Abstract
A microfluidic device with three-dimensional flow channels is fabricated by stereolithography according to a computer-aided design (CAD) model. By injecting water and oil phases into the device, a monodisperse water-in-oil emulsion is formed. We show that monodisperse thermosensitive poly(N-isopropylacrylamide) gel particles can be prepared by photopolymerization of a gelation reagent dissolved in the water phase.
Similar content being viewed by others
Introduction
The development of functional particles is extremely important for a broad range of applications, including biotechnology,1 pharmaceuticals,2 cosmetics,3 paints4 and structural materials.5, 6 Monodisperse functional particles are particularly useful because all particles exhibit identical properties and behavior owing to their surface area and volume being identical. Recently, microfluidic devices have received increased attention as versatile devices that can be used for preparing monodisperse particles.7, 8, 9 When two liquids that are immiscible, such as water and oil, are injected into the device at controlled flow rates, a highly monodisperse emulsion can be formed in the device. By using the emulsion as a template, monodisperse functional polymer and ceramic particles can be prepared.10, 11, 12, 13 To date, several types of microfluidic devices such as capillary microfluidic devices14 and polydimethylsiloxane devices by soft lithography15, 16, 17 have been developed. Capillary microfluidic devices can be fabricated at low cost by assembling tapered glass capillaries coaxially in a square capillary, although it is cumbersome to set the positions and sizes of the tapered capillaries precisely. In contrast, the soft lithography technique has a feature that facilitates accurate control of the positions and sizes of channels in the polydimethylsiloxane device through the design of mask patterns. In addition, it has the potential to be used for mass production; once a mask pattern is designed, a large number of polydimethylsiloxane devices can be fabricated by the stamping technique. However, it is difficult to fabricate a device with complex flow channels in three dimensions by soft lithography, which would limit the utility of the device for many applications.
In this study, we demonstrate the fabrication of a microfluidic device by stereolithography. Stereolithography is a method to build a three-dimensional object layer by layer through the photopolymerization of a liquid monomer resin;18 therefore, it facilitates the construction of complex flow channels in three dimensions. In addition, as the device can be directly fabricated on the basis of computer-aided design (CAD) data, fine-tuning of the channels can be easily and efficiently performed. We designed a microfluidic device with co-flow geometry using the CAD software and fabricated the device through stereolithography. Here we show that the fabricated devices can precisely produce one drop at a time, resulting in a monodisperse water-in-oil emulsion. Furthermore, we prepared monodisperse thermosensitive poly(N-isopropylacrylamide) (PNIPAM)19, 20 microgel particles for use as model functional particles by photopolymerization of a gelation reagent dissolved in the water phase.
Experimental procedure
Fabrication of microfluidic device
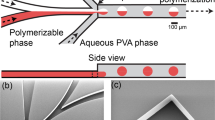
Our microfluidic device was designed on a computer using the CAD software (SolidWorks 2011, Concord, MA, USA). Figure 1 shows the designed CAD images of the microfluidic device with co-flow geometry; the intended use of the device was to generate a monodisperse water-in-oil emulsion. The device has two coaxially aligned cylindrical channels, similar to the capillary microfluidic device.14 The device size is within 1 × 1 cm2, and the internal diameters of the inner and outer channels are 50 and 1000 μm, respectively. Port a is set for injecting the water phase so that it flows through the inner channel to the outer channel, whereas port b is used for injecting the oil phase so that it flows through the outer channel in the same direction. Port c is used for collecting the water droplets formed at the tip of the inner channel together with the oil phase. The CAD data of the device were converted into a rapid prototyping format (STL file) and sliced into a set of thin layers, each with a thickness of 3 μm. The processing data were then transferred to a stereolithography machine (Sony Corp., Atsugi, Japan). On the basis of the sliced data, a ultraviolet laser beam with a wavelength of 365 nm was scanned over a liquid photopolymer epoxy resin (Sony Chemical & Information Device Corp., Tochigi, Japan), and the three-dimensional structure was built layer by layer.
(A) Computer-aided design (CAD) model of the microfluidic device with co-flow geometry. (B) Side cross-sectional view of the device along the channel axis at the center. (C) Cross-sectional view of the device normal to the channel axis at the tip of the inner channel. A full color version of this figure is available at Polymer Journal online.
