Abstract
Aim:
The aim of this study is to evaluate the short-term result of the use of an autologous scleral implant during the nonpenetrating deep sclerectomy operation instead of the more expensive collagen implant.
Design:
Prospective nonrandomized pilot study.
Patients and Methods:
This study included 20 eyes of 20 patients with a mean age of 42.3±8.1 years and the mean of preoperative best-corrected visual acuity was 0.66±6.2. All patients were indicated for conventional deep sclerectomy operation but with implantation of an autologous scleral implant. The operation was considered completely successful if the intraocular pressure (IOP) is below 18 mmHg without medications while qualified success was considered if the IOP was below 18 mmHg with medications.
Results:
This technique succeeded in declining the mean IOP from 33.1±6.2 mmHg preoperatively to 14.6±3.8 mmHg postoperatively by the end of the follow-up period (12 months). Complete and qualified successful results were seen in 85% of cases. Nd:YAG goniopuncture was needed in 45% of the cases. This intervention was needed 14.0±10.0 weeks postoperatively. The study group showed low incidence of postoperative complication with statistically nonsignificant effect on the postoperative visual acuity.
Conclusion:
The use of autologous scleral implant can be of value in controlling the IOP with low cost to the patient. Nd:YAG goniopuncture is to be performed around the third month postoperatively in cases with advanced glaucomatous changes to avoid the problem of early implant induced fibrosis. Further long-term comparative study on wider scale is needed to detect the exact value of this technique and to evaluate its long-term result.
Similar content being viewed by others
Introduction
The concept of shifting to nonpenetrating surgery in management of uncontrolled open-angle glaucoma has been recently considered. The idea of the development of this technique was to avoid ocular entry in order to limit the postoperative complications.1, 2, 3, 4, 5, 6, 7, 8, 9 In 1990, Fyodorov et al and Kozlov et al10, 11 proposed an operation which they called nonpenetrating deep sclerectomy (NPDS).
Removal of the deep scleral flap leads to formation of an empty scleral space called ‘aqueous decompression space’, wherein the aqueous humor will be collected before its drainage. In order to keep the aqueous decompression space open, different implant devices have been proposed such as collagen Aquaflow implants, reticulated hyaluronic acid implants, and the T Flux implant.8, 9, 10, 11, 12, 13, 19, 20
Those implants, however, are expensive and not easily available in every country. Therefore, cheaper materials were tried to be used as an implant, like chromic suture material.14 Another trial was to use an autologous scleral implant as an alternative to collagen implant. Sclera was chosen because of its collagen structure and at the same time it is better than homologous sclera because of the risk of transmission of diseases.15
The aim of this study is to evaluate the short-term result of the use of an autologous scleral implant during the NPDS, its success in controlling the intraocular pressure (IOP), and the rate of complications.
Patients and methods
This study included 20 eyes of 20 patients with medically uncontrolled open-angle glaucoma and indicated for surgical intervention. The mean age of them was 42.3±8.1 years (range 28–55 years). Nine patients of the study group were males and 11 patients were female. We excluded from the study the recurrent cases, single eyed patients, and patients with advanced lens opacities. Also, we excluded cases in which accidental intraoperative perforation of the thin trabeculo-Descemet's membrane occurred and the operation was converted to conventional penetrating trabeculectomy operation.
Preoperatively, the patients had clinical examination, including best-corrected visual acuity (expressed in decimal system), IOP measurement using a Goldmann applanation tonometer, gonioscopic, and fundoscopic examination. Field examination was performed on all patients using G1 program of Octopus 101 machine (Interzeag). The mean defect (MD) in the visual field (expressed in dB) was recorded and evaluated.
