Abstract
Purpose
To investigate longitudinal changes in peripapillary retinal nerve fiber layer (RNFL) thickness in patients with retinitis pigmentosa (RP).
Methods
We re-examined 103 RP patients whose RNFL thickness was previously examined and reported. RNFL thickness was measured using Stratus optical coherence tomography and was compared with the previous measurements. The results were also compared with that of previously reported normal subjects. Association between the decrease rate and visual acuity, and visual field was also investigated.
Results
The mean follow-up period was 56.9 months. After excluding the patients in whom RNFL images were of poor quality, 88 patients were eventually analyzed. The average RNFL thickness decreased from 105.8 to 98.2 μm during the period, with the average rate of decrease being 1.6 μm/year. The decrease in RNFL was more evident in superior and inferior sectors. Cross-sectional linear regression analysis also revealed an age-dependent decrease in RNFL, with the slower rate of decrease being 0.94 μm/year. The decrease in RNFL thickness was significantly faster than that reported in normal subjects. The decrease rate was not associated with visual functions.
Conclusion
Age-dependent RNFL thinning occurs at a faster rate in RP patients as compared with that in normal subjects. The result supports the notion that pathologic changes involve inner retina as well as outer retina in eyes with RP. Considering the discrepancy in the rate of RNFL thinning estimated from trend analysis and longitudinal measurement, care should be taken when interpreting the result of cross-sectional analysis.
Similar content being viewed by others
Introduction
Retinitis pigmentosa (RP) is a hereditary heterogenous disease, which primarily affects rod photoreceptors. As a consequence of rod photoreceptor death, patients experience night blindness and peripheral visual field loss in the early stages of the disease.1 Several therapeutic strategies for RP, including gene therapy, cell transplantation therapy, and retinal prosthesis have been intensively investigated in recent times.2 Each of these has demonstrated promising effects, and some are currently under clinical trials.3, 4
However, the effect of RP on the preservation of second or third neurons—which are necessary for conveying visual information to the lateral geniculate bodies or visual cortex—remains unclear.5 If the status of inner retina varies in each patient, selection of patients based on the preservation of inner retinal structures and functions would be helpful to achieve maximal effect from the treatments.6
Histology studies concerning the status of the inner retina in RP are limited.7, 8, 9, 10 These histologic reports showed that up to 75% of ganglion cell layer cells are retained in the macular area in patients with RP;7, 8 however, the percentage decreases to 20–30% in extra-macular region9 and 70–90% of total ganglion cells or their axons are lost eventually,10 suggesting that inner retinal cells are partially preserved in RP, but degenerate as the disease progresses. These reports provide important information; however, the method of post-mortem analyses cannot be used for the evaluation of patient suitability for the future treatment.
Several groups, including ours, have attempted to evaluate the inner retinal status in vivo using optical coherence tomography (OCT) (Table 1). For example, Walia et al11, 12 reported abnormal thinning and thickening of the retinal nerve fiber layer (RNFL). The abnormal thickening of the RNFL was also observed by Hood et al.5 Consistently, a recent study dealing with young subjects also showed relatively thick RNFL.13 We have previously reported wide variations in RNFL thickness in RP patients albeit with the average thickness being similar to that in normal eyes. In addition, RNFL thickness in RP patients appears to decrease faster than that observed in normal eyes.14 Anastasakis et al15 used a more recent model of OCT and confirmed the abnormal thickening and thinning of RNFL. In addition, they reported a similar rate of age-dependent decrease in RNFL thickness. The effect of using different models of OCT can be estimated by the result of another recent report.16 However, all these reports are cross-sectional studies, and information regarding the changes in RNFL thickness over time remains limited. The rate of age-dependent change estimated from trend analysis does not necessarily coincide with longitudinal measurement. In fact, a recent study concerning the RNFL thickness in normal subjects revealed discrepancies in the longitudinal and cross-sectional data;17 therefore, longitudinal measurement is necessary to evaluate time-dependent changes in RP patients in clinical practice.
In the present study, in order to evaluate longitudinal changes in RNFL thickness in RP patients, we re-examined patients in whom the RNFL thickness was previously assessed and reported ∼5 years ago. Further, we statistically analyzed these changes in RNFL thickness for age dependence and disease progress.