Materials
To prepare PNIPAM microgel particles, an aqueous solution of a gelation reagent containing N-isopropylacrylamide (monomer, 1 M), N,N′-methylene-bis-acrylamide (crosslinker, 20 mM) and a photoinitiator (0.3 wt%, Ciba, DAROCUR 1173) was used as the water phase. The oil phase used was silicone oil (50 cSt, Sigma-Aldrich, St Louis, MO, USA) containing a surfactant (3 wt%, Dow Corning 749, Midland, MI, USA) to stabilize the interface between the water and oil phases. The solutions were filtered with 5 μm filters to remove particulate impurities, and they were then infused into the microfluidic device through polyethylene tubing attached to syringes that were driven by positive displacement syringe pumps (KD Scientific, Holliston, MA, USA, KDS 100). The formation of droplets was observed through an inverted optical microscope (Olympus, Tokyo, Japan, CKX41N). After their collection, the droplets were irradiated by a ultraviolet lamp (AS One, SUV-16) for 1 h to polymerize the gelation reagent dissolved in the droplets. The polymerized gel particles were removed from the silicone oil and washed with isopropanol to remove any adsorbed oil. The PNIPAM gel particles were then washed and stored in water. The temperature-dependent change in the size of the particles was measured by using the microscope and a thermal stage (AS One, Osaka, Japan, KM-1).
Results and Discussion
Fabrication of microfluidic device
Figure 2 shows photographs of the microfluidic device fabricated by stereolithography. The three-dimensional structure was fabricated with an accuracy of less than 5 μm. The tubing for infusing the water and oil phases and collecting the emulsion was connected to ports a, b and c on the device, respectively.
Formation of monodisperse water-in-oil emulsion
Figure 3a shows a microscopic image of the formation of monodisperse droplets in the device at flow rates of 1 and 5 ml h−1 for the inner water and outer oil phases, respectively. The water phase flows through the inner channel to the outer channel, whereas the oil phase flows in the outer channel in the same direction. At the tip of the inner channel, individual monodisperse water droplets can be periodically formed without loss. The surfactant molecules added in the oil phase immediately migrate to the water–oil interface to prevent droplet coalescence. Even after its collection, the emulsion remains stable and monodisperse (coefficient of variation of the droplet size is about 2%), as evidenced by the hexagonal close packing of the droplets (Figure 3b). The droplet size can be controlled by changing the inner and outer flow rates, as shown in Figure 4. When the flow rate of the outer oil phase is increased, the size of the droplets formed at the tip decreases monotonically. For example, when the flow rate of the inner water phase is maintained constant at 0.5 ml h−1, the diameter of the droplets decreases from about 880 to 410 μm, as the flow rate of the outer oil phase is increased from 0.3 to 15 ml h−1. In contrast, when the flow rate of the inner water phase is increased, the size of the droplets increases monotonically. For example, when the flow rate of the outer oil phase is maintained constant at 5 ml h−1, the diameter of the droplets initially remains constant at about 550 μm, as long as the flow rate of the inner water phase does not exceed 1 ml h−1. As the flow rate of the water phase is increased to 3 ml h−1, the diameter gradually increases to 770 μm, and the position of drop formation gradually moves away from the tip of the inner channel. If the flow rate of the water phase is increased further, a jet—a long stream of the water phase with drops forming downstream—is formed.21 In this regime, monodisperse droplets cannot be obtained as the position at which a drop separates from the jet is not guaranteed to remain fixed.
Optical micrographs of the droplets formed at the following flow rates of the outer oil phase when the flow rate of the inner water phase is constant at 0.5 ml h−1: (a) 0.3, (b) 1, (c) 5 and (d) 15 ml h−1. (e) Plots of the diameter of the droplets at a constant outer oil phase flow rate, Qo, as a function of the flow rate of the inner water phase. A full color version of this figure is available at Polymer Journal online.
Preparation of monodisperse PNIPAM gel particles
To prepare monodisperse PNIPAM gel particles, the emulsion was irradiated with a ultraviolet lamp. Under appropriate preparation conditions, which included the gelation reagent concentration and irradiation time, the gelation reagent that is present in the dissolved form in the water droplets can be polymerized to form PNIPAM hydrogel particles, preserving the high monodispersity (coefficient of variation: about 2%). After washing the oil adsorbed on the PNIPAM particles, by using a thermal stage mounted on the microscope, we confirmed the thermosensitive behavior of the particles dispersed in water. The PNIPAM gel is a thermosensitive polymer that undergoes a volume transition at a solution temperature of 32 °C.20 As shown in Figure 5, upon heating from 25 to 40 °C, all particles begin to shrink rapidly at around 32 °C, as expected for PNIPAM gel. The size of the particles ultimately decreased by 49%, which corresponds to an 88% decrease in volume. This temperature-dependent size change is reversible and reproducible over several heating–cooling cycles. The particles have the potential for use in drug delivery2 and as actuators for biological applications.22
Conclusion
By using stereolithography, we have demonstrated the fabrication of a microfluidic device that can be used for the preparation of monodisperse particles. As stereolithography can fabricate a three-dimensional object according to a CAD model, microfluidic devices with highly complex three-dimensional flow channels can be fabricated easily and efficiently. In addition, the present method has the potential to be used for mass production. The device is a promising candidate for the preparation of monodisperse functional particles.
References
Frykman, S. & Srienc, F. Quantitating secretion rates of individual cells: design of secretion assays. Biotechnol. Bioeng. 59, 214–226 (1998).
