Abstract
Background
To propose a classification system for retinal angiomatous proliferation (RAP) on the basis of the indocyanine green angiography (ICG).
Methods
Retrospective chart review of 55 eyes of 55 patients presenting with RAP. Fluorescein angiography (FA), ICG and optical coherence tomography (OCT) were used to evaluate the patients.
Results
All RAP lesions appeared as occult or minimally classic CNV on FA without clear evidence of pigment epithelium detachment (PED). We were able to identify five different patterns of RAP on the basis of ICG. These were focal (27.2%), irregular (21.8%), circular (21.8%), multifocal (18.2%), and combined (10.9%) hyperfluorescence. The sudden termination of retinal vessel course sign was observed in 14 of 55 eyes (25.4%), which had a circular or irregular pattern on the ICG. Only the circular RAP exhibited a late hypofluorescence (‘wash out’) with staining of the surrounding tissue on the ICG. Forty eight of 55 eyes (86%) had PED according to the OCT. Out of these 48 eyes, 19 had intraretinal fluid (IRF) alone, and the rest had IRF and subretinal fluid. The eight eyes (14%) without PED belonged to the focal hyperfluorescence group and the fluid was located intraretinally in cystic spaces. In addition, in four eyes (7%) with coexisting CNV a band of tissue beneath the RAP protruding in the PED was observed.
Conclusion
We propose a classification system for RAP on the basis of ICG and present the angiographic and OCT findings of these lesions. These data may further aid in the early diagnosis of RAP and can be also used for prognosis and clinical course documentation.
Similar content being viewed by others
Introduction
In recent years, a peculiar neovascular lesion, characterized by the presence of deep retinal hemorrhages, and retinochoroidal anastomosis (RCA) has been described in patients with age-related macular degeneration (AMD). Hartnett et al1 described first in 1992, in a landmark paper, retinal neovascularization as an early finding in wet AMD. They described nine patients with pigment epithelium detachment (PED) and associated retinal vascular abnormalities, described as ‘retinal angiomatous lesions’. Later, Kuhn et al2 identified an RCA as a potential manifestation of this subtype of AMD and Hartnett et al3 suggested that the neovascularization was deep in the retina and an anastomosis connecting the retina circulation to a deep retinal vascular lesion, namely ‘deep retinal vascular anomalous complex’, was characteristic of the disorder. In 2000, Slakter et al4 recognized an RCA with or without a PED as a form of AMD resistant then to the standard wet AMD care (laser photocoagulation). Yannuzzi et al,5 coined the term retinal angiomatous proliferation (RAP), emphasizing upon the retinal origin of these lesions and proposed a three stage development. Around 2 years later, Gass6 proposed a five-stage development and advocated in favor of the choroidal origin of this lesion naming it occult chorioretinal anastomosis. More recently, Freund et al,7 reclassified these lesions suggesting a dual origin, retinal and/or choroidal.
The recognition of RAP can sometimes become challenging, as fluorescein angiography (FA) commonly reveal occult CNV, an indistinct area of stippled hyperfluorescence increasing in the late frames of the FA.5 In addition, RAP is believed to have a different natural course and response to therapy than typical CNV.8
In this study, we describe the angiographic and optical coherence tomography (OCT) findings in patients with RAP. We also propose a classification system for the angiographic pattern of RAP, on the basis of ICGA, that may be useful in diagnosis and documentation of the clinical course of the condition.
Methods
We retrospectively reviewed the angiograms and OCT images of 55 eyes of 55 consecutive patients with RAP in one of the three vasogenic stages described by Yannuzzi et al (stage I: intraretinal neovascularization, stage II: subretinal neovascularization with or without PED and stage III: CNV with RCA).5 The diagnosis of RAP was made when hemorrhages and lipid exudates were identified on dilated fundus exam in an area of occult CNV on the basis of fluoroscein angiogram and in the presence of a hot spot on the indocyanine green angiography (ICG). Patients with myopia, angioid streaks and other choroidal and retinal diseases associated with CNV, as well as patients with retinal vascular disease, such as vein occlusion, diabetic retinopathy or retinal telangiectasis were excluded. Patients that had received previous treatment of any kind for the RAP were also excluded. The study was approved by the IRB of Attikon University Hospital.