Surgical technique
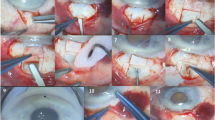
All surgeries were carried out under peri-ocular anaesthesia using lidocaine hydrochloride 4%, bupivacaine 0.75%, and hyalurounidase 50 U. One-third thickness superficial scleral flap was designed, 4 × 6 mm, and dissected till 1 mm into the clear cornea. From the posterior part of the scleral bed under this flap, a rectangle of deep sclera was removed leaving a thin layer of deep sclera over the choroids. Then, dissection of the deep scleral flap till the roof of the Schlemm's canal followed by its deroofing and peeling of the juxta-canalicular membrane were carried out as the usual steps of the deep sclerectomy operation. The part of the deep sclera removed from the posterior part of the scleral bed earlier in the operation (autologous sclera implant) was fashioned 4 × 1 mm and secured underneath the superficial scleral flap with one 10-0 nylon suture, leaving the upper edge of the graft just protruding from the superior horizontal edge of the incision (Figure 1). The superficial scleral flap was then repositioned and sutured with two loose 10-0 nylon sutures at the corners and the knots were buried. Tenectomy and closure of the conjunctiva was done. Postoperatively, patients were treated with topical steroids and antibiotics for 6–8 weeks in addition to mydriatics for the first 1–2 weeks.
The failure to achieve the target IOP at any stage of the postoperative follow-up was an indication of neodymium:yttrium–aluminum–garnet (Nd:YAG) laser goniopuncture. This procedure was performed using ARC photodisruptor, Q-Las 10 (Q-Switch 1046 nm wavelength laser, Germany), emitted via a slit-lamp microscope (Haag-Streit, Switzerland), and gonioscopy contact lens. After application of pilocarpine, we started with single burst mode and low energy of 2 mJ, then gradually increased until a small hole became visible in the trabeculo-Descemet's membrane. Goniopuncture allows direct passage of aqueous from the anterior chamber to the filtration bleb and transforms the nonperforating filtering surgery into a perforating filtering surgery. After laser treatment, patients were treated with topical steroids for 3 days.
Postoperative examinations were planned at first day, first week, first month, and then monthly till the end of the first year. During each postoperative visit, routine clinical examination was performed with recording of the IOP and detection of any postoperative complication. At the end of the follow-up period after 12 months, the best-corrected visual acuity was evaluated and at the same time visual field examination was performed with recording of the MD measured by the Octopus machine.
Statistical analysis of the pre- and postoperative data was carried out. The surgery was considered to have a complete success when IOP was ⩽18 mmHg without glaucoma medication, a qualified success when IOP was ⩽18 mmHg with or without antiglaucoma medications, whereas failure was considered if the IOP was >18 mmHg with maximum glaucoma medications or if the eye required further glaucoma drainage surgery. Neodymium:yttrium–aluminum–garnet (Nd:YAG) laser goniopuncture was considered as a part of the operative management of the patients and not a sign of failure.
Results
Our study group had a mean preoperative IOP 33.1±6.2 mmHg (range 26–48 mmHg). The mean preoperative best-corrected visual acuity was 0.66±0.2 (range 0.33–1), and the degree of the visual field defect (ie MD) measured preoperatively was 13.58±6.06 dB (range 4.2–24.5 dB).
First day postoperatively, the mean of the IOP was 8.0±3.06 mmHg (range 2–14 mmHg). In the study group, 16 cases had an IOP⩽10 mmHg, whereas the other four cases had an IOP between 11 and 18 mmHg. In the study group, we encountered two cases of shallow anterior camber with low IOP (2 and 4 mmHg) but without iridocorneal touch. One case developed mild hyphaema and another case developed moderate postoperative anterior chamber reaction. No cases of choroidal detachment were encountered. By the end of the first week postoperatively, these cases improved with proper deepening of the anterior chamber.
First month postoperatively, the mean of the IOP was 10.35±3.26 mmHg (range 4–20 mmHg). During this follow-up visit, 10 cases had IOP⩽10 mmHg, nine cases had IOP between 11 and 18 mmHg, whereas in one case, the IOP started to rise to 20 mmHg. In the later case, we decided to interfere by doing Nd:YAG goniopuncture which was done 5 weeks after surgery, and after the laser the IOP dropped to 14 mmHg.