Materials and methods
Subjects
We re-recruited RP patients whose RNFL thickness results we previously reported in 2008.14 The study sample consisted of 137 eyes from 137 RP patients (including two patients with Usher syndrome) who were first examined between January 2006 and April 2007. The exclusion criteria were as follows: best-corrected visual acuity worse than 0.1 (20/200), presence of optic nerve diseases or retinal vascular diseases, refractive errors greater than –6 D, OCT signal strength <6, or OCT image showed evident artifact. These patients were re-examined between May 2011 and April 2012 during a follow-up visit to our institution.
All procedures conformed to the tenets of the Declaration of Helsinki, and the study design was approved by the institutional review board and the ethics committee of the Kyoto University Graduate School of Medicine. The aim of the study and the measurement procedures were explained to the study participants. The review board waived the need for written informed consent. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Patients were examined using the Stratus OCT (Carl Zeiss Meditec, Inc., Dublin, CA, USA), which is not a recent OCT; however, this was the model used in the previous study and was reused to avoid interdevice variations. Peripapillary RNFL was measured with the Fast RNFL scan option, comprising three circular scans of a diameter of 3.4 mm around the optic disc. As in the previous study, we excluded the patients in whom signal strength of the image did not reach 7 or in whom segmentation of the image exhibited artifacts.
Among the measurement parameters, the RNFL thickness in the 12 divided sectors, quadrant sectors, and the average RNFL thickness were used for statistical analyses. In addition, according to the study of Wallia et al,11 we counted the number of sectors showing abnormal thinning or thickening in 12 divided sectors. When the color map image showed yellow or red, the sector was judged as ‘thinning’ and white was judged as ‘thickening’.11
We assigned a visual field score for each case based on the previously reported system14 with some modifications. Based on a previous histological report, which indicated that the central 10- and 30 degree of retina contains up to 34 and 69% of the total number of retinal ganglion cells, respectively,18 we divided the visual field into concentric circles of central 0–10 degrees, central 10–30 degrees, and >30 degrees. Each concentric circle was further divided into quadrant sectors. Theoretically, each sector contains a similar number of ganglion cells (central 0–10 degrees: 8.5%, 10–30 degrees: 8.5%, and >30 degrees: 7.8%). We assigned a score for the remaining visual field measured with the V/4e isopter of Goldmann kinetic perimetry (GP). A score of 1 was assigned for the remaining visual field in each sector. When the remaining visual field occupied more than half but not the total extent of the sector, we gave a score of 0.5 (Figure 1).
Method and an example of visual field scoring in the present study. (a) We divided the visual field into concentric areas of 0–10, 10–30, and >30 degrees. Each area was further divided into quadrant sectors. When the remaining visual field occupied a sector, 1 point was assigned. When the visual field occupied more than half but not all of each sector, a score of 0.5 was assigned. (b) In the presented case, 1 point × 4 quadrant sectors of central 10 degree, and 0.5 point × 2 quadrants to lower >30 degree were assigned. Visual field in 10–30 degree sectors and in upper sectors of >30 degree were judged as less than half of the sector, and points were not assigned; that is, visual field score for the case was 5 points.
Statistical analysis
The statistical program SPSS version 19 (IBM Japan, Tokyo, Japan) was used for the analysis. Excel 2010 version 14.0.6112.5000 (Microsoft Japan, Tokyo, Japan) was also used to compare the present data with previously reported values. Descriptive analyses are reported as means±SD, unless otherwise specified. The average RNFL thickness, RNFL thickness of each sector, and signal strength of the OCT image in the present study were compared with the previous measurements using the paired t-test. To estimate the age-dependent decrease in RNFL thickness, a linear regression model was used with the average RNFL as the dependent variable, and age, refraction error, visual acuity, and the visual field score as independent variables. To investigate the effect of the changes in each parameter for changes in RNFL thickness, another linear regression analysis was performed with the change of average RNFL thickness as the dependent variable, and observation period, change of OCT signal, change of visual acuity, and change of visual field score as independent variables. Correlations between each parameter were further analyzed with Spearman’s rank correlation test. P-values <0.05 were regarded as significant. We searched for previous reports on PubMed concerning Stratus OCT-measured RNFL of normal subjects. Among them, articles that included linear regression coefficients with 95% confidential intervals were selected for analysis.19, 20, 21, 22 We calculated the SE from the 95% confidential intervals and compared these values with the measurements in the present study using the t-test.
