Abstract
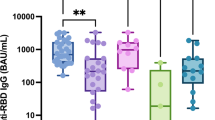
One potential setback to the use of gene therapy for the treatment of Sjögren’s syndrome is the presence of neutralizing antibodies (nAb) against adeno-associated virus (AAV) serotypes. In order to evaluate the efficacy of this treatment option, nAb titers were measured in both healthy individuals and Sjögren’s patients. Several serotypes with known transduction activity in mouse salivary glands were tested and only AAV5 showed a statistically significant change in the prevalence of nAbs between Sjögren’s and healthy participants. Both groups showed a higher rate of nAbs for AAV2 compared with most of the other serotypes tested, except for bovine AAV (BAAV). Although a similar rate of seropositivity was seen against BAAV and AAV2, the percentage of samples with high titer was significantly lower with BAAV. Furthermore, the majority of positive samples exhibited low nAb titers in the primary Sjögren’s syndrome (pSS) group for all serotypes except for AAV2. AAV5 was the only serotype that showed a statistically significant shift in the percentage of medium or high neutralizing titer. Based on these results, many serotypes are viable vectors in a gene therapy approach and pSS patients do not have a statistically significant higher rate of seropositivity or titer compared with healthy donors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Fox RI . Sjogren's syndrome. Lancet 2005; 366: 321–331.
Hansen A, Lipsky PE, Dorner T . Immunopathogenesis of primary Sjogren's syndrome: implications for disease management and therapy. Curr Opin Rheumatol 2005; 17: 558–565.
Jensen SB, Pedersen AM, Vissink A, Andersen E, Brown CG, Davies AN et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer 2010; 18: 1061–1079.
Gottenberg JE, Cagnard N, Lucchesi C, Letourneur F, Mistou S, Lazure T et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren's syndrome. Proc Natl Acad Sci USA 2006; 103: 2770–2775.
Tsunawaki S, Nakamura S, Ohyama Y, Sasaki M, Ikebe-Hiroki A, Hiraki A et al. Possible function of salivary gland epithelial cells as nonprofessional antigen-presenting cells in the development of Sjogren's syndrome. J Rheumatol 2002; 29: 1884–1896.
Mariette X, Ravaud P, Steinfeld S, Baron G, Goetz J, Hachulla E et al. Inefficacy of infliximab in primary Sjogren's syndrome: results of the randomized, controlled Trial of Remicade in Primary Sjogren's Syndrome (TRIPSS). Arthritis Rheum 2004; 50: 1270–1276.
Steinfeld SD, Demols P, Appelboom T . Infliximab in primary Sjogren's syndrome: one-year followup. Arthritis Rheum 2002; 46: 3301–3303.
Baum BJ, Alevizos I, Zheng C, Cotrim AP, Liu S, McCullagh L et al. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc Natl Acad Sci USA 2012; 109: 19403–19407.
Katano H, Kok MR, Cotrim AP, Yamano S, Schmidt M, Afione S et al. Enhanced transduction of mouse salivary glands with AAV5-based vectors. Gene Ther 2006; 13: 594–601.
Schmidt M, Katano H, Bossis I, Chiorini JA . Cloning and characterization of a bovine adeno-associated virus. J Virol 2004; 78: 6509–6516.
Schmidt M, Voutetakis A, Afione S, Zheng C, Mandikian D, Chiorini JA . Adeno-associated virus type 12 (AAV12): a novel AAV serotype with sialic acid- and heparan sulfate proteoglycan-independent transduction activity. J Virol 2008; 82: 1399–1406.
van der Marel S, Comijn EM, Verspaget HW, van Deventer S, van den Brink GR, Petry H et al. Neutralizing antibodies against adeno-associated viruses in inflammatory bowel disease patients: implications for gene therapy. Inflamm Bowel Dis 2011; 17: 2436–2442.
Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood 2003; 101: 2963–2972.
Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006; 12: 342–347.
Hildinger M, Auricchio A, Gao G, Wang L, Chirmule N, Wilson JM . Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J Virol 2001; 75: 6199–6203.
Lodde BM, Mineshiba F, Wang J, Cotrim AP, Afione S, Tak PP et al. Effect of human vasoactive intestinal peptide gene transfer in a murine model of Sjogren's syndrome. Ann Rheum Dis 2006; 65: 195–200.
Vosters JL, Roescher N, Illei GG, Chiorini JA, Tak PP . TACI-Fc gene therapy improves autoimmune sialadenitis but not salivary gland function in non-obese diabetic mice. Oral Dis 2012; 18: 365–374.
Voutetakis A, Bossis I, Kok MR, Zhang W, Wang J, Cotrim AP et al. Salivary glands as a potential gene transfer target for gene therapeutics of some monogenetic endocrine disorders. J Endocrinol 2005; 185: 363–372.
Nelson CD, Palermo LM, Hafenstein SL, Parrish CR . Different mechanisms of antibody-mediated neutralization of parvoviruses revealed using the Fab fragments of monoclonal antibodies. Virology 2007; 361: 283–293.
Kok MR, Voutetakis A, Yamano S, Wang J, Cotrim A, Katano H et al. Immune responses following salivary gland administration of recombinant adeno-associated virus serotype 2 vectors. J Gene Med 2005; 7: 432–441.
Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM . Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis 2009; 199: 381–390.
Halbert CL, Miller AD, McNamara S, Emerson J, Gibson RL, Ramsey B et al. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: Implications for gene therapy using AAV vectors. Hum Gene Ther 2006; 17: 440–447.
Burton DR . Antibodies, viruses and vaccines. Nat Rev Immunol 2002; 2: 706–713.
Segal B, Bowman SJ, Fox PC, Vivino FB, Murukutla N, Brodscholl J et al. Primary Sjogren's Syndrome: health experiences and predictors of health quality among patients in the United States. Health Qual Life Outcomes 2009; 7: 46.
Nguyen CQ, Yin H, Lee BH, Chiorini JA, Peck AB . IL17: potential therapeutic target in Sjogren's syndrome using adenovirus-mediated gene transfer. Lab Invest 2011; 91: 54–62.
Yin H, Nguyen CQ, Samuni Y, Uede T, Peck AB, Chiorini JA . Local delivery of AAV2-CTLA4IgG decreases sialadenitis and improves gland function in the C57BL/6.NOD-Aec1Aec2 mouse model of Sjogren's syndrome. Arthritis Res Ther 2012; 14: R40.
Smith RH, Afione SA, Kotin RM . Transposase-mediated construction of an integrated adeno-associated virus type 5 helper plasmid. Biotechniques 2002; 33: 204–206; 8, 10-1.
Acknowledgements
This work is supported by NIH and NIDCR intramural grants to JAC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Supplementary information
Rights and permissions
About this article
Cite this article
Corden, A., Handelman, B., Yin, H. et al. Neutralizing antibodies against adeno-associated viruses in Sjögren’s patients: implications for gene therapy. Gene Ther 24, 241–244 (2017). https://doi.org/10.1038/gt.2017.1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2017.1