All the patients underwent sequential FA and ICGA and OCT between February 2005 and June 2008 at the Retina Service of the 2nd Department of Ophthalmology of the University of Athens Medical School. A confocal laser-scanning ophthalmoscope (Heidelberg Retinal Angiograph, Heidelberg Engineering, Heidelberg, Germany) was used in all cases. The same technique was applied in all cases by a single experienced photographer. All angiograms were screened and analyzed by two masked observers. In cases of a disagreement, a third opinion was obtained and the majority opinion was accepted.
Stratus OCT evaluation was also performed in all patients and consisted of six linear 6.00 mm scans oriented at intervals of 30° and centered on the foveal region. To verify the accuracy of the OCT findings, the fundus area encompassing the RAP was additionally scanned using several vertical and horizontal 6.00-mm length scans.
Results
The results of 55 eyes of 55 patients with the diagnosis of RAP were reviewed. 22 (40%) were men and 33 (60%) were women. The mean age of the patients was 73 years (range 68–82). Two (3.6%) of the patients had stage I RAP, 49 (89%) stage II RAP and four (5.5%) stage III RAP, according to the Yannuzzi classification.5 There was agreement between the masked observers in 46 eyes (84%) and nine eyes went onto the third observer (16%). In five eyes (9%) the RAP lesions were located superiorly to the fovea, in 30 eyes (54.5%) temporally to the fovea, nasally in 14 eyes (25.4%) and inferiorly in six eyes (10.9%). On the basis of the FA angiograms all eyes demonstrated a poorly defined leakage producing an indistinct continuous zone of leakage within and beneath the retina, which was interpreted as occult or minimally classic CNV. In 35 eyes of 55 eyes (63.6%) there was dye leakage into the retina in cystic spaces on late FA frames. According to the ICG angiograms, there are five patterns of RAP lesions during the transit phase of high-speed ICG. These are: (1) focal hyperfluorescence in 15 eyes (27.2%, Figure 1), (2) irregular hyperfluorescence in 12 eyes (21.8%, Figures 2,3,4), (3) circular hyperfluorescence in 12 eyes (21.8%, Figure 5), (4) multifocal hyperfluorescence in 10 eyes (18.2%, Figure 6) and (5) combined hyperfluorescence in six eyes (10.9%, Figure 7). More specifically, four eyes had a combination of focal (1) and irregular (2) hyperfluorescence and the other two eyes demonstrated a combination of focal (1), irregular (2), and circular (3) hyperfluorescence. For more information about the characteristics of the RAP lesions refer to the Table 1.
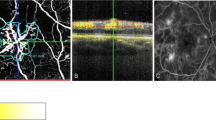
Focal RAP. The FA shows a small hyperfluorescence in early frames (a, red arrow) with late diffuse leakage of the dye in cystic spaces near and inside the fovea with stippled hyperfluorescence beneath the fovea, which has the angiographic signature of occult CNV (b, the hyperfluorescence located superiorly and temporally is an area of geographic atrophy, white arrow). The ICG shows a focal pattern in early and late frames (c and d, respectively). A PED is evident on the OCT (e) that was otherwise not detectable on the FA.
RAP stage II classified as irregular hypefluorescence. The fluorescein leaks into the retina in cystic spaces (a, b) surrounding the fovea and without distinct margins superiorly and temporally to the fovea. The ICG reveals the presence of a RAP classified as irregular hyperfluorescence; there is also a sudden termination of a retina vessel course in the RAP (c, red arrow). A PED is demonstrated on the OCT, which is undetectable on the FA. There is no direct evidence on the FA (a, b) and the ICG (c, d) of a CNV. However, the sudden termination of the retina vessel (possible RCA), the band of tissue extending into the PED detected on the OCT (e, red arrow) and the presence of subretinal fluid (SRF) raise the possibility of a CNV.
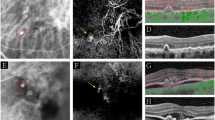
RAP stage II classified as irregular. There is leakage with diffuse, indistinct margins on the FA (a, b). The ICG (c) shows a RAP lesion classified as irregular. In addition, the sudden termination with angulation of retinal vessels is apparent on the ICG (c, red arrows). There is no wash out of the dye in the late ICG (d). The OCT (e) shows a PED with IRF in cystic spaces and subretinal fluid.
