Abstract
Purpose
To assess the effect of antiviral treatment on corneal graft survival following penetrating keratoplasty for herpetic keratitis.
Methods
Retrospective cohort study of 454 patients receiving primary penetrating keratoplasties (PKs) for viral infection reported to NHS Blood and Transplant (NHSBT) between April 1999 and June 2005. Follow-up data were available on 403 PKs. Kaplan–Meier survival estimates were used to determine graft survival for the three treatment groups: no medication, topical antiviral, and oral antiviral medication. A Cox regression model was used to investigate the combined effects of all additional factors on graft failure. The model was fitted using all pre-operative factors first and then post-operative factors including type of antiviral medication were included.
Results
Patients who received oral antiviral medication post-operatively had consistently better graft survival than those receiving no medication or only topical medication. Patients receiving oral antivirals were less than a third as likely to have a failed graft at 5 years compared with those on no antiviral medication (relative risk (RR) 0.3, CI: 0.2–0.7, P=0.002). Other factors that were found to influence the risk of graft failure were the presence of deep corneal vascularisation (P=0.009), PK performed for therapeutic reasons (P=0.03), large diameter grafts (P=0.04), and experiencing a rejection episode (P=0.003).
Conclusion
Oral antiviral treatment reduces the risk of graft failure in patients undergoing primary PK for herpetic keratitis and should be routinely used in this group of patients post-operatively unless contra-indicated.
Similar content being viewed by others
Introduction
Herpetic keratitis is a major cause of corneal scarring and consequent visual loss in developed countries.1 Penetrating keratoplasty (PK) may be undertaken to visually rehabilitate patients, yet graft survival in patients with herpetic keratitis remains lower than that of other common corneal conditions such as keratoconus, Fuchs' endothelial dystrophy, and non-herpetic corneal scarring.2 This reduced survival may be attributable to the indefinite potential for recurrence of herpetic keratitis, which represents a leading cause of graft failure in patients who undergo PK for this indication.3
Clinical practice has therefore moved towards the use of antiviral prophylaxis against reactivation of herpes simplex virus (HSV)— a concept that was initially used for reducing recurrences of genital herpes simplex.4 In ocular herpetic infection, antiviral treatment was initially focused on topical medication, but in view of complications from epithelial toxicity and persistent epithelial defects, its use did not extend to routine prophylactic treatment following PK. The Herpetic Eye Disease Study Group found that using prophylactic oral acyclovir helped to prevent relapses in patients with recurrent herpetic eye disease, but the study group excluded patients who had previously undergone keratoplasty surgery.5 Smaller scale studies have since found reduced graft failure in patients receiving prophylactic post-operative oral acyclovir,3, 6, 7 although no benefit in rates of graft failure have also been reported.8
In the United Kingdom, data on corneal transplants are routinely collected by NHS Blood and Transplant (NHSBT) through the United Kingdom Ocular Tissue Transplant Audit. This study aims to analyse these data for patients undergoing primary PK for herpetic infection to determine whether the use of oral antiviral medication affects graft survival, after allowing for other confounding factors.
Patients and methods
NHSBT routinely collect data on patients undergoing PKs through the Unite Kingdom Ocular Tissue Transplant Audit. These data are collected from across the United Kingdom, although excludes corneas supplied by East Grinstead and Moorfields eye banks. Data submission by surgeons is through a standard set of forms. A form is completed at the time of surgery, recording details such as age, sex, indication for transplantation, type of procedure, donor and recipient trephine size, pre-operative risk factors, and best corrected visual acuity (BCVA). Surgeons are required to submit follow-up data at 1, 2, and 5 years postoperatively, recording whether the graft has failed and, if so, the reason for failure, postoperative complications (recurrence of original disease, infection, loose/broken sutures, wound leak), subsequent cataract, or other ocular surgery, rejection episodes, topical and oral medications (antiviral, steroid, glaucoma, or other immunosuppressant therapy), and details of BCVA, best day-to-day visual acuity and refractive outcomes.
We retrospectively analysed patients who received a primary PK for viral infection between April 1999 and June 2005. Statistical analysis was performed using SAS V9.1 software (SAS Institute Inc., Cary, NC, USA). Three treatment groups were defined: no antiviral medication, only topical antiviral, and oral antiviral (with or without topical antiviral). Kaplan–Meier survival estimates were used to determine graft survival and a Cox proportional hazards regression model was used to investigate the combined effects of all additional factors on graft failure. The model was fitted using all pre-operative factors first and then post-operative factors were included (post-operative antiviral medication, steroid medication, recurrence of original disease, and time to first rejection episode). The factors considered are listed in Table 1.
