Abstract
So far very few patients with sequence variants in the closely related tectonic genes TCTN1-3 have been described. By multi-gene panel next-generation sequencing (NGS) in patients with Joubert syndrome, we identified two more patients and summarize what is currently known about the phenotypes associated with sequence variants in these genes. In a boy aged 12 years with intellectual disability and the classical molar tooth sign on MRI, a homozygous splice-site sequence variant in TCTN3 leading to in-frame skipping of exon 7 was detected. A previously described non-truncating sequence variant in TCTN3 was also associated with Joubert syndrome, whereas four truncating sequence variants were detected in patients with Meckel–Gruber or Mohr–Majewski syndrome. The second patient, a boy aged 7 years with severe psychomotor retardation, was found to carry a homozygous canonic splice-site sequence variant in TCTN2. So far, only three sequence variants associated with Joubert syndrome and two with Meckel–Gruber syndrome have been described in this gene. Reviewing the clinical data on patients with sequence variants in the tectonic genes TCTN1-3 reveals that all of them have a neurological phenotype with vermis hypoplasia or occipital encephalocele associated with severe intellectual disability in the surviving patients. In contrast, other features frequently seen in patients with ciliopathies such as nephronophthisis, liver fibrosis, retinal dystrophy or coloboma have not been reported. Our patients emphasize the usefulness and efficacy of a comprehensive NGS panel approach. A concise genetic diagnosis may help to prevent unnecessary investigations and improve the clinical management of these patients.
Similar content being viewed by others
Introduction
Joubert syndrome [MIM 213300] was first described by Dr Marie Joubert in 1968 in four siblings who displayed a phenotype that would nowadays be considered as Joubert syndrome-related disorder.1 Other than the typical signs of Joubert syndrome such as abnormal breathing pattern in infancy, irregular eye movements, nystagmus, ataxia, psychomotor retardation and agenesis of the vermis, one child also showed an occipital meningoencephalocele, characteristic of Meckel–Gruber syndrome (MKS), highlighting the broad phenotypic spectrum and allelic character of these disorders. Many patients with cerebellar vermis hypoplasia, which in conjunction with other mid-hindbrain abnormalities leads to the typical ‘molar tooth sign’ (MTS) on MRI, have additional symptoms like cystic kidney disease, liver fibrosis, polydactyly, retinal dystrophy or coloboma.2 There is conflicting data if the brain malformation leading to the MTS is also seen in other syndromes that are caused by gene defects which impair primary cilia and associated structures. Joubert-related phenotypes are cerebellar-ocular-renal syndrome, Dekaban–Arima syndrome, acrocallosal syndrome, cerebellar vermis hypo-aplasia, oligophrenia, congenital ataxia, ocular coloboma and hepatic fibrosis, and MKS, the most severe disorder of the spectrum. In other ciliopathies such as orofaciodigital syndrome (OFD) an MTS might be observed as part of the spectrum, but not constantly, and in Senior–Løken syndrome and Bardet–Biedl syndrome (BBS) it is not part of the clinical phenotype. To date, 22 genes, among them TCTN1, TCTN2 and TCTN3, have been described to cause Joubert syndrome. To facilitate optimal patient care and enable reliable prognostic predictions, an accurate description of the range of clinical symptoms associated with defects in a gene is essential. So far, homozygous and compound heterozygous sequence variants in TCTN2 and TCTN3 have been described in only very few patients.3, 4, 5 We here describe two additional Joubert syndrome cases with homozygous sequence variants in these genes and summarize the clinical and genetic findings reported for the tectonic gene family members TCTN1, TCTN2 and TCTN3.