Third month postoperatively, the mean of the IOP started to rise to 16.2±5.47 mmHg (range 6–22 mmHg). During this period, we encountered six cases with IOP above 18 mmHg, and decision to interfere by Nd:YAG goniopuncture was taken. The laser was carried out on all the six cases during the third and fourth months. The IOP dropped in all the cases after the procedure. After the goniopuncture procedures, one case developed partial iris incarceration, but it did not cause complete occlusion of the filtering pathway or distortion of pupil shape, and IOP remained controlled. Also, three cases developed mild hyphaema which improved spontaneously within 5 days.
By the end of the fourth month postoperatively, the mean IOP dropped to 11.75±2.29 mmHg (range 5–15 mmHg). Also, the distribution of the IOP in the group showed lower values as five cases had IOP⩽10 mmHg, whereas the remaining 15 patients had IOP between 11 and 18 mmHg (four of them needed antiglaucoma medications to reach this target level of IOP).
Additional two cases experienced elevation of IOP above 18.0 mmHg 5 months and 9 months postoperatively. These patients were managed by laser goniopuncture that succeeded in controlling IOP in both of them.
By the end of the follow-up period, 12 months after the operation, the mean IOP was 14.6±3.8 mmHg (range 9–24 mmHg) (Figure 2). One case had IOP⩽10 mmHg, 16 patients had IOP between 11 and 18 mmHg (seven cases of them needed medical treatment to reach this level; ie qualified success). Three cases had IOP higher than 18 mmHg in spite of the Nd:YAG laser treatment and antiglaucoma medical treatment, and these patients were scheduled for re-operation (ie failure) (Figure 3).
One case of our study group had progression of the lens opacity. This patient 52 years old and his best-corrected visual acuity was 6/12 preoperatively. Six months after the operation, his cataract progressed and his visual acuity dropped to 6/60. The patient was scheduled for cataract surgery and after the surgery, the IOP remained controlled till the end of the follow-up period.
By the end of the follow-up period, the mean of the best-corrected visual acuity was 0.6±0.2 (range 0.1–1). Field examination was performed for all the patients in the study group and the MD of the visual field was 13.92±6.63 dB (Range 5–25.9 dB).
Discussion
Trabeculectomy has been the standard surgical approach for lowering IOP until NPDS was introduced. Although the reported efficacy of nonpenetrating filtration surgery for open-angle glaucoma is widely different among the studies carried out over the past few years, all of them proved the effectiveness and safety of this procedure especially with the use of the collagen implant.1, 2, 3, 4, 5, 6, 7, 8, 9, 16, 17, 18, 19, 20
In our study, we evaluated the deep sclerectomy operation using the cheaper autologous scleral implant instead of the more expensive collagen implants. Comparison between the preoperative mean IOP (33.1±6.2 mmHg) and postoperative mean IOP at the end of follow-up period (14.6±3.8 mmHg) showed high statistically significant difference between both values (P<0.001) indicating the success of this technique in controlling the IOP.
