Abstract
Dilated cardiomyopathy (DCM) and malignant ventricular arrhythmias are important causes of congestive heart failure, heart transplantation, and sudden cardiac death in young patients. Cypher/ZASP is a cytoskeletal protein localized in the sarcomeric Z-line that has a pivotal role in maintaining adult cardiac structure and function. The putative mutation p.(D117N) in Cypher/ZASP has been suggested to cause systolic dysfunction, dilated left ventricle with hypertrabeculated myocardium, and intraventricular conduction disturbance, based on two reported sporadic cases. In two unrelated Bedouin families, one with pediatric DCM and the other with DCM and ventricular arrhythmias at young adulthood searching for the causative mutation by exome sequencing we identified the p.(D117N) variant in Cypher/ZASP. However, p.(D117N) did not segregate as the causative mutation in these families, i.e. it was not present in some patients and was found in several individuals who had no clinical manifestations. Furthermore, the carrier frequency in the Bedouin population of origin is estimated to be 5.2%, which is much higher than the incidence of idiopathic DCM in this population. Thus, our data support the notion that the p.(D117N) variant in Cypher/ZASP is not a causative mutation in the families tested by us. The results also indicates that at least in some cases, the p.(D117N) in Cypher/ZASP is not a causative mutation and the role of D117N in Cypher/ZASP in cardiac pathologies should be further clarified and re-evaluated.
Similar content being viewed by others
Introduction
Dilated cardiomyopathy (DCM) is genetically transmitted in 30–40% of cases. Genetic heterogeneity has been identified with mutations in multiple cytoskeletal and sarcomeric genes causing the phenotype. Isolated non-compaction of the left ventricular myocardium (INLVM) with a hypertrophic dilated left ventricle, ventricular dysfunction, and deep trabeculations, is also inherited, and some of the genes identified to date differ from those causing DCM. The mechanistic links between genetic cardiomyopathies and arrhythmias are not fully defined. While cardiac dysfunction by itself clearly increases the risk of malignant arrhythmias, some patients die suddenly before symptomatic heart failure develops. In others, the arrhythmic phenotype can occur without any sign of mechanical dysfunction.1 Mutations in the Z-band alternatively spliced PDZ motif (ZASP1/ Cypher:LDB3) gene that encodes a Z-disc protein have been identified in patients with DCM, INLVM, and various cardiac arrhythmias.2, 3 ZASP1 is one of the major components of the Z-Disc proteins in cardiac muscle,4 which has an important role in stabilizing the Z-Disc structure through its PDZ-mediated interaction with α-actinin-2, the main component of the Z-Disc actin cross-linker, and F-actin, the main cyto-architectural protein of cardiomyocytes5 as well as others.6 Global loss of normal ZASP1 expression or ablation of the murine ZASP homolog cypher can disorganize both sarcomere and cytoskeleton, leading to severe cardiomyopathy and skeletal myopathy in mice and humans, termed zaspopathy.7 Cardiac-specific ablation of the murine cypher led to dilated cardiomyopathy and death before 23 weeks of age.8
A putative mutation p.(D117N) in ZASP1 was reported in two white individuals who suffered from systolic dysfunction, dilated left ventricle with hypertrabeculated myocardium, and intraventricular conduction disturbance. Both patients showed sporadic heterozygote mutation. One of the patients had a family history of sudden cardiac death.2 The ZASP1-D117N also served to suggest a mechanism by which a mutation that disrupts a structural protein can cause electrophysiological remodeling that may contribute to conduction abnormalities. In this context, the effects of ZASP1-D117N on the sodium channel Nav1.5 were studied by patch-clamp recordings of human embryonic kidney-293 cells and neonatal rat cardiomyocytes by Xi and colleagues.9 Their results demonstrated that ZASP1-D117N attenuated INa by 27 and 32%, respectively, and right shifted the activation and inactivation curves compared with ZASP1-wild-type. Based on computational modeling, these effects of ZASP1-D117N on the function of Nav1.5 were suggested to uncover mechanisms leading to conduction disturbances in zaspopathy.9
Here we present compelling evidence that although ZASP1-p.(D117N) is rather prevalent in the Bedouin population of the Negev in Israel, it does not segregate as a causative mutation of DCM, conduction abnormalities or arrhythmic phenotype in our families of interest. Our results indicate that the linkage between ZASP1-p.(D117N) and cardiac pathologies should be further clarified.
