Abstract
Hydrogel microspheres (microgels), which consist of crosslinked hydrophilic or amphiphilic polymer chains, are components of stable colloidal dispersions. Their typical size is below ~10 μm, and they exhibit fascinating properties in aqueous solution. Owing to their attractive properties, they have been used for a variety of applications (for example, as templates, sensors, catalysts, and coatings) and have promising prospects for advanced chemical technologies such as drug carriers. In this context, we have been conducting research on microgels, including their synthesis, characterization, assembly, and application. In this focus review, we summarize recent results of microgel research conducted mainly by our group as well as work by our collaborators.
Similar content being viewed by others
Introduction
In contrast to bulk or macroscopic materials, small materials (sizes:<10 μm) show unique colloidal behavior. Polymeric hydrogel microspheres (microgels) are a good example for such materials. Microgels are water-swellable colloidal particles consisting of cross-linked polymeric networks. Consequently, microgels are soft and deformable, albeit that such phenomena are in general difficult to observe with the naked eye or by optical microscopy. Considering that research on microgels has been ongoing for more than 30 years, and that interest has been steadily growing during this period, it is hardly surprising that the body of relevant scientific literature reported is vast.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 Especially the biomedical applications of microgels12, 13 have received much attention in recent years, owing to the tunability of their size, surface charge, softness and stimuli-responsiveness, as well as on account of their biocompatibility. In this focus review, we summarize recent results mainly from our research group and from those of our collaborators, whereby particular emphasis is put on how to control the spatiotemporal structures of polymeric microgels.
Nanocomposite microgels: synthesis and properties
The design and control of the chemical composition and morphology of microgels are important to realize various applications for microgels. Since Pelton and Chibante first reported the synthesis of uniform poly(N-isopropyl acrylamide; pNIPAm) microgels by precipitation polymerization,14 various types of functional microgels, such as core-shell microgels15 and hollow microgels16 have been reported. Suzuki and Kawaguchi reported a different approach to control the size and spatial distribution of metal nanoparticles using pNIPAm-based microgels as templetes.17, 18, 19, 20 As the metal nanoparticles are formed within the microgels, and are thus covered with a hydrated gel layer, the nanocomposite microgels exhibit high colloidal stability in aqueous solution. Moreover, such hybrid microgels exhibit color changes on account of their thermosensitive properties, for example, the surface plasmon resonance adsorption of Au, Au@Ag, and Au@Ag@Au nanoparticles can be changed by controlling interparticle interactions between these nanoparticles within the microgels. Such hybrid microgels are thus expected to serve as functional sensors.
More recently, our group has focused on the hydrogel-solid polymer nanocomposite microgels synthesized by seeded ‘dispersion’ or ‘emulsion’ polymerization (SEP) in the presence of functional microgels (Figure 1).21, 22, 23, 24, 25 In contrast to the functionalized or core-shell microgels prepared by (seeded) precipitation polymerization with water-soluble monomers, the morphology of our nanocomposite microgels is non-spherical, and they also assume the functions of the seed microgels, for example, response to external stimuli and high colloidal stability. Additionally, the relationship between the local environment in microgels, i.e., the polarity and hydrophobicity, and the hydrophobic monomer used for the polymerization can be investigated by evaluating the spatial distribution of solid spheres formed in the resulting nanocomposite microgels using electron microscopy (Figure 1).
Multi-layered composite microgels were obtained using glycidyl methacrylate (GMA; solubility in water: 120 mm) as a monomer,22 while raspberry-shaped composite microgels were obtained using styrene (solubility in water: 2.9 mm).23, 24, 25 For example, when pure (non-functionalized) pNIPAm microgels were used as cores for the SEP of styrene, raspberry-shaped composite microgels, which contain polystyrene particles of different size attached to the core of microgels, were obtained (Figure 2a). These particles developed irregular surfaces (D=137 nm, CV=16%) with increasing polymerization time on account of the fusion of neighboring polystyrene nanoparticles.23 Moreover, when the anionic surfactant SDS was added during the polymerization, polystyrene nanoparticles (D=46 nm, CV=13%) were formed on the surface and also within the core microgels (Figure 2b), indicating the formation of aggregates of SDS within the microgels during the SEP, where they work as polymerization fields for hydrophobic polystyrene.25 These results indicate that the monomer styrene can be dissolved and polymerized in deswollen pNIPAm microgels, and that SDS aggregates are formed within the pNIPAm microgels.23 Interestingly, when poly(NIPAm-co-methacrylic acid) microgels, whose charge groups are localized at the center of the microgels,26 were used as cores, polystyrene nanoparticles were localized on the surface of the microgels (Figure 2c).24 In contrast, Watanabe et al., found that when poly(NIPAm-co-fumaric acid) microgels, whose charge groups are localized on the surface, were used as cores, the core microgels were covered almost entirely with polystyrene (Figure 2d). The thus obtained composite microgels exhibited high colloidal stability, as they are covered with a thin hydrogel layer.25 It should be noted that cryo-TEM measurements are very important for the structural analysis of swollen composite microgels. These results indicate that monomeric styrene can penetrate the polyelectrolyte hydrogel layers. However, the resulting polystyrene nanoparticles do not readily combine with polyelectrolyte hydrogels.25 To control the morphology of microgels, including the size distribution of the hydrophobic particles (~CV=5%), the structural anisotropy and the hierarchy of gel-solid layers, as well as the effects of microgel properties on the polymerization behavior of hydrophobic monomers needs to be investigated in more detail. The details of such studies should provide key factors for the design of the morphology of nanocomposite microgels, and thus further the understanding of the properties of such microgels. We believe that this conclusion should be a key factor for the design of the morphology of nanocomposite microgels, and we will continue to develop nanocomposite microgels with unique structures and functions.