RAP stage III classified as irregular (a). The underlying CNV (black arrows) has a surface area greater than the overlying RAP (red arrows), which makes its detection possible. The OCT (b) shows the band of tissue that protrudes into the PED, a finding present in all RAP stage III and in one stage II. There is also subretinal fluid at the edge of the PED and over the CNV.
RAP stage II classified as circular hyperfluorescence. The FA demonstrates an indistinct poorly defined area of leakage (a). The ICG shows a circular RAP. A retina-retina anastomosis is also present (b, red arrow: artery, blue arrow: vein) and the RAP shows a ‘wash-out’ phenomenon at the late frames of the ICG with staining of the surrounding tissue in a circular fashion (c). A PED is detected on the OCT (FA again is inconclusive regarding the presence of a PED or not) (d). The RAP is shown on the OCT as an area of relative hyperreflectivity in the inner retina (d, red bold arrow).
RAP stage II classified as combined (multifocal and irregular). There is an ill-defined leakage on the FA (a, b). The ICG (c) reveals the presence of focal (red arrows) RAP lesions and of an irregular (blue arrow) RAP lesion. The yellow arrows on the ICG (c) represent telangiectatic changes. The OCT (d) shows a RAP (red bold arrow) with PED, IRF (cystic spaces) and subretinal fluid.
The OCT revealed PED in 48 eyes (86%). Out of these 48 eyes, 19 eyes had intraretinal fluid (IRF) alone (in 18 eyes the fluid was located in cystic spaces, whereas in one eye there were no cystic spaces). No patient had subretinal fluid (SRF) alone. The rest 29 eyes had a combination of intraretinal and SRF. All the eyes with RAP lesions classified (based on the ICG) to the categories 2–5 and eight eyes with RAP belonging to type 1 had PED. On the other hand, in the rest seven eyes that had focal (1) RAP lesions there was no PED and the fluid was located intraretinally in cystic spaces (two of these were RAP stage I). Furthermore, in four eyes with RAP stage III (according to Yannuzzi), in which the surface area of the underlying CNV is obviously larger compared with the surface area of the overlying RAP in the ICG (Figure 4), and in one eye with RAP stage II the OCT showed a large notched PED with SRF at the edges and a band of tissue with medium reflectivity protruding in the PED (Figures 2 and 4).
Discussion
On the basis of the FA, RAP mimics the angiographic signature of the occult or minimally classic CNV. In addition, the vascularized PED in eyes with RAP does not have the appearance on the FA according to Gass (pooling of the dye in late frames with or without notched margins).9 Thus, the FA alone may not be able to reveal the presence of a PED in all RAP lesions (Figures 2, 3, 5 and 6). A possible explanation is the different sequence of events in CNV and RAP. The CNV originates from the choroid, next a PED is formed and later on fluid leaks into the retina. On the other hand, RAP originates from the inner retina demonstrating leakage in the retina and in a subsequent stage a PED can be formed. The presence of this early IRF can be confusing in terms of detection of an underlying PED.
On the basis of the ICG we were able to identify five distinct types of RAP lesions. Despite the fact that all lesions were newly diagnosed, we are not sure whether these types represent time dependent points in the course of the vasogenesis. A prospective follow-up study would be helpful, but its design carries significant difficulties, as these patients require immediate treatment after the diagnosis, as the prognosis of RAP is generally poor.