Materials and methods
Patient 1
The male patient originates from consanguineous Persian parents and has two healthy younger sisters. Father and mother are first-degree cousins. In the extended family no other affected members are known. The propositus was born by cesarian section due to breech presentation at 41 weeks of gestation. Birth weight was 3750 g and length 51 cm. At birth a postaxial polydactyly of the left foot was noted. At age 5 months, developmental delay and hypotonia leading to head lag were noted. Sitting was achieved at age 18 months and unsupported walking at 4 years of age. At presentation, at age 12 years, he had an ataxic gait and was unable to stand on one leg. Weight was 51 kg (90th centile), height 156 cm (75th centile) and head circumference 57 cm (1 cm >the 97th centile). Muscle tone was reduced but strength normal. His fine motor abilities were good, eye movements normal. Ultrasound of liver and kidneys as well as laboratory routine investigations in blood and urine were normal. He was severely intellectually disabled and only spoke two words spontaneously, but was reportedly able to repeat longer sentences and follow simple requests in German or Persian. He was friendly, did not, however, interact with other children. An initial diagnosis of autism had been made on the basis of his lack of social interaction, his special interests and repetitive behavior and rituals. MRI of the brain showed enlargement of the fourth ventricle and the typical MTS resulting from vermian hypoplasia and thickened superior cerebellar peduncles (Figure 1).
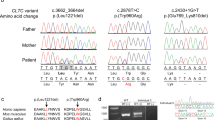
Facial aspect of patient 1 (a); axial T1-weighted image shows the molar tooth sign with vermis hypoplasia and thickened cerebellar peduncles (b); sagittal T1-weighted image shows an enlarged fourth ventricle, vermis hypoplasia and a deepened interpeduncular fossa (c); genomic sequencing results for patient 1 (above) showing the homozygous c.853-1G>T sequence variant and his heterozygous mother (below) (d); cDNA sequencing of a control (above) and patient 1 showing that the splice-site sequence variant leads to skipping of exon 7 (e).
Patient 2
The male patient is the first living child of second-degree consanguineous Turkish parents. Two previous pregnancies of the couple ended in early abortions within the first trimester. The propositus was born in the 34th gestational week (gw) (length 46 cm, weight 2310 g, head circumference 31 cm, all values P50–75, APGAR 9/10/10). Postaxial hexadactyly of all four extremities was noted but no further dysmorphic features. Initial muscular hypertonia at birth was replaced by considerable hypotonia shortly after. From early on the patient’s development was considerably delayed and he never learned to speak more than two words. Chromosome analysis and other clinical examinations were unremarkable. At the age of 6 months, a cranial MRI was performed and showed complex supra- and infratentorial abnormalities such as frontally pronounced polymicrogyria, cerebellar hypoplasia and myelinisation defects. Eye examination revealed nystagmus and hyperopia. At the age of 3½ years, cranial MRI was repeated and showed slight pachygyric alterations with mildly dilated ventricles, particularly in the frontotemporal region and an MTS. At the age of 7½ years, muscle tone was generally increased with signs of spasticity and brisk reflexes. Nevertheless, he learned to walk at age 7 years, his gait was, however, ataxic and dysmetric. EEG was normal. The couple’s fourth pregnancy was terminated after a brain malformation in the posterior fossa and postaxial hexadactyly of hands and feet were noted on ultrasound at the 20th gw suggesting that the fetus was also affected.
Methods
Next-generation sequencing (NGS)
All exons and adjacent intronic boundaries of 129 or 131 genes (including TCTN2 (NM_024809.4, NG_030442.1) and TCTN3 (NM_015631.5, NG_032953.1) in total 2216 or 2335 coding exons, respectively, due to different versions of our ciliopathy multi-gene panel) known or hypothesized to cause ciliopathies were targeted by a custom SeqCap EZ choice sequence capture library (NimbleGen, Madison, WI, USA) and subsequently sequenced on a Roche GS FLX (Roche Diagnostics GmbH, Mannheim, Germany) or an Illumina MiSeq platform (Illumina, INC, San Diego, CA, USA) (2 × 150 PE) according to the manufacturer’s protocol and as described previously.6 The first patient described here was analyzed with an average coverage of 100X, the second patient with an average coverage of about 46X (Supplementary Table 1). Potential sequence variants were confirmed by Sanger sequencing and shown to segregate.