Qiu, Y. & Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliver. Rev. 53, 321–339 (2001).
Lademann, J., Richter, H., Schanzer, S., Knorr, F., Meinke, M., Sterry, W. & Patzelt, A. Penetration and storage of particles in human skin: perspectives and safety aspects. Eur. J. Pharm. Biopharm. 77, 465–468 (2011).
McDonald, C. J. & Devon, M. J. Hollow latex particles: synthesis and applications. Adv. Colloid Interface Sci. 99, 181–213 (2002).
Bonderer, L. J., Studart, A. R. & Gauckler, L. J. Bioinspired design and assembly of platelet reinforced polymer films. Science 319, 1069–1073 (2008).
Kanai, T., Sawada, T., Toyotama, A. & Kitamura, K. Air-pulse-drive fabrication of photonic crystal films of colloids with high spectral quality. Adv. Funct. Mater. 15, 25–29 (2005).
Shah, R. K., Shum, H. C., Rowat, A. C., Lee, D., Agresti, J. J., Utada, A. S., Chu, L. Y., Kim, J.- W., Fernandez-Nieves, A., Martinez, C. J. & Weitz, D. A. Designer emulsions using microfluidics. Mater. Today 11, 18–27 (2008).
Shum, H. C., Kim, J.- W. & Weitz, D. A. Microfluidic fabrication of monodisperse biocompatible and biodegradable polymersomes with controlled permeability. J. Am. Chem. Soc. 130, 9543–9549 (2008).
Kanai, T., Lee, D., Shum, H. C., Shah, R. K. & Weitz, D. A. Gel-immobilized colloidal crystal shell with enhanced thermal sensitivity at photonic wavelengths. Adv. Mater. 22, 4998–5002 (2010).
Kim, S.- H., Cho, Y.- S., Jeon, S.- J., Eun, T. H., Yi, G.- R. & Yang, S.- M. Microspheres with tunable refractive index by controlled assembly of nanoparticles. Adv. Mater. 20, 3268–3273 (2008).
Lee, D. & Weitz, D. A. Double emulsion-templated nanoparticle colloidosomes with selective permeability. Adv. Mater. 20, 3498–3503 (2008).
Kim, J.- W., Utada, A. S., Fernández-Nieves, A., Hu, Z. B. & Weitz, D. A. Fabrication of monodisperse gel shells and functional microgels in microfluidic devices. Angew. Chem. Int. Ed. 46, 1819–1822 (2007).
Kanai, T., Lee, D., Shum, H. C. & Weitz, D. A. Fabrication of tunable spherical colloidal crystals immobilized in soft hydrogels. Small 6, 807–810 (2010).
Utada, A. S., Lorenceau, E., Link, D. R., Kaplan, P. D., Stone, H. A. & Weitz, D. A. Monodisperse double emulsions generated from a microcapillary device. Science 308, 537–541 (2005).
Whitesides, G. M. & Stroock, A. D. Flexible methods for microfluidics. Physics Today 54, 42–48 (2001).
Abate, A. R., Lee, D., Do, T., Holtze, C. & Weitz, D. A. Glass coating for PDMS microfluidic channels by sol-gel methods. Lab on a Chip 8, 516–518 (2008).
Romanowsky, M. B., Heymann, M., Abate, A. R., Krummel, A. T., Fraden, S. & Weitz, D. A. Functional patterning of PDMS microfluidic devices using integrated chemo-masks. Lab on a Chip 10, 1521–1524 (2010).
Rosochowski, A. & Matuszak, A. Rapid tooling: the state of the art. J. Mater. Process. Tech. 106, 191–198 (2000).
Saunders, B. R. & Vincent, B. Microgel particles as model colloids: theory, properties and applications. Adv. Colloid Interface Sci. 80, 1–25 (1999).
Shah, R. K., Kim, J. W., Agresti, J. J. & Weitz, D. A. Fabrication of monodisperse thermosensitive microgels and gel capsules in microfluidic devices. Soft Matter 12, 2303–2309 (2008).
Utada, A. S., Fernández-Nieves, A., Stone, H. A. & Weitz, D. A. Dripping to jetting transitions in coflowing liquid streams. Phys. Rev. Lett. 99, 094502-1–094502-4 (2007).
Pelah, A. & Jovin, T. M. Polymeric actuators for biological applications. Chem. Phys. Chem. 8, 1757–1760 (2007).
Acknowledgements
This work was supported by the JSPS Grant-in-Aid for Scientific Research (22686063) and the Kanagawa R&D Network Plan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanai, T., Ohtani, K., Fukuyama, M. et al. Preparation of monodisperse PNIPAM gel particles in a microfluidic device fabricated by stereolithography. Polym J 43, 987–990 (2011). https://doi.org/10.1038/pj.2011.103
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2011.103