The ICG is a useful tool in determining the nature of the indistinct hyperfluorescence seen on the FA. However, it is a study that gives us information in two dimensions, such as the FA. Thus, someone cannot exclude the presence of a lesion that originates from the choroid (CNV) that has a smaller surface area compared with the overlying lesion we detect on the ICG (RAP).10 This is more important for the circular and irregular RAP lesions and not for the focal or multifocal RAP as the latter have a relatively small surface area, which almost excludes the presence of an even smaller underlying CNV. The sudden termination of the retinal vessels course in the lesion, which may suggest the presence of a RCA with an underlying CNV with smaller surface area compared with the RAP, was observed only in circular or irregular RAP (Figures 2 and 3). This is in favor of the fact that the focal or multifocal RAP is less likely to be accompanied by a CNV and thus, they may have a better prognosis. We believe that, with the help of the high-resolution spectral domain OCT we will be able to study these lesions in greater detail and uncover the presence of these small accompanying CNVs. We did not include the spectral domain technology, as it was not available in 2005, when we began this study. On the basis of all the above observations, the classic staging of RAP according to Yannuzzi5 carries a significant amount of uncertainty regarding the true nature of RAP lesions. The term ‘type 3 neovascularization’, proposed by the same group in 2008,7, 11 is a more appropriate term that should be used for RAP lesions. We used the Yannuzzi staging and not the Freund terminology because when we started our study this was the standard staging for RAP. In addition, the fact that all the RAP without PED demonstrated only IRF on the OCT, whereas the RAP with PED had predominantly IRF and/or SRF may provide additional evidence for the coexistence, along with the RAP, of an underlying CNV with surface area smaller than the overlying RAP. So, the presence of a PED and/or SRF may suggest the coexistence of a CNV (when ICG fails to reveal it). To further support this notion, as the CNV breaks through the RPE, the subretinal space is the first space that fluid will be extravasated, whereas IRF suggests extravasion from a lesion of retinal origin. The band of tissue that extends into the PED (Figures 2 and 4) was observed in all stage III RAP and thus may be a sign indicative of an accompanying CNV when angiography fails to demonstrate it. This finding was also present in one eye with RAP stage II (Figure 2) (classified as II, as there was no angiographic evidence for coexisting CNV). We believe that the early identification of these small accompanying CNVs in RAP is very important because of the different prognosis, as an RCA will eventually form in these lesions. Consequently, the presence of a lesion that is perfused by two vascular networks (retinal and choroidal) with different hemodynamics poses a challenge to the retina specialist regarding its treatment.
Matsumoto et al12 reported the OCT findings in nine eyes (one with stage I and eight with stage II) with RAP using Spectral Domain technology (Cirrus, Zeiss, Dublin, CA, USA). Intraretinal neovascularization appeared as a highly reflective mass from the outer plexiform layer to the deeper layer in seven eyes and the underlying retinal pigment epithelium was disrupted beneath the intraretinal neovascularization in all eyes. All eyes had retinal edema around the intraretinal neovascularization, whereas serous retinal detachments were seen in only two eyes with stage II disease with pigment epithelial detachment. In another study13 comparing the morphological findings of the SD-OCT (Cirrus) to the time-domain OCT (Stratus), the authors reported the presence of RPE breaks only in eyes with RAP stage III and not with stage II.
Another important finding in our study is that the dye wash out in the late phases of the ICG, as previously described,4, 5 was present only in the cases with circular hyperfluorescence (Figure 5). This can be explained by the fact that these lesions may originate solely from the retina and not the choroid. This way, the ICG dye is washed out at the late phases because there is no fibrovascular tissue (due to a CNV) in the lesion that the dye will stain. The dye wash out that is observed in RAP is accompanied by a late staining at the rim and surrounding space of the lesion, probably due to the existence of exudated fibrin in this area.14
In conclusion, we classify the RAP lesions in five different types (Figure 8) on the basis of ICG. This classification system can be potentially used to document the clinical course of the condition after diagnosis and any subsequent treatment, and determine prognosis and optimal management plan in each different subtype. We also show that the PED on the FA in RAP does not have the classic appearance according to Gass, the wash out phenomenon is observed only in circular RAP and the RAP without PED exhibit only IRF on the OCT and have a focal pattern. The sudden termination of retinal vessel course sign on the ICG is present only in circular or irregular RAP. Moreover, someone cannot exclude the presence of CNV with smaller surface area compared with the overlying retinal neovascularization with the current standard imaging technology (Stratus OCT, FA and ICG). The detection in the OCT of a band of tissue protruding in the PED may be indicative of an accompanying CNV when FA or ICG fail to demonstrate it. Further larger prospective studies using spectral-domain OCT technology with long follow-up are necessary to study RAP and develop effective treatments.

