Abstract
Purpose
To evaluate the effect of controlled conjunctival cautery on the frequency and extent of subconjunctival haemorrhage (SCH) associated with sub-Tenon anaesthetic (STA) injection in patients with various clotting states.
Methods
One hundred forty-four patients suitable for cataract surgery with STA were prospectively divided into four groups: group A (n=36) were on warfarin (INR 1.8–4.2); group B (n=48) on aspirin (75 mg); group C (n=12) on clopidogrel (75 mg); and group D (n=48) on no anticoagulant or antiplatelet agents. All patients had no other known coagulopathy. Each group was randomly divided into two subgroups, one of which received localised bipolar cautery under microscope control to the STA conjunctival entry site before tissue dissection, whereas the other served as a control. χ2 and Fisher's exact tests were used to analyse the data with the SPSS package (v11.0).
Results
Conjunctival cautery reduced the frequency of SCH from 67 to 6% in group A (P=0.0001); from 37.5 to 4% in group B (P=0.005); from 50 to 0% in group C (P=0.9); and from 17 to 0% in group D (P=0.55). This overall reduction in SCH was highly significant (P<0.0001), especially in groups A and B. No statistically significant reduction in the extent of SCH was found.
Conclusions
Controlled localised bipolar conjunctival cautery before STA injection may significantly reduce the frequency of SCH, especially in patients on warfarin or aspirin.
Similar content being viewed by others
Introduction
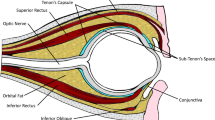
Sub-Tenon's anaesthesia (STA) is an increasingly popular technique used to achieve adequate anaesthesia during cataract surgery. It provides good levels of analgesia and akinesia via a blunt cannula technique with relatively small volumes of local anaesthetic, if administered correctly.1, 2 The frequency of subconjunctival haemorrhage (SCH) during STA injections is often a consequence of the procedure and has been reported to occur in up to 56% of cases.3, 4 The most likely source of bleeding are the conjunctival vessels, which get injured, cut, or sheared during the process of creating a conjunctival entry site for the cannula to pass into the sub-Tenon space. Although this does not necessarily have any direct morbidity associated with it, a chemosed haemorrhagic conjunctiva not only results in an unsightly postoperative eye but can also make visualisation during cataract surgery more challenging owing to pooling of ocular surface fluids.
Coagulation status has long been a focus of discussion for cataract surgeons and ophthalmic anaesthetists alike. The practice of routine anticoagulant cessation before cataract surgery was found to have an associated increased risk of thromboembolic complications.5, 6 This led to general acceptance that anticoagulation and antiplatelet agents should be continued before cataract surgery.4, 6, 7 However, patients on anticoagulant or antiplatelet agents have been reported to experience increased haemorrhagic complications,8, 9 such as SCH,10 and as such are at ‘higher risk’ of this occurring during STA injection.
Cauterisation of the conjunctiva has been recommended in previous descriptions of parabulbar anaesthesia;11 however, there has been no objective, controlled, or standardised data to support its use, especially in patients with altered clotting states. Recent controversy over the benefits of conjunctival cautery during STA12, 13 has raised further questions over the validity of this intervention. This study looks at the use of bipolar cautery on the conjunctiva before any manipulation or dissection of the conjunctival entry site. This technique was investigated to assess whether it would be a suitable adjuvant procedure to reduce the frequency and extent of SCH associated with STA injection, specifically looking at its impact on various clotting states.
Materials and methods
Cataract surgery patients that were suitable for STA were prospectively identified over a finite data collection period of 9 months from November 2003 to July 2004 in two sequential eye departments. Patient recruitment was subject to the following exclusions: not suitable for local anaesthesia; on multiple anticoagulant or antiplatelet agents (including chemicals known to affect clotting, eg Ginkgo biloba); on regular oral steroids or NSAIDs; if there was any other known coagulopathy or bleeding diathesis; or if there was any conjunctival pathology identified preoperatively.
Patients were allocated into one of four groups depending on whether they were on regular warfarin (group A), aspirin (75 mg; group B), clopidogrel (75 mg; group C), or on no antiplatelet or anticoagulation agents (group D, also referred to as ‘no agent’). Patients within each group were randomly allocated to a ‘cautery’ or ‘no cautery’ subgroup using block randomisation where the block size was randomly varied (4–10). The subgroups were age- and sex-matched with the ‘no cautery’ subgroups serving as controls. All patients on warfarin had INR measurement within 24 h of surgery.
The protocol for sub-Tenon injection and conjunctival cautery is described below. The ‘no cautery’ subjects had the same protocol, except for the omission of step 3. All procedures were performed by a single ophthalmic surgeon. All patients went on to have clear corneal phacoemulsification cataract extraction.
Sub-Tenon's protocol
-
1
Topical anaesthetic drops (proxymethocaine 0.5% minims) were administered and a wire speculum was used to retract the lids.