Subjects and methods
Patients
The study was approved by the Soroka Medical Center Review Board and the subjects gave informed consent. The patients’ medical records were carefully reviewed, and details of their somatic growth, psychomotor development, clinical course, hospitalizations, and laboratory results were recorded. Their parents and siblings were interviewed and underwent a complete physical examination (particularly focused on the cardiac and neuromuscular systems). The patients’ evaluation included: echocardiology: transthoracic two-dimensional and Doppler echocardiography performed using a System Vivid 7 echocardiograph (GE Medical Systems, Hatfield, UK). Measurements of left ventricular (LV), LV end-diastolic dimension (LVED), and LV end-systolic dimension (LVES) were obtained in accordance with the recommendations of the American Society of Echocardiography.10 Fractional shortening (FS) was calculated as ((LVED-LVES)/LVED) × 100. Dimensions were corrected for age and body surface area (BSA) according to the formula of Henry et al11 (LVED (percent predicted)=(measured LVED/predicted LVED) × 100; predicted LVED= (45.3 × body surface area0.3)−(0.03 × Age)−7.2). LV abnormalities were classified as follows: DCM, LVED≥117% predicted, and FS<25% in the absence of known causes of ventricular dilatation.1112 LV noncompaction (INLVM) diagnosis was determined according to the echocardiographic criteria described by Jenni et al.13, 14
DNA preparation and next-generation sequencing and analysis
Genomic DNA was extracted from peripheral blood and submitted to Otogenetics Corporation (Norcross, GA, USA) for exome capture and sequencing. Data were analyzed for data quality, exome coverage, and exome-wide SNP/InDel using the platform provided by DNAnexus (DNAnexus, Inc, Mountain View, CA, USA).
Basic bioinformatics analysis was performed at DNAnexus and Omicia Opal (Oakland, CA, USA; http://www.omicia.com/) was used to search for cardiomyopathy known mutations.
Verification of the variant
To verify the variant by Sanger sequencing, PCR amplification of exon 6 was performed using primers F: 5′-ACCCACCATCTGGAGACTTG-3′.
R: 5′-GTCCAACGTGAGGGAAGAAA-3′. PCR conditions were 94 °C for 3 min followed by 35 cycles of 94 °C for 40 s, 62 °C for 40 s and 72 °C for 1 min, respectively, with final extension step of 7 min at 72 °C. Direct sequencing the PCR products was performed on an ABIPRISM3100DNA Analyzer with the BigDye Terminator v.1.1 Cycle Sequencing Kit (Applied Biosystems, CA, USA) according to the manufacturer’s protocol.
The data on the population screening was submitted to the LVOD database, individual ID 00046334.
Results and discussion
Clinical findings
Family A – following several independent hospitalizations of pediatric patients from a single large Bedouin family (Figure 1a), we identified a total of five patients at ages 2 months–18 years who were diagnosed with DCM, focal INLVM, and sustained ventricular tachycardia (VT). Two additional family members who passed away before the study (siblings of patients II-3 and II-4, Figure 1a) were reported to have died from sudden death. Two-dimensional echocardiography in the apical four chamber and parasternal short axis images at the level of the ventricles showed dilatation of both ventricles, with global severe depression of the LV function, 4 and 5 trabeculae and intertrabecular recesses in inferior and lateral walls of the left ventricle with normal origins of the coronary arteries. In these lacunar regions, the ratio of compacted versus non-compacted myocardium was 1:2. Cardiac MRI revealed, in short axis, axial and four-chamber images severe dilatation of the left ventricle with severely depressed function. Ejection fraction was calculated to be around 30–65%, with evidence of focal multiple recesses in LV lateral wall. The two older patients were frequently suffering from ventricular tachycardia and on multiple occasions underwent recurrent electrical cardioversion and intravenous administration of amiodarone. They have undergone implantable cardioverter-defibrillator (ICD) implantation and one patient had a heart transplant. The patients’ cardiac manifestations are presented in Table 1.