Results
We successfully re-evaluated 103 (75.2%) patients out of the original population of 137 patients. Generally, the examination was performed smoothly; however, 15 patients were excluded due to poor OCT image quality (7 or worse; nine patients) and evident artifacts in RNFL segmentation (six patients). Poor image quality was due to progression of cataract, vitreous opacity, or fixation loss. A decrease in signal strength, which affects thickness measurement,23, 24 was observed even in the included subjects (9.0±1.1–8.5±1.1, P=0.002). Thus, the final study population consisted of 88 patients (39 men and 49 women). At the initial examination, the mean age was 50.4±13.8 years (range, 20–77 years); the refractive error, –1.5±2.4 D (range, +3.75 to –5.875); logMAR, 0.18±0.33 units (range, –0.18–1.0); and GP score, 7.6±2.8 (range, 2–12). The average duration between the previous and the present examination was 56.9±4.4 months (range, 50.8–68.7 months).
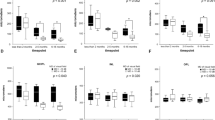
The average RNFL thickness decreased from 105.8±22.7 to 98.3±23.2 μm in the 5-year period (Figure 2a). Linear regression model showed that the change of OCT signal is partly responsible for the decrease of measurement. (2.1 μm of decrease/1 unit of change, P=0.007) The mean rate of decrease was calculated as 1.63±2.0 μm/year (range, −8.3 to +3.4 μm/year). The decrease was more evident in the superior and inferior sectors (Figure 2b, exact values are presented in Supplementary Table 1). OCT images from a representative case are shown in Figure 3.
Changes in RNFL thickness in patients with retinitis pigmentosa. RNFL thickness was measured after ∼5 years. The scatter plot demonstrates that most of the patients experienced a decrease in RNFL thickness (a). The line chart shows the changes in the RNFL thickness in each sector (b). The upper and lower lines represent the baseline and the follow-up (after 5 years) measurements of RNFL thickness, respectively. Decrease in RNFL thickness was statistically significant in the inferior and superior sectors. Figure 2 (c and d) shows correlation between age and RNFL thickness. The regression line indicates a slope of –0.938 for the multiple linear regression model (c). Baseline age and the rate of RNFL changes calculated from longitudinal measurements did not show evident trend (d). T, temporal; S, superior; N, nasal; I, inferior; *P<0.05, **P<0.01.
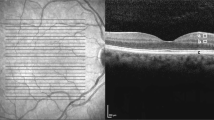
A representative case with retinitis pigmentosa showing marked thinning of the RNFL. RNFL thickness was measured in this 51-year-old woman after a 5-year interval. Fundus photographs show the progression of the disease as indicated by pigmentation and retinal pigment epithelium atrophy. (a and e) Scan alignment, image quality, and segmentation of RNFL were confirmed for each measurement. (b, c, f, and g) The average RNFL thickness decreased from 102.88 (d) to 75.82 μm (h), at the end of 5 years follow-up in this patient.
A negative correlation was noted between the average RNFL and age;, using cross-sectional multivariate linear regression analysis, the RNFL thickness was noted to decrease by 0.94 μm/year (P<0.001, 95% CI: 0.64–1.24, Figure 2c). Baseline age was also associated with change of RNFL in the observation period (ρ=–0.2, P=0.05). Refractive errors, visual acuity, and visual field had no significant effects on RNFL thickness in multivariate linear regression analysis. The result was consistent with the previous result that RNFL thinning is not necessarily associated with the degree of visual impairment.14 However, refractive error and the rate of decrease in RNFL thickness showed a weak association (ρ=–0.30, P=0.004; hyperopic eyes showed a faster rate of decrease). GP score did not show significant effect on average RNFL thickness, but was associated with decrease of RNFL in the inferior sector (ρ=0.21, P=0.048; better GP score showed a smaller decrease of the inferior sector RNFL).
Figure 2d shows the longitudinal measurements for the decrease in RNFL thickness in each patient based on the baseline age. Most patients showed a decrease in the RNFL thickness at a rate of 0 to –4 μm/year; however, 13 patients (14.8%) showed an increase in RNFL thickness. We compared these 13 patients and the rest of the subjects in age, sex, refractive error, visual acuity, and visual field score, but there was no significant differences (P=0.38, 0.20, 0.07, 0.44, and 0.14, respectively).