-
2
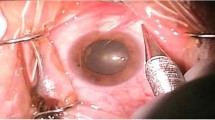
The conjunctival entry site was identified, under microscope control, where the STA injection was to be administered. This was performed in the inferonasal quadrant to achieve akinesia and anaesthesia2 (Figure 1a).
-
3
The bipolar cautery was applied to approximately 5 mm of conjuctiva in a curvilinear manner. The cautery power was increased until the conjunctiva and any visible vessels in the treated area were blanched (Figure 1a).
-
4
A pair of Westcott scissors were then used to cut through tented conjunctiva and Tenons within the area of conjunctival cautery (Figure 1b).
-
5
A blunt 1.1 × 25 mm rigid curved posterior sub-Tenon cannula (BD Visitec™) was then passed into the sub-Tenon space and approximately 3 ml of lignocaine 2% (without epinephrine) was administered (Figure 1c).
-
6
Two minutes of gentle digital pressure was applied to the anaesthetised eye.
Eight minutes after the administration of the STA, the presence or absence of SCH, the quadrants of the conjunctival surface involved (1–4), and the incidence of nonhaemorrhagic chemosis were objectively recorded by two independent assessors that were not present during the STA injection process. The cataract operation was then commenced. The degree of akinesia and anaesthesia was subjectively assessed by the operating surgeon.
Statistical analysis of the data was performed with the SPSS package (version 11.0). Discrete data were analysed using χ2 and Fisher's exact test. Statistical significance was determined at P<0.05.
Results
A total of 144 eyes of 144 patients were enrolled in the study. These were distributed in four groups as follows: 36 patients in group A, 48 patients in group B, 12 patients in group C, and 48 patients in group D. The age range was 56–82 years with a mean of 71 years. The findings are summarised in Tables 1, 2 and Figure 2.
Of the 72 patients randomised to have conjunctival cautery before STA, 18 were on warfarin, 24 on aspirin, six on clopidogrel, and 24 were in the ‘no-agent’ group. Only a single patient (1.4%) was noted to have SCH, which involved less than two quadrants of ocular surface, and was from the warfarin group. The other 72 patients in the study underwent routine STA without conjunctival cautery. Of these, SCH was noted in 26 patients (36%), of which the greatest proportion was in the warfarin control subgroup. This overall reduction in SCH by conjuctival cautery was highly significant (P<0.0001). Individual group analysis revealed that the reduction in SCH by conjunctival cautery in group A was from 67 to 6% (P=0.0001), group B from 7 to 0% (P=0.005), group C from 50 to 0% (P=0.9), and group D from 17 to 0% (P=0.055).
No statistically significant difference was found in the extent of SCH among the control subgroups. These results are summarised in Table 2. There was also no significant difference evident in the subjective degree of akinesia or anaesthesia among the ‘cautery’ and ‘no cautery’ subgroups. No ‘top-up’ STA was required; however, two patients received intraoperative topical proxymethocaine 0.5% for ocular surface discomfort. All conjunctival wounds appeared well healed at the 2-week postoperative check. No cases of damage to the sclera or deeper ocular tissues owing to cautery were noted. The incidence of nonhaemorrhagic conjunctival chemosis remained unaffected by conjunctival cautery and was noted in approximately 18% of cases. The INR range in the warfarin group was 1.8–4.1 (mean 2.6).
Discussion
SCH is a well-recognised complication of the STA technique.2 Often this has prompted surgeons to consider topical anaesthetic techniques;14 however, the lack of akinesia and patient discomfort15 have been dissuading factors.
Previously employed procedures to reduce SCH include the use of vasoconstrictive topical agents or simple avoidance of conjunctival vessels during tissue dissection; however, these have not been objectively assessed or reported in the literature in the context of STA. Other concerns regarding the continuation of antiplatelet and anticoagulant agents before cataract surgery, such as suprachoroidal or retrobulbar haemorrhage, are beyond the scope of this study.
An audit reported by Kumar and Williamson12 concluded that the use of disposable diathermy by the anaesthetist did not reduce the incidence of SCH during STA. This audit excluded all patients on anticoagulant or antiplatelet agents which, in our study, were the groups that gained most statistical benefit from conjunctival cautery. A report by Chung and Chua13 on the benefits of conjunctival cautery before STA did not use a randomised or controlled data set and their technique involved the use of hand-held cautery. Both the reports did not use microscope control or bipolar cautery. The use of microscope control not only allows detailed visualisation of the area of treatment but also allows better assessment of the coagulation reaction produced by the cautery thereby ensuring adequate treatment is performed with minimal energy required. Indeed, the use of the microscope for detailed conjunctival visualisation may be replaced by high-power loupe spectacles enabling the procedure to be carried out in the induction room with the use of a bipolar machine. Hand-held or disposable cautery delivers a variable and unmeasured degree of coagulation that unchecked may lead to inadvertent globe damage, and has been reported to be a fire hazard.16 The use of bipolar cautery allows a more controlled and graduated delivery of coagulation, and once set up, it can be used intraoperatively to cauterise ‘late’ conjunctival capillary bleeders that may not be evident during the initial injection. Such ‘late’ cautery was not performed during this study to allow assessment of the initial cautery procedure alone.
