Abstract
Purpose
To investigate the relationship between subfoveal choroidal thickness, severity of diabetic retinopathy (DR), and the presence of diabetic macular oedema (DMO) using enhanced depth imaging spectral domain optical coherence tomography (EDI-OCT) in patients with type 2 diabetes.
Methods
A retrospective study of 145 eyes from untreated, type 2 diabetic patients who attended clinic at the Oxford Eye Hospital between January 2012 and February 2013, and underwent fundus photography and EDI-OCT imaging. Eyes were divided into two groups based on the presence or absence of foveal involving DMO and classified according to retinopathy grade: R1 (mild non-proliferative diabetic retinopathy (NPDR), R2 (moderate-severe NPDR), and R3 (proliferative diabetic retinopathy (PDR). Subfoveal choroidal thickness was measured on the EDI-OCT images and statistically analysed using Student’s t-test.
Results
In mild NPDR (n=87), the mean subfoveal choroidal thickness was 217.7 microns. In moderate–severe NPDR (n=37), the mean subfoveal choroidal thickness was 221.7 microns. In PDR (n=21), the mean subfoveal choroidal thickness was 242.1 microns. There was a statistically significant increase in choroidal thickness in PDR when compared with the mild NPDR group, P=0.027. DMO was associated with a non-statistically significant increase in choroidal thickness (225.4 microns) compared with eyes without DMO (209.3 microns), P=0.13.
Conclusion
Subfoveal choroidal thickness increased with the severity of diabetic retinopathy but showed no statistically significant association with the presence of DMO. This suggests that the choroidal layer is responsive to retinal vascular changes.
Similar content being viewed by others
Introduction
Diabetic retinopathy (DR) is a leading cause of visual loss in the working age population, yet its pathogenesis is incompletely understood.1 Alongside retinal vascular damage, choroidal vascular abnormalities have long been implicated in the development of DR and maculopathy.2 The choroidal layer is crucial for the metabolic function of the outer layers of the retina, including the retinal pigment epithelium and photoreceptors. Owing to the poor visibility of the choroid in vivo, there has been little clinical study in diabetic patients to date and choroidal anatomical changes have not been well characterised. Thus far, histological analysis has shown increased tortuosity, focal vascular dilations and narrowing, capillary dropout, and scarring in DR.3, 4
Spectral domain optical coherence tomography (SD-OCT) has recently been used to examine choroidal morphology, revealing an irregular shape to the choroidoscleral interface.5 The development of enhanced depth imaging (EDI) SD-OCT has improved visualisation of the choroid with high resolution cross-sectional imaging, enabling reliable and reproducible measurements of full choroidal thickness.6, 7
Although there have been a few small studies measuring choroidal thickness in diabetic patients, not all have used EDI-OCT imaging and the relationship between choroidal thickness and progression of DR remains unclear with reports of both a thicker8, 9 and a thinner choroidal layer in diabetic subjects.5, 10, 11, 12, 13 In addition, it is unknown whether diabetic macular oedema (DMO) influences the degree of choroidopathy with the above studies finding eyes with DMO to have choroidal layers thicker, thinner, and no different to controls. In patients with DR and DMO, we hypothesised that choroidal thickness would correlate more closely with DR, increasing with the severity. If this measurement of choroidopathy represents a diffuse pathological process, we did not expect it to be related to the discrete process of DMO.
The present study therefore aimed to investigate whether there is an association between subfoveal choroidal thickness, severity of DR, and the presence of DMO, specifically in type 2 diabetic patients.
Materials and methods
This was a retrospective study of patients attending the diabetic clinic over a 1-year period. Data were collected using the Medisoft (Medisoft, Leeds, UK) Ophthalmology Electronic Medical Records system. The local ethics committee approved the study protocol, and we certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research. As the data were sourced from existing retinal imaging, individual consent was not deemed necessary.
A complete list of all patients (2271) who attended the diabetic eye clinic at Oxford Eye Hospital between January 2012 and February 2013 was generated. Patient names were erased from the record and the hospital number and date of birth were used as two unique identifiers. Patients with ‘mild visual loss’ (vision better than 6/12 in at least one eye), ‘type 1’ or ‘not specified’ types of diabetes were excluded, to leave a cohort of patients with ‘type 2’ diabetes and moderate-to-severe visual loss. Patients had to meet the following inclusion criteria: a documented history of foveal involving DMO in at least one eye, no prior treatment for diabetic eye disease, and no other retinal pathologies. This generated a patient population of 412. Finally, the requirement for EDI-OCT images reduced the study population to 145 eyes from 95 patients (58 males and 37 females, mean age of 70 years; range 44–99 years).