The idea of the use of autologous scleral implant is similar to that of the other collagen implants used in this operation. It is supposed to promote the filtration by keeping the ‘subscleral aqueous decompression space’ open. Also, it has the same problem of the collagen implant in inducing fibrosis, which limits the postoperative filtration and causes recurrence of the elevation of the IOP. This implant-induced fibrosis can be solved with Nd:YAG goniopuncture to reach the target IOP. As a nondevitalized structure, it seems that this scleral implant induces more fibrosis than the collagen implant.15
This increased implant-induced fibrosis is known by the timing of laser goniopuncture. In our study group using the autologous scleral implant, this implant-induced fibrosis and consequently the elevation of the IOP was started in most of our cases around the third month postoperatively (mean 14.0±10 weeks) (Figures 2 and 3). This duration is much earlier when compared to other studies using the collagen implant in which the implant induced-fibrosis started around 12 months postoperatively.1, 2, 3, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18
Forty-five per cent of our cases (nine out of 20 cases) needed this goniopuncture, which is nearly similar to the rate needed for laser in cases with collagen implant, as documented in previous studies (around 40%).1, 2, 3, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18
Although a controversy may arise about the value of nonpenetrating surgery because of the need for laser interference postoperatively converting the procedure to perforating surgery again, we did not find any major risk after laser goniopuncture in our small study group. In addition, our idea that this stepped intervention is less risky than surgical opening of the anterior chamber during trabeculectomy from the start was supported by most of the previous studies.1, 3, 5, 7
After goniopuncture, we have encountered only one case of iris incarceration in addition to three cases of mild hyphaema shortly after the procedure and these cases did not need any further interference (Nd:YAG or surgical goniosynechiolysis).15, 16, 17, 18
Similar to other studies using the collagen implant1, 2, 3, 4, 5, 6, 7, 8, 9, 13, 15, 16, 17 we obtained low rates of hyphaema, hypotony, flat anterior chamber, and postoperative cataract. No cases of choroidal detachment, endophthalmitis, or cystic bleb formation were seen in our study group. Of course, this is due to the small study group, but generally it gives an idea about the low rate of complications after using the autologous scleral implant similar to the use of collagen implant.
On comparing the mean of the pre- and postoperative visual acuity (0.66 and 0.6, respectively), we noticed that the mean of the visual acuity postoperatively was slightly lower but this difference was statistically nonsignificant (P>0.1) indicating the value of this technique in preserving the visual function of the eyes of the patients. Also, we noticed a statistically nonsignificant difference (P>0.1) between the preoperative MD measured by the visual field (13.58 dB) and the postoperative MD (13.92 dB). This also indicates the value of this technique in preserving the function of the optic nerve owing to proper control of the IOP. These results are similar to the results shown in previous studies evaluating the effect of NPDS on the visual acuity and visual field sensitivity.20
Using the autologous scleral implant at the end of the follow-up period yielded complete success in 50% of cases (10 out of 20 eyes) and qualified success in 85% of cases (additional seven cases). This approximate the success rate of using collagen implant reported in previous studies which was around 63% complete success and 94% qualified success.2, 11, 15, 16, 17, 18
Although there is a difference in the method of the study and the ethnic properties of the study group from the other previous studies carried out, but our results give a preliminary idea about the effectiveness of this technique in managing cases of open-angle glaucoma.
Conclusion
It would appear that the use of autologous scleral implant can give a result comparable to the use of the expensive collagen implant in controlling IOP, but with less operative cost to the patient. It appears to be safe and efficient procedure. The problem of implant-induced fibrosis can be solved by the early use of Nd:YAG goniopuncture if the target IOP was not achieved at any stage of the follow-up of the patients. Further long-term comparative study on wider scale is needed to detect the exact value of this technique and to evaluate its long-term result.
References
Ambresin A, Shaarawy T, Mermoud A . Deep sclerectomy with collagen implant in one eye compared with trabeculectomy in the other eye in the same patient. J Glaucoma 2002; 11: 214–220.
Karlen ME, Sanchez E, Schnyder CC, Sickenberg M, Mermoud A . Deep sclerectomy with collagen implant: medium term results. Br J Ophthalmol 1999; 83 (January): 6–11.
Mermoud A, Schnyder CC, Sickenberg M, Chiou AG, Hediguer SE, Faggioni R . Comparison of deep sclerectomy with collagen implant and trabeculectomy in open-angle glaucoma. J Cataract Refract Surg 1999; 25: 323–331.
