique called X-ray tomography has revealed that a tendon of one steering muscle buckles during the wing’s upstroke for wing beats that have high amplitudes, suggesting that contraction of this muscle must be effective mainly during the downstroke and hence activity of this muscle would limit the wing amplitude at the lowest part of the downstroke7. Collectively, these approaches have considerably enhanced our knowledge of flight control. However, the biomechanical underpinnings of the musculoskeletal interactions that lead to wing motion during flight remain far from clear.
Melis and colleagues recorded the calcium activity in the fruit fly’s steering muscles and simultaneously measured its wing movements. Using a neural-network approach, the authors successfully evaluated and predicted wing movements on the basis of the muscles’ calcium activity. Interestingly, Melis et al. used a specific architecture of the neural network to map all 13 inputs — 12 for the steering-muscle activities and one for wing-beat frequency — onto output variables that were used to reconstruct 4 angles that defined the wing position and shape. This architecture included an internal ‘bottleneck’ layer of five nodes, corresponding to the four sclerites and one for the wing beat frequency8, such that each node receives inputs from its associated muscles, thus building the hinge’s biomechanical groups into the neural architecture.
The predicted muscle-activity patterns were used to model wing movements that could simulate short flight sequences, resembling those of a real fly. Using a robotic flapper as a model, the authors tested the aerodynamic effect of controlling the four angles used to define the wing position and shape.
Machine-learning strategies for analysing the neuromechanical principles of joints bring researchers a step closer to building a purely physics-based model that explains how muscle activity causes deformation in the sclerites, which ultimately produces the diversity in wing movement. Crucially, this work provides a computer platform for performing virtual experiments. Melis et al. used their model to alter the activity of single muscles and mapped the effects these changes had on wing movements.
Trade-offs between stability and manoeuvrability in bird flight
In addition to increasing and decreasing the activity of one muscle in their virtual experiments, the authors simultaneously changed the activity in the other muscles in the same proportions as those of the synergies they found in a live fly. It is unclear whether these synergies occur randomly or whether they represent a strategy in which the activities of differing sets of muscles are always coupled. Another possibility is that these muscle groups and sclerites are physically constrained to move in a coupled manner and that, therefore, they must act on the wing in ways that are more limited than the full set of combinations of muscles and sclerites.
The authors not only investigated aspects of wing movement, such as the wing-stroke angle, but also examined changes in the arching of the wing surface (wing camber). The effect of wing camber on aerodynamic output has been debated for years9, and computer models will help scientists to test whether flies control their wing camber during flight manoeuvres, and if so, how they do this. Results from such computer analyses might also reveal the mechanical basis for the clutch mechanism that has been proposed previously for the wing hinge10. Such a system could enable each wing to be independently decoupled from the oscillating thorax during some behaviours, such as courtship, but remain physically connected to each other during others, including flight. During courtship, male D. melanogaster extend and vibrate only one wing away from the thorax to generate a mating song; the other is folded and disengaged. Understanding how such physical strains are transferred from the thorax to the wing through the hinge will shed light on how the wings are actively engaged and disengaged during flight.
Previous work on steering muscles indicates that millisecond differences in the firing phase of neuronal inputs into muscles affect their output on a stroke-to-stroke basis4,11. However, calcium levels in the muscles, measured by Melis and colleagues using a calcium-indicator protein called GCaMP7f, reveal a relatively slow and integrated signal over time. Although a useful measure of activity, it remains unclear whether such analyses represent the entire behavioural repertoire and whether they can be used to predict a complete map of all the relevant interactions between muscles and the thorax. Moreover, although the authors studied fruit flies, their results might be applicable to other insects, even though each species has its own hinge architecture. For instance, some parts of fly wing hinges (sclerites and steering muscles) are similar to those of bees and wasps12. A generalized model with properly adjusted parameters and measurements will enable us to understand the wing hinge as well as its evolutionary importance to the mechanisms for flight control.

 Read the paper: Machine learning reveals the control mechanics of an insect wing hinge
Read the paper: Machine learning reveals the control mechanics of an insect wing hinge
 Trade-offs between stability and manoeuvrability in bird flight
Trade-offs between stability and manoeuvrability in bird flight
 Flight of the RoboBee
Flight of the RoboBee