As we did not have healthy controls who were followed up for 5 years, we compared the previously reported rates of decrease in RNFL thickness in normal eyes. We found four studies that examined normal subjects using Stratus OCT, and reported the rate of decrease in RNFL thickness along with 95% confidence intervals (Table 2). The reported rate of decrease in RNFL thickness ranged between –0.16 and –0.26 μm/year.19, 20, 21, 22 We calculated SDs and SEs from the reported means and 95% CIs, and we then compared the value with the present result. The regression coefficient in the present study indicated a significantly faster rate of decrease in RNFL thickness than that in these four reports (P<0.001 for all four comparisons).
We also investigated the abnormal thinning and thickening noted in certain patients. At the baseline examination, abnormal thinning was noted in 1.1±1.9 sectors, whereas thickening was noted in 2.7±2.6 sectors. In the present assessment, the number of sectors with abnormal thinning increased to 1.8±2.2 (P<0.001), and with thickening decreased to 2.4±2.3 with non-significant P-value (P=0.057).
Discussion
The present study investigated time-dependent changes in RNFL thickness in RP patients. The average RNFL thickness decreased by 7.1% in ∼5 years in these patients, and the rate of RNFL thinning was higher than that previously reported in healthy subjects.
The linear regression model showed a decrease in RNFL thickness of 0.94 μm/year in the RP patients, which was similar to that previously reported (–0.65 μm/year within the 95% CI in the present study) using a different OCT model.15 However, longitudinal RNFL thickness measurements demonstrated that RNFL thickness in RP patients decreased at a rate of 1.63 μm/year. A similar discrepancy in longitudinal measurements and linear regression coefficient measurements for RNFL thickness was recently reported in normal subjects,17 suggesting that applying the linear regression model may not always be valid for evaluating RNFL thinning in RP patients. Leung et al17 clarified that age-related changes in individuals should be determined from longitudinal data and not based on the extrapolation of cross-sectional data;17 this can also be applied in the present case.
The RNFL thinning did not progress equally in each quadrant. The sectoral difference was already reported in normal subjects, but it is not consistent as to which quadrant significantly decreases with age.17, 19, 21 In the present study, inferior and superior sector showed significant thinning, but nasal and temporal sector did not. In addition, association between visual field score and RNFL decrease rate was found only in the inferior quadrant, implying the correlation between disease stage and progression pattern of RNFL thinning. Sectoral difference can be an important issue when considering the site of visual prosthesis implantation or cell transplantation. It should be further investigated.
Abnormal thickening as well as thinning of the RNFL has been previously reported in RP patients.5, 11, 12 In fact, some patients showed thick RNFL at baseline and after the follow-up. However, the number of sectors with thinning or thicknening also showed the trend of thinning; the number of sectors with RNFL thinning increased, whereas that with RNFL thickening tended to decrease. These findings suggest that abnormal RNFL thickening certainly occurs in RP patients; however, the overall RNFL thickness continues to decrease over time. If patients are examined over longer follow-up periods or only in advanced stages, a predominance of abnormal thinning may be noted with no findings of RNFL thickening. Although the patients whose RNFL thickness increased during the follow-up period did not show specific characteristics in the present study and the change can be a variability of the measurement, investigating which patients and when these patients show thickening of RNFL would be of interest as it would highlight the pathological process of RP in the retinal cells other than photoreceptors.
RNFL thickness is reported to be affected by signal strength, with low signal strength being associated with RNFL thinning.23, 24 Generally, obtaining good-quality OCT images in RP patients is more difficult than that in normal subjects due to cataract, vitreous opacity, unstable fixation, and so on. Increasing patient age and disease progression render obtaining of OCT images even more difficult. In the present study, 15 patients were excluded due to signal strength <6 or the presence of evident artifacts. Moreover, the average signal strength of the included patients was also observed to have decreased in comparison to the baseline. According to the linear regression analysis, around 1 μm of the decrease observed in the present study could be explained by the change of signal strength.
In the present study, we used the Stratus OCT instead of a later model. Although the latest models of spectral domain OCT provide better resolution and higher reproducibility,16 previous reports comparing measurements from these models showed that the results from differing models, although highly correlated, are not interchangeable.25, 26, 27, 28 As the Stratus OCT was used in our previous study of the same study population, we used the same model for the present study. The Stratus OCT does not have an eye-tracking system and automated registration. In addition, acquisition of fundus image is done after the OCT measurement is finished, thus, the placement of scan circle is not completely precise. These limitations in variation of measurement should be noted. We now examine patients with spectral domain OCT, and longitudinal analyses in the future will be based on the result from the latest OCT models.