Massy J, Gruber D, Muraine M, Brasseur G . Non-penetrating deep sclerectomy in the surgical treatment of chronic open-angle glaucoma; midterm results. J Fr Ophthalmol 1999; 22: 292–298.
Chiselita D . Non-penetrating deep sclerectomy versus trabeculectomy in primary open-angle glaucoma surgery. Eye 2001; 15: 197–201.
Demailly P, Lavat P, Kretz G, Jeanteur-Lunel MN . Non-penetrating deep sclerectomy (NPDS) with or without collagen device (CD) in primary open-angle glaucoma; middle-term retrospective study. Int Ophthalmol 1996–1997; 20 (1–3): 131–140 [Medline].
El Sayad F, Helal M, El-Kholify H, El-Maghraby A . Non-penetrating deep sclerectomy versus trabeculectomy in bilateral primary open-angle glaucoma. Ophthalmology 2000; 107: 1671–1674.
Sanchez E, Schnyder CC, Sickenberg M, Chiou AG, Hediguer SE, Mermoud A . Deep sclerectomy: results with and without collagen implant. Int Ophthalmol 1996-97; 20 (1–3): 157–162 [Medline].
Shaarawy T, Karlen M, Schnyder C, Achache F, Sanchez E, Mermoud A . Five-year results of deep sclerectomy with collagen implant. J Cataract Refract Surg 2001; 27: 1770–1778.
Kozlov VI, Bagarov SN, Anisimoba SY . Nonpenetrating deep sclerectomy with collagen. Ophthalmosurgery 1990; 3: 44–46.
Fyodorv SN, Kozlov VI, Timoshkina NT . Nonpenetrating deep sclerectomy in open angle glaucoma. Ophthalmosurgery 1990; 3: 52–55.
Delarive T, Rossier A, Rossier S, Ravinet E, Shaarawy T, Mermoud A . Aqueous dynamic and histological findings after deep sclerectomy with collagen implant in an animal model. Br J Ophthalmol 2003; 87: 1340–1344.
Shaarawy T, Nguyen C, Schnyder C, Mermoud A . Comparative study between deep sclerectomy with and without collagen implant: long-term follow-up. Br J Ophthalmol 2004; 88: 95–98.
Wevill MT, Meyer D, Van Aswegen E . A pilot study of deep sclerectomy with implantation of chromic suture material as a collagen implant: medium-term results. Eye 2005; 19 (5): 549–554.
Devloo S, Deghislage C, Van Malderen L, Goethals M, Zeyen T . Non-penetrating deep sclerectomy without or with autologous scleral implant in open-angle glaucoma; medium-term results. Graefes Arch Clin Exp Ophthalmol 2005; 8: 1–7.
Shaarawy T, Mansouri K, Schnyder C, Ravinet E, Achache F, Mermoud A . Long-term results of deep sclerectomy with collagen implant. J Cataract Refract Surg 2004; 30: 1225–1231.
Chiou AGY, Mermoud A, Underdahl JP, Schnyder CC . An ultrasound biomicroscopic study of eyes after deep sclerectomy with collagen implant. Ophthalmology 1998; 105: 746–750.
Vuori ML . Complications of neodymium: YAG laser goniopuncture after deep sclerectomy. Acta Ophthalmol Scand 2003; 81: 573–576.
CHENG Jin-wei, MA Xiao-ye, WEI Rui-li . Efficacy of non-penetrating trabecular surgery for open angle glaucoma: a meta-analysis. Chin Med J 2004; 117 (7): 1006–1010.
Lachkar Y, Neverauskiene J, Jeanteur-Lunel MN . Nonpenetrating deep sclerectomy: a 6-year retrospective study. Eur J Ophthalmol 2004; 14: 26–36.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mousa, A. Preliminary evaluation of nonpenetrating deep sclerectomy with autologous scleral implant in open-angle glaucoma. Eye 21, 1234–1238 (2007). https://doi.org/10.1038/sj.eye.6702571
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6702571