Read mapping and variant analysis
Demultiplexed reads from the GS FLX platform were mapped against the hg19 human reference genome using SMALT (Wellcome Trust Sanger Institute, Cambridge, UK) and paired end reads (2 × 150 bp) from the Illumina MiSeq instrument were mapped against the hg19 human reference genome using BWA.7 Both mapping algorithms were used with the recommended standard settings. The mapped reads were preprocessed with SAMtools and duplicate reads were marked by Picard (http://picard.sourceforge.net). Finally, GATK (Broad Institute, Cambridge, MA, USA) was applied for a local realignment and base quality score recalibration of the mapped reads. Mapping and coverage statistics were generated from the mapping output files using the SeqCap analysis toolkit provided by Roche 454 (Branford, CT, USA) as well as GATK. For the Roche 454 data, variants were detected using SAMtools and for Illumina data, variants were detected using GATK, respectively. Detected variants were checked against the dbNSFP v2.4 as well as dbSNP v138 and HGMDH Professional 2014.1 database (Biobase, Beverly, MA, USA). Single-nucleotide variations (SNVs) and indels were filtered depending on their allele frequency focusing on rare variants with a minor allele frequency of 1% or less. Nonsense, frame shift and canonical splice-site variants were considered pathogenic. Pathogenicity of a rare non-synonymous SNV (nSNV), scores of which were not yet predicted in dbNSFP, was assessed using five in silico prediction software tools: SIFT (Fred Hutchinson Cancer Research Center, Seattle, WA, USA), MutationTaster, PolyPhen-2, AlignGVGD and PMut. An nSNV was considered likely pathogenic when at least three of these algorithms predicted that the variant is probably damaging and when it was predicted as conserved with the conservation prediction algorithms PhyloP and GERP++. Variants not listed in HGMD were considered novel. For visualization of the identified SNVs, SFF files (Roche 454) or FASTQ files (Illumina, INC) of the patients’ sample were loaded into the SeqPilot SeqNext module (v4.1.2, JSI Medical Systems GmBH, Kippenheim, Germany) and reads were mapped against the genomic sequences of the genes in the indicated subpanels JBTS/MKS.v3 (JSI Medical Systems GmBH). SNVs were filtered by their occurrence in at least 25% of the reads. Distinct variations were checked against the in-house database. Owing to inaccurate sequencing of homopolymers by Roche 454 pyrosequencing, small indels in homopolymer stretches were filtered using stringent criteria (bidirectional occurrence in at least 20% of the forward reads and 40% of the reverse reads or vice versa) and visual inspection in the SeqNext software. Identified sequence variants were annotated according to the guidelines published by the Human Genome Variation Society.
RNA analysis
RNA isolation and cDNA synthesis: Lymphocytes of the first patient described were isolated from heparinized whole blood using lymphocyte separation medium (LSM 1077, PAA, Cölbe, Germany) followed by total RNA extraction by the use of peqGOLD TriFast reagents (Peqlab, Erlangen, Germany). Extracted RNA was stored at −80 °C until usage. cDNA was synthesized by the use of TaqMan Reverse Transcription Reagents (Invitrogen, Life Technologies, Darmstadt, Germany) and 200 ng of total RNA and random hexamers for each reaction. To detect the skipped exon, 50 ng of cDNA and the following primers (T6-forward: 5′-GTA CAA CTT GCA CTC GTT TTT TC-3′ and T7-reverse: 5'-CAT AGG TGA CCT GAG AAA CTA CA-3') were used for PCR and analyzed on a 1% agarose gel. For sequencing, specific bands were eluted from the gel by the use of NucleoSpin Gel and PCR Clean-up (Machery-Nagel, Düren, Germany) and subsequently processed for direct dye terminator sequencing with BigDye Terminator Ready Reaction chemistry 3.1 on an ABI PRISM 3130-Avant genetic analyser (Life Technologies). All reactions were performed according to the instructions provided by the manufacturer.
Results
By NGS an average coverage of 100X was achieved for patient 1 and of about 46X for patient 2 (Supplementary Table 1). After filtering, only one variant for patient 1 (TCTN3) and four variants for patient 2 remained that required closer examination (Supplementary Table 2, 3, 4). Apart from the causative sequence variants identified in TCTN3 and TCTN2, respectively, three variants were neglected due to their character as polymorphism with dbSNP annotation or sequencing artifact caused by Roche 454 sequencing.