The present study was limited by its non-prospective design and lack of normal control subjects, as we did not have access to healthy subjects who were followed up for 5 years; this is probably the case for most institutions. Age-, gender-, and ethnicity-matched control would provide more robust conclusion. A prospective study that strictly compares the rates of change in RNFL thickness between normal controls and RP patients is required in the future.
The results of the present study demonstrated progressive age-related loss of RNFL thickness in RP patients based on a longitudinal analysis of OCT images. Our results indicate that the integrity of the inner retina should be carefully evaluated in each RP patient before determining the therapeutic strategy.

References
Hartong DT, Berson EL, Dryja TP . Retinitis pigmentosa. Lancet 2006; 368 (9549): 1795–1809.
Sahni JN, Angi M, Irigoyen C, Semeraro F, Romano MR, Parmeggiani F . Therapeutic challenges to retinitis pigmentosa: from neuroprotection to gene therapy. Curr Genomics 2011; 12 (4): 276–284.
Weiland JD, Cho AK, Humayun MS . Retinal prostheses: current clinical results and future needs. Ophthalmology 2011; 118 (11): 2227–2237.
Kuno N, Fujii S . Biodegradable intraocular therapies for retinal disorders: progress to date. Drugs Aging 2010; 27 (2): 117–134.
Hood DC, Lin CE, Lazow MA, Locke KG, Zhang X, Birch DG . Thickness of receptor and post-receptor retinal layers in patients with retinitis pigmentosa measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci 2009; 50 (5): 2328–2336.
Huang Q, Chowdhury V, Coroneo MT . Evaluation of patient suitability for a retinal prosthesis using structural and functional tests of inner retinal integrity. J Neural Eng 2009; 6 (3): 035010.
Stone JL, Barlow WE, Humayun MS, de Juan E, Milam AH . Morphometric analysis of macular photoreceptors and ganglion cells in retinas with retinitis pigmentosa. Arch Ophthalmol 1992; 110 (11): 1634–1639.
Santos A, Humayun MS, de Juan E, Greenburg RJ, Marsh MJ, Klock IB et al. Preservation of the inner retina in retinitis pigmentosa. A morphometric analysis. Arch Ophthalmol 1997; 115 (4): 511–515.
Humayun MS, Prince M, de Juan E, Barron Y, Moskowitz M, Klock IB et al. Morphometric analysis of the extramacular retina from postmortem eyes with retinitis pigmentosa. Invest Ophthalmol Vis Sci 1999; 40 (1): 143–148.
Eng JG, Agrawal RN, Tozer KR, Ross-Cisneros FN, Dagnelie G, Greenberg RJ et al. Morphometric analysis of optic nerves and retina from an end-stage retinitis pigmentosa patient with an implanted active epiretinal array. Invest Ophthalmol Vis Sci 2011; 52 (7): 4610–4616.
Walia S, Fishman GA, Edward DP, Lindeman M . Retinal nerve fiber layer defects in RP patients. Invest Ophthalmol Vis Sci 2007; 48 (10): 4748–4752.
Walia S, Fishman GA . Retinal nerve fiber layer analysis in RP patients using Fourier-domain OCT. Invest Ophthalmol Vis Sci 2008; 49 (8): 3525–3528.
Hwang YH, Kim SW, Kim YY, Na JH, Kim HK, Sohn YH . Optic nerve head, retinal nerve fiber layer, and macular thickness measurements in young patients with retinitis pigmentosa. Curr Eye Res 2012; 37 (10): 914–920.
Oishi A, Otani A, Sasahara M, Kurimoto M, Nakamura H, Kojima H et al. Retinal nerve fiber layer thickness in patients with retinitis pigmentosa. Eye 2009; 23 (3): 561–566.
Anastasakis A, Genead MA, McAnany JJ, Fishman GA . Evaluation of retinal nerve fiber layer thickness in patients with retinitis pigmentosa using spectral-domain optical coherence tomography. Retina 2012; 32 (2): 358–363.
Garcia-Martin E, Pinilla I, Sancho E, Almarcegui C, Dolz I, Rodriguez-Mena D et al. Optical coherence tomography in retinitis pigmentosa: reproducibility and capacity to detect macular and retinal nerve fiber layer thickness alterations. Retina 2012; 32 (8): 1581–1591.
Leung CK, Yu M, Weinreb RN, Ye C, Liu S, Lai G et al. Retinal nerve fiber layer imaging with spectral-domain optical coherence tomography: a prospective analysis of age-related loss. Ophthalmology 2012; 119 (4): 731–737.