Two masked observers analysed all 145 eyes (RR and AW: medical student and junior doctor, respectively). A third masked observer (VC: consultant ophthalmologist) evaluated any disparate grades or measurements.
Diabetic retinopathy
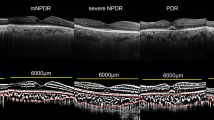
In accordance with the UK National Health Service Diabetes Eye Screening Revised Grading Definitions,14 DR was graded using a combination of clinical examination and imaging. Retinal photographs were taken on the Optomap (Optos, Dunfermline, Scotland, UK) system. This divided the eyes into three grades of retinopathy (Table 1): R1—mild non-proliferative diabetic retinopathy (NPDR); R2—moderate–severe NPDR; and R3—proliferative diabetic retinopathy (PDR).
Diabetic macular oedema
Heidelberg Spectralis (Heidelberg, Germany) EDI-OCT images were obtained according to the previously explained method7 and a horizontal section through the fovea was examined. DMO was present if cystic change or serous retinal detachment were detected at the fovea (500 micron region). A summary of the results is shown in Table 1.
Subfoveal choroidal thickness
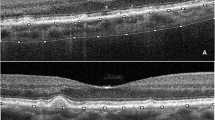
Subfoveal choroidal thickness was manually assessed on EDI-OCT images using the ImageJ tool (National Institute of Health, Bethesda, MD, USA) tool to measure the distance from the outer edge of the hyper-reflective retinal pigment epithelium to the inner sclera. A representative scan demonstrating the measurement of choroidal thickness is shown in Figure 1.
Statistical analysis
Statistical analysis was performed using unpaired Student’s t-tests on Microsoft (Microsoft, Redmond, WA, USA) Excel 2007, with significance set at P<0.05.
Results
Subfoveal choroidal thickness increases with the severity of diabetic retinopathy
Mean subfoveal choroidal thickness measured: 217.7 microns in mild NPDR (n=87), 221.7 microns in moderate–severe NPDR (n=37), and 242.1 microns in PDR (n=21) (Table 2).
The PDR group demonstrated a statistically significant increase in subfoveal choroidal thickness when compared with the mild NPDR group (P=0.027). The increase in subfoveal choroidal thickness in the moderate–severe NPDR group compared with the mild NPDR group was not statistically significant (P=0.17).
Subfoveal choroidal thickness is not associated with the presence of DMO
Mean subfoveal choroidal thickness was 209.3 microns in the group without DMO (n=52) and 225.4 microns in the group with DMO (n=93) (Table 3). The increased choroidal thickness in the presence of DMO, however, was not statistically significant (P=0.13).
Discussion
This study assessed subfoveal choroidal thickness using EDI-OCT imaging in relation to the severity of DR and the presence of DMO in a Western population of untreated type 2 diabetic patients. Subfoveal choroidal thickness increased with the severity of retinopathy but showed no statistically significant association with the presence of DMO.
The majority of studies measuring choroidal thickness thus far have been in Asian populations. Our findings replicate the correlation between choroidal thickness and progression of DR found by Kim et al8 in their Korean study of 195 untreated eyes from type 2 diabetic patients. Kase et al15 also noted increased central choroidal thickness in eyes with DR compared with those without retinopathy, although it was not significant in their small cohort. A population-based Beijing study of 246 eyes similarly found increased choroidal thickness in diabetic eyes, but this was not further affected by DR stage.9 However, there were only 23 eyes with DR and it is likely that the average stage of DR in the community was lower than that in our hospital-based study population.
In contrast, a separate Korean study by Lee et al10 of 203 diabetic eyes reported overall choroidal thinning, which remained stable with increasing severity of DR. It is not completely clear why the results contradict those found in our population—racial differences are unlikely to be significant,16 but it may reflect the inclusion of patients with type 1 diabetes and differences in classification of DR. The most plausibly contributory factor is previous photocoagulation; studies including eyes treated with pan retinal photocoagulation (PRP) generally report reduced choroidal thickness in PDR.5, 11, 17 Although PRP appears to initially increase choroidal thickness, over time it leads to choroidal thinning.8, 18, 19
A further study of 63 diabetic eyes with NPDR also found overall choroidal thinning.12 This inconsistency might be explained by Kim et al8 who demonstrated an initial reduction in choroidal thickness (244.6 microns) compared with healthy controls (262.3 microns) in early diabetic eye disease, followed by an increase in choroidal thickness in severe NPDR (291.1 microns) and PDR (363.5 microns). This is similar to the finding that systemically untreated diabetic patients exhibit thinner central choroidal thickness in mild–moderate NPDR than treated patients.15 The effect of treatment was demonstrated in a small study in which 17 subjects underwent a programme of intensive glycaemic control.20 After only 2 weeks, choroidal thickness significantly increased, suggesting a highly responsive choroidal layer. If there is indeed an initial change in the choroidal vasculature that thins the choroid in early diabetic eye disease, then it could account for the non-significant increase in choroidal thickness between mild NPDR and moderate–severe NPDR in our study.