Analysis of our results revealed that the highly statistically significant reduction of SCH with conjunctival cautery, which in individual group analysis was only significant in the warfarin and aspirin groups. This may well be due to the smaller numbers in the clopidogrel group and the inherently low rate of SCH in the ‘no-agent’ group, despite both showing a trend towards significance. Indeed, the same applies to studying the extent of SCH, which was limited by there being only one case of SCH in the ‘cautery’ group. A larger study could help better delineate these relationships and further assess the impact of cautery on the extent of SCH.
The results from this study provide some degree of objective evidence to support the use of bipolar conjunctival cautery before STA injection, especially in patients at ‘high risk’ of SCH. The selective use of this adjuvant procedure can also limit the cost implication involved. We therefore suggest that the use of controlled conjunctival cautery before STA injection be considered to help reduce the incidence of SCH, particularly in patients on aspirin or warfarin.
References
Crandall AS . Anesthesia modalities for cataract surgery. Curr Opin Ophthalmol 2001; 12: 9–11.
Stevens JD . A new local anaesthesia technique for cataract extraction by one quadrant sub-Tenon's infiltration. Br J Ophthalmol 1992; 76 (11): 670–674.
Guise PA . Sub-Tenon anesthesia. Anesthesiology 2003; 98 (4): 964–968.
Roman SJ, Chong Sit DA, Boureau CM, Auclin FX, Ullern MM . Sub-Tenon's anesthesia: an efficient and safe technique. Br J Ophthalmol 1997; 81 (8): 673–676.
Konstantatos A . Anticoagulation and cataract surgery: a review of the current literature. Anaesth Intens Care 2001; 29 (1): 11–18.
Moll AC, van Rij G, van der Loos LJM . Anticoagulant therapy and cataract surgery. Doc Ophthalmol 1989; 72: 367–373.
Hall DL, Steen Jr WH, Drummond JW, Byrd WA . Anticoagulants and cataract surgery. Ophthalmic Surg 1988; 19: 221–222.
Carter K, Miller KM . Phacoemulsification and lens implantation in patients treated with aspirin or warfarin. J Cataract Refract Surg 1998; 24 (10): 1361–1364.
Gainey SP, Robertson DM, Fay W, Ilstrup D . Ocular surgery on patients receiving long-term warfarin therapy. Am J Ophthalmol 1989; 108: 142–146.
Saitoh AK, Saitoh A, Taniguchi H, Amemiya T . Anticoagulation therapy and ocular surgery. Ophthalmic Surg Lasers 1998; 29 (11): 909–916.
Greenbaum S . Parabulbar anesthesia. Am J Ophthalmol 1992; 114 (6): 776.
Kumar CM, Williamson S . Diathermy does not reduce subconjunctival haemorrhage during sub-Tenon's block. Br J Anaesth 2005; 95 (4): 562.
Chung RSH, Chua CN . Reduction of subconjunctival hemorrhage with sub-Tenon's anesthesia. J Cataract Refract Surg 2005; 31 (10): 2031.
Wirbelauer C, Weller A, Haberle H, Pham DT . Cataract surgery under topical anesthesia with oral anticoagulants. Klin Monatsbl Augenheilkd 2004; 221 (9): 749–752 (German).
Ruschen H, Celaschi D, Bunce C, Carr C . Randomised controlled trial of sub-Tenon's block vs topical anaesthesia for cataract surgery: a comparison of patient satisfaction. Br J Ophthalmol 2005; 89 (3): 291–293.
Curtin JW . The disposable cautery: a fire hazard. Plast Reconstr Surg 1989; 84 (5): 853.
Author information
Authors and Affiliations
Corresponding author
Additional information
Fully informed patient consent was obtained and the procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 1983
Presentations: ESCRS meeting, Paris 2004; EVER meeting, Portugal 2004
The authors have no propriety or financial interest in this research.
Rights and permissions
About this article
Cite this article
Gauba, V., Saleh, G., Watson, K. et al. Sub-Tenon anaesthesia: reduction in subconjunctival haemorrhage with controlled bipolar conjunctival cautery. Eye 21, 1387–1390 (2007). https://doi.org/10.1038/sj.eye.6702447
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702447
Keywords
This article is cited by
-
Anästhesieformen in der Augenheilkunde
Der Ophthalmologe (2021)
-
Sub-Tenon's anaesthesia: complications and their prevention
Eye (2011)