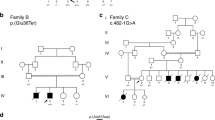
Family B – in the adult cardiac clinic of our institute we identified a large family of Bedouin origin in which an unusually high incidence of non-ischemic DCM in young adulthood and a family history of sudden cardiac death and ventricular arrhythmias are present (Figure 1b). Acquired cardiomyopathy was further ruled out based on past medical history, physical examination, and laboratory tests. Echocardiographic signs of non-compaction were also noted in several siblings. The cardiac manifestations of the family members are depicted in Table 2.
The malignant nature of the disease in this family started after subject II 4 first presented to our cardiology clinic 8 years ago with unexplained syncope, heart failure New York Heart Association class II–III symptoms and non-sustained VT. History revealed that one of the siblings (II 11) died suddenly at the age of 29. Echocardiography revealed severe LV dysfunction, atrial septal defect, and apical trabeculations consistent with a variant of non-compaction syndrome. Coronary angiography revealed normal coronary anatomy and an ICD was implanted for primary prevention of sudden cardiac death. Given his young age and a family history of sudden cardiac death, all other siblings underwent cardiac evaluation for cardiomyopathy, revealing an extremely high incidence of non-ischemic cardiomyopathy in both males and females at the ages of 20–40 years, as depicted in Table 2. Two brothers were diagnosed with moderate to severe LV dysfunction at the ages of 40 and 43 years, without symptoms of heart failure (subjects II 2 and II 8). Both underwent ICD implantation for primary prevention of SCD. Their younger brother (II 3) was found to have mild LV dysfunction with apical trabeculations compatible with a mild variant of non-compaction syndrome. Three sisters (II 5, II 6, and II 7) at the ages of 29, 31, and 37 years, were diagnosed with moderate to severe LV dysfunction, and underwent ICD implantation for primary prevention of SCD. One sister had non-sustained ventricular tachycardia that was recorded by the ICD during follow-up. A fourth sister (II 1) presented with symptomatic right ventricular outflow tract tachycardia that required radiofrequency ablation. An ICD was not implanted yet, given mildly reduced LV function and no evidence of heart failure. Finally, a nephew (III 2) presented at the age of 21 years with severely depressed LV function and non-sustained ventricular tachycardia which necessitated an ICD implantation.
Genetic analysis
The DNA of patients II 1 of family A and II 3 of family B (Figure 1) were exome sequenced. Mutations in genes known to cause cardiomyopathy were verified (100 genes at top of list from genecards (http://www.genecards.org/). No novel variants were found in these genes. The only putative mutation found in heterozygosity in both patients was in Chr10:88446830 (GRCh37) in ZASP1/ Cypher:LDB3 [NM_001080114.1] c.(349G>A), p.(D117N). This variant was reported to be associated with systolic dysfunction, dilated left ventricle with hypertrabeculated myocardium, and intraventricular conduction disturbance in two white patients,2 a 44-year-old female with INLVM and left bundle branch block and a 33-year-old male with INLVM, LV hypertrophy, ventricular bigeminy, and intraventricular conduction delay. We have verified the segregation of this variant in our pedigrees by PCR amplification of exon 6 and sequencing of the PCR products by the Sanger method and found that the variant is not present in all patients and is also present in five healthy members who were thoroughly evaluated, although three of the healthy members could be too young to be informative (Table 3). The segregation of p.(D117N) in ZASP1/ Cypher in the families is presented in Figure 1 and Table 2.
Owing to the above findings we have further verified the prevalence of the variant in unrelated individuals from the general Bedouin population from the same region of southern Israel. Eleven out of 210 chromosomes (5.2%) were found to carry p.(D117N) (all in heterozygous state). Although exact epidemiologic data on the prevalence of DCM, ILNCN, idiopathic ventricular arrhythmias, or ventricular conduction abnormalities in this population is lacking, the majority of pediatric and adult cardiac patients are treated by us and we have not observed that sporadic cases of these cardiac presentations are markedly prevalent in the Bedouin population of the region in comparison with other populations or in comparison with numbers in the literature. Thus, the possibility that the p.(D117N) variant is an actual mutation which increases DCM prevalence in the general Bedouin population of the region is highly unlikely. We cannot exclude however, a modifier effect of p.(D117N), that in the genetic background of the Bedouin population has a very low penetrance, making the p.(D117N) in ZASP1/ Cypher less clinically significant in this specific population. However, the fact that we observed no obvious correlation between disease severity and the presence of p.(D117N) in the multiple patients of family B, make this option less likely.