The association between choroidal thickness and DMO is also controversial. Although we found a small increase in choroidal thickness in the presence of DMO, this might reflect the higher proportion of severe retinopathy in the DMO group (Table 1). Kim et al,8 however, demonstrated a significantly thicker choroid in their DMO subset, which might be because there were more eyes with serous retinal detachment and these show the thickest choroidal layers. Varying stages of DR and differing classifications of macular oedema could account for the considerable discrepancies between the studies, and the repeated finding that DMO does not further influence choroidal thickness10, 11, 12, 13 suggests that choroidal thickness may be more affected by DR. DMO results from breakdown of the blood–retinal barrier and subsequent leakage of intraretinal fluid.21 It is thus possible that the choroid, with its initial abundance of blood vessels, may be relatively resistant to macular oedema.
The aetiology of these changes in choroidal thickness may relate to changes in choroidal blood flow and potentially occur before the onset of retinopathy. A postmortem examination of 10 diabetic eyes without evidence of retinopathy found choriocapillary degeneration, suggesting that choroidopathy might be responsible for the deterioration in sight found in patients without overt DR changes.3 Although technically difficult to measure, there is some in vivo evidence: laser Doppler flowmetry demonstrated impaired choroidal blood flow in diabetic eyes, which was further reduced in NPDR and even more so in NPDR with DMO.22 The authors hypothesised that hypoxia might initially thin the choroid with the resultant choroidal ischaemia damaging the retinal pigment epithelium. Subsequent production of vascular endothelial growth factor, neovascularisation, and increased blood flow might then account for the development of a thicker choroid with advancing retinopathy.22 In support of this, a study using Langham pneumotonometry showed increased choroidal blood flow in severe NPDR and PDR and decreased flow in treated PDR.23
Conclusions and further work
The results presented here suggest a positive relationship between subfoveal choroidal thickness and the severity of DR but no significant association between choroidal thickness and DMO. However, the retrospective nature of our study means that cause and effect cannot be clearly separated. A longitudinal study with serial measurements of choroidal thickness in relation to the progression of DR will therefore be important. In addition, further investigation will be necessary to correlate the anatomical findings on EDI-OCT with functional studies of the choroidal vasculature. Assessment of the choroid may prove to be a useful tool in the clinical assessment of diabetic eye disease and as a marker of compromised systemic vasculature.
Limitations
The main limitation of this study is the small number of eyes included. Unfortunately, despite a large cohort of type 2 diabetic patients available, the study could only include those with EDI-OCT imaging, and at the start of data collection, EDI imaging was not the standard protocol. The study cohort was further reduced owing to the exclusion of poor quality images. Second, in order to assess choroidal thickness in patients with diabetic retinopathy and macular oedema, all patients had DMO in at least one eye. As a result, those classified under ‘DMO absent’ were taken from patients with DMO in the fellow eye; the effect of this is unknown. Third, we acknowledge that this study has not assessed choroidal thickness in relation to other measures such as refractive error or diabetic control, which were not available on our database, and may influence choroidal thickness.

References
Antonetti D, Klein R, Gardner T . Diabetic retinopathy. N Engl J Med 2012; 366 (13): 1227–1239.
Hidayat A, Fine B . Diabetic choroidopathy: light and electron microscopic observations of seven cases. Ophthalmology 1985; 92 (4): 512–522.
Cao J, McLeod D, Merges C, Lutty G . Choriocapillaris degeneration and related pathologic changes in human diabetic eyes. Arch Ophthalmol 1998; 116 (5): 589–597.
Fukushima I, McLeod D, Lutty G . Intrachoroidal microvascular abnormality: a previously unrecognized form of choroidal neovascularization. Am J Ophthalmol 1997; 124 (4): 473–487.
Adhi M, Brewer E, Waheed N, Duker J . Analysis of morphological features and vascular layers of choroid in diabetic retinopathy using spectral-domain optical coherence tomography. JAMA Ophthalmol 2013; 131 (10): 1267–1274.
Sim D, Keane P, Mehta H, Fung S, Zarranz-Ventura J, Fruttiger M et al. Repeatability and reproducibility of choroidal vessel layer measurements in diabetic retinopathy using enhanced depth optical coherence tomography. Invest Ophthalmol Vis Sci 2013; 54 (4): 2893–2901.
Spaide R, Koizumi H, Pozzoni M . Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 2008; 146 (4): 496–500.