Rizzo JF . Embryology anatomy and physiology of the afferent visual pathway. In: Miller NR, Newman NJ, (eds), Walsh, Hoyt’s Clinical Neuro-Ophthalmology 6th edn. Lippincott Williams & Wilkins: Philadelphia, 2005 pp 3–82.
Parikh RS, Parikh SR, Sekhar GC, Prabakaran S, Babu JG, Thomas R . Normal age-related decay of retinal nerve fiber layer thickness. Ophthalmology 2007; 114 (5): 921–926.
Budenz DL, Anderson DR, Varma R, Schuman J, Cantor L, Savell J et al. Determinants of normal retinal nerve fiber layer thickness measured by Stratus OCT. Ophthalmology 2007; 114 (6): 1046–1052.
Sung KR, Wollstein G, Bilonick RA, Townsend KA, Ishikawa H, Kagemann L et al. Effects of age on optical coherence tomography measurements of healthy retinal nerve fiber layer, macula, and optic nerve head. Ophthalmology 2009; 116 (6): 1119–1124.
Feuer WJ, Budenz DL, Anderson DR, Cantor L, Greenfield DS, Savell J et al. Topographic differences in the age-related changes in the retinal nerve fiber layer of normal eyes measured by Stratus optical coherence tomography. J Glaucoma 2011; 20 (3): 133–138.
Cheung CY, Leung CK, Lin D, Pang CP, Lam DS . Relationship between retinal nerve fiber layer measurement and signal strength in optical coherence tomography. Ophthalmology 2008; 115 (8): 1347–1351 1351 e1341-1342.
Vizzeri G, Bowd C, Medeiros FA, Weinreb RN, Zangwill LM . Effect of signal strength and improper alignment on the variability of stratus optical coherence tomography retinal nerve fiber layer thickness measurements. Am J Ophthalmol 2009; 148 (2): 249–255 e241.
Knight OJ, Chang RT, Feuer WJ, Budenz DL . Comparison of retinal nerve fiber layer measurements using time domain and spectral domain optical coherent tomography. Ophthalmology 2009; 116 (7): 1271–1277.
Sung KR, Kim DY, Park SB, Kook MS . Comparison of retinal nerve fiber layer thickness measured by Cirrus HD and Stratus optical coherence tomography. Ophthalmology 2009; 116 (7): 1264–1270 1270 e1261.
Vizzeri G, Weinreb RN, Gonzalez-Garcia AO, Bowd C, Medeiros FA, Sample PA et al. Agreement between spectral-domain and time-domain OCT for measuring RNFL thickness. Br J Ophthalmol 2009; 93 (6): 775–781.
Seibold LK, Mandava N, Kahook MY . Comparison of retinal nerve fiber layer thickness in normal eyes using time-domain and spectral-domain optical coherence tomography. Am J Ophthalmol 2010; 150 (6): 807–814.
Tamaki M, Matsuo T . Optical coherence tomographic parameters as objective signs for visual acuity in patients with retinitis pigmentosa, future candidates for retinal prostheses. J Artif Organs 2011; 14 (2): 140–150.
Sliesoraityte I, Troeger E, Bernd A, Kurtenbach A, Zrenner E . Correlation between spectral domain OCT retinal nerve fibre layer thickness and multifocal pattern electroretinogram in advanced retinitis pigmentosa. Adv Exp Med Biol 2012; 723: 471–478.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
N Yoshimura is a paid advisory board member of Nidek and Topcon. The other authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Eye website
Supplementary information
Rights and permissions
About this article
Cite this article
Oishi, A., Ogino, K., Nakagawa, S. et al. Longitudinal analysis of the peripapillary retinal nerve fiber layer thinning in patients with retinitis pigmentosa. Eye 27, 597–604 (2013). https://doi.org/10.1038/eye.2013.34
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2013.34
Keywords
This article is cited by
-
Analysis of imaging biomarkers and retinal nerve fiber layer thickness in RPGR-associated retinitis pigmentosa
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)
-
Optical Coherence Tomography Angiography to Estimate Retinal Blood Flow in Eyes with Retinitis Pigmentosa
Scientific Reports (2017)
-
Structure-function correlations in Retinitis Pigmentosa patients with partially preserved vision: a voxel-based morphometry study
Scientific Reports (2017)
-
Increased aqueous flare is associated with thickening of inner retinal layers in eyes with retinitis pigmentosa
Scientific Reports (2016)