The putative variant designated rs121908338 appears 13 times in the 1000 genomes database (0.65%) and has a prevalence of 1% in the 662 participants of European descent from the ClinSeq project. The Exome Variant Server (http://evs.gs.washington.edu/EVS/) presents a prevalence of 0.3% in European Americans and 1.2% in African Americans.
As Bedouins and Arabs are probably under-represented in these variant databases, our study demonstrates that care should be taken when assigning pathogenicity to variants based on low representation in these databases for patients of different populations.
Conclusion
Since the segregation pattern in the pedigrees of both families does not show the expected pattern of a causative mutation and this genetic variant occurs in a relatively large proportion of the general population, our results suggest that at least in the Bedouin population (and possibly in other populations) p.(D117N) in ZASP1/ Cypher is not the causative mutation for these cardiac abnormalities. This conclusion is further supported by the demonstration that heterozygous patients are not more severely affected than the patients without the p.(D117N) in ZASP1/ Cypher. Thus, other causative mutations should be sought.
References
Iacoviello M, Monitillo F : Non-invasive evaluation of arrhythmic risk in dilated cardiomyopathy: from imaging to electrocardiographic measures. World J Cardiol 2014; 6: 562–576.
Vatta M, Mohapatra B, Jimenez S et al: Mutations in Cypher/ZASP in patients with dilated cardiomyopathy and left ventricular non-compaction. J Am Coll Cardiol 2003; 42: 2014–2027.
Xing Y, Ichida F, Matsuoka T et al: Genetic analysis in patients with left ventricular noncompaction and evidence for genetic heterogeneity. Mol Genet Metab 2006; 88: 71–77.
Faulkner G, Pallavicini A, Formentin E et al: ZASP: a new Z-band alternatively spliced PDZ-motif protein. J Cell Biol 1999; 146: 465–475.
Klaavuniemi T, Ylanne J : Zasp/Cypher internal ZM-motif containing fragments are sufficient to co-localize with alpha-actinin–analysis of patient mutations. Exp Cell Res 2006; 312: 1299–1311.
Martinelli VC, Kyle WB, Kojic S et al: ZASP interacts with the mechanosensing protein Ankrd2 and p53 in the signalling network of striated muscle. PLoS One 2014; 9: e92259.
Zhou Q, Chu PH, Huang C et al: Ablation of Cypher, a PDZ-LIM domain Z-line protein, causes a severe form of congenital myopathy. J Cell Biol 2001; 155: 605–612.
Zheng M, Cheng H, Li X et al: Cardiac-specific ablation of Cypher leads to a severe form of dilated cardiomyopathy with premature death. Hum Mol Genet 2009; 18: 701–713.
Xi Y, Ai T, De Lange E et al: Loss of function of hNav1.5 by a ZASP1 mutation associated with intraventricular conduction disturbances in left ventricular noncompaction. Circ Arrhythm Electrophysiol 2012; 5: 1017–1026.
Schiller NB, Shah PM, Crawford M et al: Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989; 2: 358–367.
Henry WL, Gardin JM, Ware JH : Echocardiographic measurements in normal subjects from infancy to old age. Circulation 1980; 62: 1054–1061.
Richardson P, McKenna W, Bristow M et al: Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation 1996; 93: 841–842.
Jenni R, Oechslin EN, van der Loo B : Isolated ventricular non-compaction of the myocardium in adults. Heart 2007; 93: 11–15.
Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA : Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart 2001; 86: 666–671.
Acknowledgements
We thank the family members for their participation. The study was supported by a nuclear grant of Ben-Gurion University of the Negev (AL, JMW, YE, and RP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Levitas, A., Konstantino, Y., Muhammad, E. et al. D117N in Cypher/ZASP may not be a causative mutation for dilated cardiomyopathy and ventricular arrhythmias. Eur J Hum Genet 24, 666–671 (2016). https://doi.org/10.1038/ejhg.2015.195
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejhg.2015.195