Kim J, Lee D, Joe S, Kim J, Yoon Y . Changes in choroidal thickness in relation to the severity of retinopathy and macular edema in type 2 diabetic patients. Invest Ophthalmol Vis Sci 2013; 54 (5): 3378–3384.
Xu J, Xu L, Du K, Shao L, Chen C, Zhou J et al. Subfoveal choroidal thickness in diabetes and diabetic retinopathy. Ophthalmology 2013; 120 (10): 2023–2028.
Lee H, Lim J, Shin M . Comparison of choroidal thickness in patients with diabetes by spectral-domain optical coherence tomography. Korean J Ophthalmol 2013; 27 (6): 433–439.
Regatieri C, Branchini L, Carmody J, Fujimoto J, Duker J . Choroidal thickness in patients with diabetic retinopathy analyzed by spectral-domain optical coherence tomography. Retina 2012; 32 (3): 563–568.
Querques G, Lattanzio R, Querques L, Del Turco C, Forte R, Pierro L et al. Enhanced depth imaging optical coherence tomography in type 2 diabetes. Invest Ophthalmol Vis Sci 2012; 53 (10): 6017–6024.
Vujosevic S, Martini F, Cavarzeran F, Pilotto E, Midena E . Macular and peripapillary choroidal thickness in diabetic patients. Retina 2012; 32 (9): 1781–1790.
Taylor D . Diabetic eye screening revised grading definitions. NHS Screening Programmes, 2012, v1.3. www.gov.uk.
Kase S, Endo H, Yokoi M, Kotani M, Katsuta S, Takahashi M et al. Choroidal thickness in diabetic retinopathy in relation to long-term systemic treatments for diabetes mellitus. Eur J Ophthalmol 2016; 26 (2): 158–162.
Karapetyan A, Ouyang P, Tang LS, Gemilyan M . Choroidal thickness in relation to ethnicity measured using enhanced depth imaging optical coherence tomography. Retina 2016; 36 (1): 82–90.
Ünsal E, Eltutar K, Zirtiloğlu S, Dinçer N, Erkul SO, Güngel H . Choroidal thickness in patients with diabetic retinopathy. Clin Ophthalmol 2014; 8: 637–642.
Lee S, Kim J, Chung H, Kim H . Changes of choroidal thickness after treatment for diabetic retinopathy. Curr Eye Res 2014; 39 (7): 736–744.
Zhang Z, Meng X, Wu Z, Zou W, Zhang J, Zhu D et al. Changes in choroidal thickness after panretinal photocoagulation for diabetic retinopathy: a 12-week longitudinal study. Invest Ophthalmol Vis Sci 2015; 56 (4): 2631–2638.
Jo Y, Ikuno Y, Iwamoto R, Okita K, Nishida K . Choroidal thickness changes after diabetes type 2 and blood pressure control in a hospitalized situation. Retina 2014; 34 (6): 1190–1198.
Bhagat N, Grigorian R, Tutela A, Zarbin M . Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol 2009; 54 (1): 1–32.
Nagaoka T, Kitaya N, Sugawara R, Yokota H, Mori F, Hikichi T et al. Alteration of choroidal circulation in the foveal region in patients with type 2 diabetes. Br J Ophthalmol 2004; 88 (8): 1060–1063.
Savage H, Hendrix J, Peterson D, Young H, Wilkinson C . Differences in pulsatile ocular blood flow among three classifications of diabetic retinopathy. Invest Ophthalmol Vis Sci 2004; 45 (12): 4504–4509.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
VC is a consultant of Allergan, Bayer, Novartis, and Quantel Medical and has received speaker fees from Heidelberg Engineering. The other authors declare no conflict of interest.
Additional information
This study was presented as a poster at The Association for Research in Vision and Ophthalmology in 2014.
Rights and permissions
About this article
Cite this article
Rewbury, R., Want, A., Varughese, R. et al. Subfoveal choroidal thickness in patients with diabetic retinopathy and diabetic macular oedema. Eye 30, 1568–1572 (2016). https://doi.org/10.1038/eye.2016.187
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/eye.2016.187
This article is cited by
-
Choroidal vascularity index as a predictor for the development of retinopathy in diabetic patients
Journal of Endocrinological Investigation (2023)
-
Morphological changes of foveal cysts as a predictor for visual response to anti-vascular endothelial growth factor treatments in diabetic macular edema
International Ophthalmology (2023)
-
Higher choroidal thickness and lower choriocapillaris blood flow signal density based on optical coherence tomography angiography in diabetics
Scientific Reports (2021)
-
Diabetic retinopathy and diabetic macular oedema pathways and management: UK Consensus Working Group
Eye (2020)
-
Alteration of choroidal vascular structure in diabetic macular edema
Graefe's Archive for Clinical and Experimental Ophthalmology (2020)