The type of antiviral medication may change from one visit to the next, so the effect of this factor was modelled as a time-dependent variable. The same approach was used to assess the impact of rejection by including time to first rejection episode in the model. The level of significance was set at 5%. Relative risks from the Cox model are quoted with 95% confidence intervals (95% CI).
Results
Patients
During the study period, 454 primary PKs for viral infection were reported to NHSBT. Follow-up data were available for 403 PKs; there were 235 males and 168 females with median ages, respectively, of 63 years (IQ 49–74) and 65 years (IQ 50–78).
Univariate graft survival
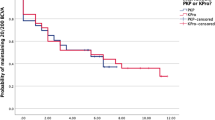
Univariate Kaplan–Meier survival curves for the three treatment groups are shown in Figure 1.
Log-rank tests revealed differences in graft survival between the three groups (P=0.04). Patients who received oral medication postoperatively had consistently better graft survival than those receiving no medication or only topical medication. At 5 years, the graft survival rates for the three groups were 75% (95% CI: 64–84) for oral antivirals, 65% (95% CI: 44–80) for topical antivirals only, and 70% (95% CI: 56–80) where no antivirals had been used postoperatively.
Multivariate analysis of graft failure
There was a more marked reduction in risk of graft failure in the group receiving oral antivirals after adjusting for the effects of all additional factors on graft failure, using the Cox model. These patients were less than a third as likely to have a failed graft at 5 years compared with those on no antiviral medication (RR 0.3, CI: 0.2–0.7, P=0.002). The impact of topical medication was less certain but cannot be dismissed (RR 0.2, CI: 0.02–1.2, P=0.07). The other factors found to significantly influence graft failure are shown in Table 2.
These factors increasing the risk of graft failure included the presence of deep corneal vascularisation (110 (27%) with 1 quadrant, 57 (14%) with 2 quadrants, 3 (4%) with 3 quadrants, and 4 (4%) with 4 quadrants), PK performed for therapeutic reasons (that is, not just to improve vision) and large diameter grafts (>8 mm). Postoperatively, the 76 patients who experienced a rejection episode were more than twice as likely to have a failed graft at 5 years (RR 2.3, 95% CI: 1.3–3.8, P=0.003).
Discussion
Herpetic keratitis is a common indication for corneal transplantation, but carries an increased risk of graft failure,2 prompting investigation into the use of postoperative antiviral medication to improve graft survival. Antiviral treatment following PK initially focused on topical antivirals, although complications from epithelial toxicity, delayed wound healing and persistent epithelial defects limited routine prophylactic use.9 Ficker et al10 in 1988 found that topical antiviral prophylaxis during intensive steroid treatment for graft-rejection episodes both reduced the incidence of HSV recurrence complicating rejection, and improved the outcome following rejection. Beyer et al11 in 1989 went on to investigate the use of systemic acyclovir in a rabbit model of HSV keratitis and demonstrated a reduction in viral shedding, geographical ulceration and stromal keratitis in the treatment group after keratoplasty. Barney and Foster3 subsequently performed a small prospective randomised trial of oral acyclovir after PK for herpes simplex keratitis and found a reduction in both HSV recurrence and graft failure.
Van Rooij et al8 found prophylactic oral acyclovir reduced the risk of HSV recurrence following PK, but did not find a benefit in overall graft failure compared with placebo, although other studies have found reduced rates of graft failure in patients receiving oral acyclovir.3, 6, 7 Our study found a graft survival rate of 75% at 5 years in patients receiving oral antiviral treatment. These patients showed a more than threefold reduction in risk of graft failure at 5 years compared with those not receiving any antiviral medication when the bias from additional variables were accounted for. Our analysis therefore, adds to the weight of evidence favouring the use of oral antiviral treatment and supports its routine use following PK for HSV in all patients unless contra-indicated.
Acyclovir is the most commonly used oral antiviral and this is generally accepted to be a safe, well-tolerated drug. Acyclovir is an acyclic purine nucleoside analogue that inhibits DNA replication, thereby reducing the risk of reactivation of latent HSV in the trigeminal ganglion. Topical antivirals will only function locally on the cornea and before common usage of postoperative systemic antivirals, Moyes et al9 demonstrated decreased rates of HSV recurrence and rejection episodes with topical antiviral prophylaxis, but the benefit did not extend to an improvement in overall graft survival. Our analysis also did not provide unequivocal support for the routine use of topical antiviral medication. Kaplan–Meier univariate analysis showed no benefit, and although multivariate analysis was suggestive of a reduced risk of graft failure with topical treatment, this did not reach the 5% level of significance.