NGS in patient 1, confirmed by Sanger sequencing, resulted in detection of the homozygous c.853-1G>T sequence variant in TCTN3 altering the respective canonic acceptor splice site (Figure 1). To determine the consequences of the sequence variant, we analyzed cDNA and found that the sequence variant causes exon skipping of exon 7 (NG_032953.1, nt 12614–12649) leading to an in-frame deletion of 10 amino acids. Exon 7 is part of the extracellular domain and is conserved in higher primates as Macaca fascicularis, M. mulatta, Nomascus leucogenys, Pan troglodytes, Pongo abelii and Gorilla gorilla gorilla, but is absent in rodents (http://www.uniprot.org/blast/).
In patient 2, the TCTN2 splice-site sequence variant c.1235-1G>A was detected by NGS and subsequently confirmed by Sanger sequencing (Figure 2). This sequence variant affects the canonic acceptor splice site of intron 10 and presumably leads to in-frame skipping of exon 11 (NG_030442.1, nt 29108–29185) in both transcript variants and thereby loss of 26 amino acids. Unfortunately, no cDNA was available from this patient and we could not validate the consequences on transcript level. In silico mining predicted two alternative splice acceptors after 22 bp (http://wangcomputing.com/assp/) and 35 bp (http://spliceport.cbcb.umd.edu/). Both splice acceptors were located in exon 11, which would result in a loss of 3 and 11 amino acids, respectively. ‘NetGene2’ (Center for Biological Sequence Analysis, Lyngby, Denmark; http://www.cbs.dtu.dk/services/NetGene2/) predicted no alternative splice acceptor sites.
Frontal view of patient 2 (a); axial and sagittal T1-weighted images showing the molar tooth sign and the enlarged fourth ventricle (b, c); pedigree of the family of patient 2 (d); genomic DNA sequences traces of patient 1 (above) showing the homozygous c.1235-1G>A and heterozygous in his mother (below) (e).
Both sequence variants were bioinformatically validated as outlined above and neither of them have been reported in any public database or our in-house database.
The clinical phenotypes associated with sequence variants in TCTN1, TCTN2 and TCTN3 are summarized in Table 1.
Discussion
The primary cilium, a microtubule-based organelle, has an important role in vertebrate development and is crucial for Hedgehog signal transduction.8 In 2006, a new protein, tectonic, was described to be involved in the Hedgehog-mediated patterning of the neural tube.9 The encoding gene TCTN1 was found to be a member of a novel gene family with two other members involved, TCTN2 and TCTN3 that share 49% and 58% similarity, respectively. It was shown that the proteins coded for by TCTN1, TCTN2 and TCTN3 interact with each other and with other genes involved in the pathogenesis of MKS in the transition zone of primary cilia, a region between the basal body and the ciliary axoneme.10 Subsequently, it was shown that the disruptions of TCTN2 and TCTN3 also disrupt the Hedgehog signaling pathway.5, 10 Hedgehog signaling is involved in the patterning of the embryo and defects of the pathway can lead to symptoms like polydactyly, skeletal and central nervous system anomalies.8
Subsequently, sequence variants in all three genes have been reported to cause disorders of the ciliopathy spectrum. So far, sequence variants in TCTN3 have been described in six fetuses with OFD IV and MKS and two siblings with Joubert syndrome.5 The patient we describe here with Joubert syndrome clearly confirms TCTN3 as a gene for Joubert syndrome. Our patient was found to have a homozygous splice-site sequence variant that leads to exon skipping of exon 7 coding for 10 amino acids. However, this deletion does not cause a frame shift and the resulting protein might therefore have residual activity. The nature of the sequence variant further supports the preliminary genotype–phenotype correlation seen in the original publication on TCTN3 sequence variants. Sequence variants leading to lethal phenotypes such as OFD IV and MKS were either early nonsense sequence variants (c.1222C>T; c.1327C>T) or frameshifting deletions (c.650_653del; c.566_567del; c.1348_1349del). In contrast, the described Joubert syndrome patients were shown to carry a (probably hypomorphic) homozygous missense sequence variant (c.940G>A).5
For TCTN2, a genotype–phenotype correlation is not that obvious. The four patients with MKS described thus far all carry a homozygous splice-site sequence variant causing a frame shift (c.1506-2A>G) compatible with a severe phenotype and presumed loss-of-function.4 Patients with Joubert syndrome described so far carry different sequence variant types ranging from a homozygous insertion (c.76_77insG) and a homozygous nonsense sequence variant (c. 1873C>T) to two splice-site sequence variants.3 Although it is likely that these sequence variants result in loss-of-function alleles and lead to nonsense-mediated decay (NMD) with premature termination of translation, circumvention of NMD and production of a shortened protein product with residual function cannot be excluded without further studies. Transcriptional complexity and NMD are well known to have crucial roles in defining the resulting phenotypes of patients.11 All sequence variants described so far in TCTN1 are splice-site sequence variants that result in a frame shift.10, 12 As all of these sequence variants are associated with Joubert syndrome, this might indicate that the loss of Tectonic, the protein coded for by TCTN1, is less severe than defects in TCTN2 or TCTN3.