References
Hartnett ME, Weiter JJ, Garsd A, Jalkh AE . Classification of retinal pigment epithelial detachments associated with drusen. Graefes Arch Clin Exp Ophthalmol 1992; 230: 11–19.
Kuhn D, Meunier I, Soubrane G, Coscas G . Imaging of chorioretinal anastomoses in vascularized retinal pigment epithelium detachments. Arch Ophthalmol 1995; 113: 1392–1398.
Hartnett ME, Weiter JJ, Staurenghi G, Elsner AE . Deep retinal vascular anomalous complexes in advanced age-related macular degeneration. Ophthalmology 1996; 103: 2042–2053.
Slakter JS, Yannuzzi LA, Schneider U, Sorenson JA, Ciardella A, Guyer DR et al. Retinal choroidal anastomoses, occult choroidal neovascularization in age-related macular degeneration. Ophthalmology 2000; 107: 742–753; discussion 753-744.
Yannuzzi LA, Negrão S, Iida T, Carvalho C, Rodriguez-Coleman H, Slakter J et al. Retinal angiomatous proliferation in age-related macular degeneration. Retina 2001; 21: 416–434.
Gass JD, Agarwal A, Lavina AM, Tawansy KA . Focal inner retinal hemorrhages in patients with drusen: an early sign of occult choroidal neovascularization, chorioretinal anastomosis. Retina 2003; 23: 741–751.
Freund KB, Ho IV, Barbazetto IA, Koizumi H, Laud K, Ferrara D et al. Type 3 neovascularization: the expanded spectrum of retinal angiomatous proliferation. Retina 2008; 28: 201–211.
Bottoni F, Massacesi A, Cigada M, Viola F, Musicco I, Staurenghi G . Treatment of retinal angiomatous proliferation in age-related macular degeneration: a series of 104 cases of retinal angiomatous proliferation. Arch Ophthalmol 2005; 123: 1644–1650.
Gass JD . Serous retinal pigment epithelial detachment with a notch. A sign of occult choroidal neovascularization. Retina 1984; 4: 205–220.
Rouvas AA, Papakostas TD, Vavvas D, Vergados I, Moschos MM, Kotsolis A et al. Intravitreal ranibizumab, intravitreal ranibizumab with PDT, intravitreal triamcinolone with PDT for the treatment of retinal angiomatous proliferation: a prospective study. Retina 2009; 29: 536–544.
Yannuzzi LA, Freund KB, Takahashi BS . Review of retinal angiomatous proliferation or type 3 neovascularization. Retina 2008; 28: 375–384.
Matsumoto H, Sato T, Kishi S . Tomographic features of intraretinal neovascularization in retinal angiomatous proliferation. Retina 2010; 30: 425–430.
Krebs I, Glittenberg C, Hagen S, Haas P, Binder S . Retinal angiomatous proliferation: morphological changes assessed by Stratus, Cirrus OCT. Ophthalmic Surg Lasers Imaging 2009; 40: 285–289.
Lafaut BA, Aisenbrey S, Vanden Broecke C, Bartz-Schmidt KU . Clinicopathological correlation of deep retinal vascular anomalous complex in age related macular degeneration. Br J Ophthalmol 2000; 84: 1269–1274.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Rouvas, A., Papakostas, T., Ntouraki, A. et al. Angiographic and OCT features of retinal angiomatous proliferation. Eye 24, 1633–1643 (2010). https://doi.org/10.1038/eye.2010.134
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2010.134
Keywords
This article is cited by
-
Neovascular age-related macular degeneration in which exudation predominantly occurs as a subretinal fluid during anti-vascular endothelial growth factor treatment
Scientific Reports (2022)
-
Circumscribed choroidal haemangioma: Indocyanine green angiography features on scanning laser ophthalmoscopy versus traditional digital fundus photography
Eye (2021)
-
Distinguishing retinal angiomatous proliferation from polypoidal choroidal vasculopathy with a deep neural network based on optical coherence tomography
Scientific Reports (2021)
-
Impact of macular fluid features on outcomes of anti-vascular endothelial growth factor treatment for type 3 macular neovascularization
Scientific Reports (2021)
-
One year results of intravitreal ranibizumab monotherapy for retinal angiomatous proliferation: a comparative analysis based on disease stages
BMC Ophthalmology (2015)