A recent study by Goldblum et al12 also investigated the use of valacyclovir as an alternative to oral acyclovir. This found prophylactic valacyclovir to be at least as effective as acyclovir in preventing recurrence of HSV keratitis following corneal transplantation and equally effective concerning graft survival. The potential benefits include higher plasma levels of the drug and reduced frequency of administration, although in practice the actual dosage and frequency of antiviral medication required remains uncertain. Treatment with acyclovir 400 mg twice daily for at least 1 year postoperatively has been previously recommended6, 8, 13 but longer treatment may be indicated. Conversely, Jansen et al14 have reported follow-up data for patients given only 6 months postoperative oral acyclovir following PK for HSV keratitis. At 5 year follow-up, the benefit was still significant with a lower cumulative rate of herpetic recurrence, although the incidence of graft failure was too low to analyse differences between treatment groups. Unfortunately our study does not provide further information on the optimal duration of treatment, as the precise duration of individuals' treatment with antivirals is not recorded: this is a shortcoming of our data and a consequence of the retrospective data collection.
This retrospective nature of our study is its principal limitation. We were confined to data that could be extracted at the 1, 2, and 5 year follow-up points. In the case of medications, if patients were not receiving any of the specified medications, respondents were asked the date of the last reported usage if applicable, but we were unable to collect further data beyond these details specified on the form at each follow-up point. Additionally, although the study group is assumed to represent HSV infection, the inclusion of some patients with HZV cannot be completely ruled out. Herpes zoster virus (HZV) keratitis is an infrequent indication for PK,2 but clinical differentiation from HSV is not always straightforward15 and ideally in a prospective study, confirmation of the presence of HSV by culture, PCR, or immunohistochemistry would be sought.
Besides the issue of postoperative antiviral treatment, several other factors were found to have an effect on graft failure. Of the pre-operative factors considered, whether the eye was grafted for therapeutic reasons, the presence of deep neovascularisation, and larger diameter grafts were all associated with a higher risk of graft failure. It would be expected that grafts performed for therapeutic reasons would have a higher risk of graft failure as actively inflamed eyes have been shown to be a risk factor for graft failure in herpetic keratitis.10, 16, 17 This lends support to the principle of temporising measures such as gluing or lamellar patch grafting in eyes with, or threatened with perforation. This may allow for PK to be performed at a later date when the eye is quiet.
Many studies have reported on the influence of graft diameter18, 19, 20 and corneal vascularisation15, 21, 22 on graft survival; a recent meta-analysis of 24 944 grafts found corneal vascularisation increased the risk of graft failure with a risk ratio of 1.32, with incrementally increasing risk as more quadrants are involved.23 In addition in our study, patients who had experienced a rejection episode were more than twice as likely to have a failed graft at 5 years (Table 2). Rejection episodes following PK for HSV are as high as 46% during the first 2 years postoperatively16 and subsequent graft failure following a rejection episode has previously been reported to be similar to our results.9 Treatment of rejection episodes necessitates the use of intensive topical steroid treatment, thereby increasing the risk of HSV recurrence and graft failure, thus supporting the role of postoperative antiviral treatment.
To our knowledge, this is the largest reported study investigating the effect of antiviral treatment on graft survival in herpetic keratitis. We conclude that our study strongly supports the use of oral antiviral treatment to reduce the risk of graft failure in patients undergoing primary PK for herpetic keratitis and that this should be routinely used postoperatively unless contra-indicated.

References
Cohen EJ, Laibson PR, Arentsen JJ . Corneal transplantation for herpes simplex keratitis. Am J Ophthalmol 1983; 95: 645–650.
Williams KA, Lowe MT, Bartlett CM, Kelly L, Coster DJ . The Australian Corneal Graft Registry 2007 report. Flinders University Press: Adelaide, 2007.
Barney NP, Foster CS . A prospective randomized trial of oral acyclovir after penetrating keratoplasty for herpes simplex keratitis. Cornea 1994; 13: 232–236.
Mertz GJ, Jones CC, Mills J, Fife KH, Lemon SM, Stapleton JT et al. Long term acyclovir suppression of frequently recurring genital herpes simplex virus infection: a multicentred double blind trial. JAMA 1988; 260: 201–206.
Herpetic Eye Disease Study Group. Oral acyclovir for herpes simplex virus eye disease. Effect on prevention of epithelial keratitis and stromal keratitis. Arch Ophthalmol 2000; 118: 1030–1036.