To better describe the clinical phenotypes associated with sequence variants in TCTN1, TCTN2 and TCTN3, we have summarized what has been reported for these genes (Table 1). Overall, sequence variants in one of the TCTN genes represent rare causes for Joubert syndrome and MKS. Interestingly, all patients have a clear neurological phenotype with either vermis hypoplasia or occipital encephalocele. In the cases without fetal lethality intellectual disability seems to be a constant symptom. On the other hand, frequent symptoms in patients with ciliopathies, such as nephronophthisis, liver fibrosis, retinal dystrophy or coloboma, have not been reported so far. TCTN3 sequence variants seem to be very frequently associated with polydactyly even in the milder cases, whereas kidney cysts and bile duct proliferation were only described in severely affected patients. However, in most of these publications the main focus was on genetic and molecular and cell biology aspects and not on description of the clinical phenotype. Thus, more detailed clinical descriptions are needed that the exciting new genetic and molecular findings can help clinicians to diagnose and treat these rare disorders.
References
Joubert M, Eisenring JJ, Andermann F : Familial dysgenesis of the vermis: a syndrome of hyperventilation, abnormal eye movements and retardation. Neurology 1968; 18: 302–303.
Sattar S, Gleeson JG : The ciliopathies in neuronal development: a clinical approach to investigation of Joubert syndrome and Joubert syndrome-related disorders. Dev Med Child Neurol 2011; 53: 793–798.
Sang L, Miller JJ, Corbit KC et al: Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell 2011; 145: 513–528.
Shaheen R, Faqeih E, Seidahmed MZ et al: A TCTN2 mutation defines a novel Meckel Gruber syndrome locus. Hum Mutat 2011; 32: 573–578.
Thomas S, Legendre M, Saunier S et al: TCTN3 mutations cause Mohr-Majewski syndrome. Am J Hum Genet 2012; 91: 372–378.
Hoff S, Halbritter J, Epting D et al: ANKS6 is a central component of a nephronophthisis module linking NEK8 to INVS and NPHP3. Nat Genet 2013; 45: 951–956.
Li H, Durbin R : Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25: 1754–1760.
Goetz SC, Anderson KV : The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 2010; 11: 331–344.
Reiter JF, Skarnes WC : Tectonic, a novel regulator of the Hedgehog pathway required for both activation and inhibition. Genes Dev 2006; 20: 22–27.
Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS et al: A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet 2011; 43: 776–784.
Cartegni L, Chew SL, Krainer AR : Listening to silence and understanding nonsense: exonic mutations that affect splicing. Nat Rev Genet 2002; 3: 285–298.
Alazami AM, Alshammari MJ, Salih MA et al: Molecular characterization of Joubert syndrome in Saudi Arabia. Hum Mutat 2012; 33: 1423–1428.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
HJB and CB are employees of Bioscientia/Sonic Healthcare.
Additional information
Supplementary Information accompanies this paper on European Journal of Human Genetics website
Rights and permissions
About this article
Cite this article
Huppke, P., Wegener, E., Böhrer-Rabel, H. et al. Tectonic gene mutations in patients with Joubert syndrome. Eur J Hum Genet 23, 616–620 (2015). https://doi.org/10.1038/ejhg.2014.160
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2014.160
This article is cited by
-
Loss of Tctn3 causes neuronal apoptosis and neural tube defects in mice
Cell Death & Disease (2018)