Garcia DD, Farjo Q, Musch DC, Sugar A . Effect of prophylactic oral acyclovir after penetrating keratoplasty for herpes simplex keratitis. Cornea 2007; 26: 930–934.
Ghosh S, Jhanji V, Lamoureux E, Taylor HR, Vajpayee RB . Acyclovir therapy in prevention of recurrent herpetic keratitis following penetrating keratoplasty. Am J Ophthalmol 2008; 145: 198–202.
Van Rooij J, Rijneveld WJ, Remeijer L, Volker-Dieben HJM, Egginnk CA, Geerards AJM et al. Effect of oral acyclovir after penetrating keratoplasty for herpetic keratitis. Ophthalmology 2003; 110: 1916–1919.
Moyes AL, Sugar A, Musch DC, Barnes RD . Antiviral therapy after penetrating keratoplasty for herpes simplex keratitis. Arch Ophthalmol 1994; 112: 601–607.
Ficker LA, Kirkness CM, Rice NSC, Steele ADM . Longterm prognosis for corneal grafting in herpes simplex keratitis. Eye 1988; 2: 400–408.
Beyer CF, Arens MQ, Hill GA, Rose BT, Beyer LR, Schanzlin DJ . Oral acyclovir reduces the incidence of recurrent herpes simplex in rabbits after penetrating keratoplasty. Arch Ophthalmol 1989; 107: 1200–1205.
Goldblum D, Bachman C, Tappeiner C, Garweg J, Frueh BE . Comparison of oral antiviral therapy with valacyclovir or acyclovir after penetrating keratoplasty for herpetic keratitis. Br J Ophthalmol 2008; 92: 1201–1205.
Tambasco FP, Cohen MJ, Nguyen LH, Rapuano CJ, Laibson PR . Oral acyclovir after penetrating keratoplasty for herpes simplex keratitis. Arch Ophthalmol 1999; 117: 445–449.
Jansen AFG, Rijneveld WJ, Remeijer L, Volker-Dieben HJM, Eggink CA, Geerards AJM et al. Five year follow-up on the effect of oral acyclovir after penetrating keratoplasty for herpetic keratitis. Cornea 2009; 28: 843–845.
Kaye SB, Baker K, Bonshek R, Maseruka H, Grinfeld E, Tullo A et al. Human herpesviruses in the cornea. Br J Ophthalmol 2000; 84: 563–571.
Cobo LM, Coster DJ, Rice NSC, Jones BR . Prognosis and management of corneal transplantation for herpetic keratitis. Arch Ophthalmol 1980; 98: 1755–1759.
Lomholt JA, Baggesen K, Ehlers N . Recurrence and rejection rates following corneal transplantation for herpes simplex keratitis. Acta Ophthalmol Scand 1995; 73: 29–33.
Volker-Dieben HJ, D'Amaro J, Kok-Van Alphen CC . Hierarchy of prognostic factors for corneal graft survival. Aust NZ J Ophthalmol 1987; 15: 11–18.
Williams KA, Roder D, Esterman A, Muehlberg SM, Coster DJ . Factors predictive of corneal graft survival. Report from the Australian Corneal Graft Registry. Ophthalmology 1992; 99: 403–414.
Vail A, Gore SM, Bradley BA, Easty DL, Rogers CA, Armitage WJ . Conclusions of the corneal transplant follow up study. Br J Ophthalmol 1997; 81: 631–636.
Foster CS, Duncan J . Penetrating keratoplasty for herpes simplex keratitis. Am J Ophthalmol 1981; 92: 336–343.
Niederkorn JY . Immune privilege and immune regulation in the eye. Adv Immunol 1990; 48: 191–226.
Bachmann B, Taylor RS, Cursiefen C . Corneal neovascularisation as a risk factor for graft failure and rejection after keratoplasty: an evidence-based meta-analysis. Ophthalmology 2010; 117: 1300–1305.
Acknowledgements
We thank all the ophthalmologists who contributed to NHS Blood and Transplant for their continued support and active participation.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work has previously been presented at the Royal College of Ophthalmologists Annual Congress, Birmingham, May 2009.
Rights and permissions
About this article
Cite this article
Goodfellow, J., Nabili, S., Jones, M. et al. Antiviral treatment following penetrating keratoplasty for herpetic keratitis. Eye 25, 470–474 (2011). https://doi.org/10.1038/eye.2010.237
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2010.237
Keywords
This article is cited by
-
Ocular surgery after herpes simplex and herpes zoster keratitis
International Ophthalmology (2020)