Characterization of the morphology, microstructure and function of swollen microgels
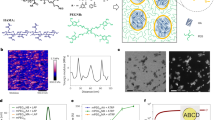
As microgels are colloidal in nature, they can be characterized by their size, polydispersity, shape, and the state of dispersion/flocculation. In addition, microgels possess gel properties, and therefore, the degree of swelling and the network structure are equally important features. The size and size distribution of microgels are usually visualized by optical microscopy. Microgels with diameters approximating or largely exceeding 1 μm are generally considered suitable for observation by optical microscopy.27 If higher resolution is required, microscopy techniques based on radiation of much smaller wavelength are required. Thus far, dried microgels have frequently been characterized by electron microscopy, such as scanning electron microscopy (SEM) and transmission electron microscopy (TEM).28 However, it is difficult to observe the swollen microgels on the submicron or decanano scale using these techniques. Although swollen microgels exhibit unique properties, for electron microscopy, samples must be dried, and sample chambers in electron microscopes are normally maintained under high vacuum. To overcome these limitations, Horigome et al.29 have proposed the swelling of microgels with ionic liquids, as these are nonvolatile even under high-vacuum conditions. Indeed, the aforementioned hybrid microgels could be visualized using conventional SEM techniques (Figure 3a). Particularly, the degree of dispersion of the embedded metal nanoparticles in the microgels could be evaluated on account of the swelling caused by the ionic liquids.
(a) SEM images of hybrid microgels containing metal nanoparticles. Pt/Pd sputtering was performed prior to the measurement (left). The microgels were swollen using 0.5 μl of [dema][OTf] (diethylmethylamine trifluorosulfonate) (right). Reprinted with permission from29 Copyright (2016) Nature Publishing Group. (b) Schematic diagram of the microscopic structural changes in poly(NIPAm-co-acrylic acid) microgels in the presence of cationic organic dyes. Reprinted with permission from33 Copyright (2016) American Chemical Society. A full color version of this figure is available at Polymer Journal online.
However, the microscopic structural parameters of these microgels (for example, mesh size) cannot be easily characterized by these techniques, although the characterization of the microscopic structures is important for the understanding of the microgel properties.30 Therefore, our group collaborated with that of Sato, who had already established techniques for the structural analyses of microgels and other colloids using small- and wide-angle X-ray scattering (SWAXS).31 Indeed, Sato et al. discovered for the first time two interference peaks in the high-q regime of SWAXS profiles that allowed the calculation of a characteristic distance (d*) for pNIPAm microgels. The characteristic distance d* is related to the hydrodynamic diameter as a function of temperature.
Kureha et al.32 have investigated the relationship between the uptake/release of organic dyes and revealed microscopic structures for pNIPAm microgels. In the case of non-functionalized pNIPAm microgels that are slightly negatively charged by initiator residues, hydrophobic interactions occur between the association of hydrophobic isopropyl groups in the pNIPAm microgels and rhodamine 6 G or erythrosine, which represent cationic and anionic organic dyes, respectively. Thus, an increasing uptake of dye with growing hydrophobic domains in the pNIPAm microgels was observed. More recently, the uptake/release behavior of anionic poly(NIPAm-co-acrylic acid) microgels, which are the most extensively studied microgels on account of their responsiveness to pH and temperature changes, have been investigated by relating microscopic structural information on the anionically charged microgel to the quantity of incorporated cationic molecules.33 At pH=7, where the acrylic acid groups are deprotonated, highly swollen microgels were observed and their volume transition, originating from pNIPAm segments in the microgels, was suppressed by the presence of charged carboxyl groups. Conversely, at 25 °C in the presence of cationic dyes, the electrostatic interactions between the charged groups and the cationic dyes dominate the uptake behavior and the changes in the microscopic structure of the microgels and result in a contraction of the microgels. When the temperature was increased above the lower critical solution temperature of pure pNIPAm (~33 °C), the charge of the carboxyl groups was screened by cationic dyes in the first step, followed by a growth of hydrophobic domains, which was accompanied by a coil-to-globule transition of the pNIPAm chains, resulting in an increased uptake (Figure 3b). Accordingly, the combination of hydrophobic and electrostatic partitioning of the cationic dyes by the negatively charged microgels affects the separation and volume transition behavior of the microgel. Such studies are ongoing in our group in order to improve the design of microgels as separation carriers to achieve a selective separation, high recyclability, and suppression of structural changes in the presence of target molecules.
Microgel assembly in bulk solutions, pastes and at interfaces
Assemblies of microgels exhibit unique properties that cannot be achieved by individually dispersed microgels in a liquid. Regularly arranged microgels diffract specific visible light, and as a result, exhibit structural colors.34, 35 In 2006, Suzuki joined the Lyon group at Georgia Institute of Technology, and fabricated thermosensitive colloidal crystals of the aforementioned nanocomposite microgels, wherein gold nanoparticles are localized in the first shell of the core/shell/shell microgels synthesized by seeded precipitation polymerization.36 Several years later, Okubo and Suzuki et al. found that pNIPAm microgels form such photonic materials at ultralow concentrations in the microgels (~0.04 wt.%) after careful purification with resins.37, 38 These results indicate that soft microgels, whose interfaces are vague, may afford unique regularly arranged structures that can diffract visible light, that is, high concentrations of microgels (pastes) due to steric stabilization from highly hydrated superficial polymer chains35, 36 in combination with low concentrations of microgels due to electric double layers formed on the vague interface.37, 38
Recently, the Urayama group has studied the rheological behavior of microgel pastes,39, 40 and discovered that the yield strain of microgel pastes is virtually independent from the crosslink density, microgel diameter, and microgel concentration in a limited concentration range. Moreover, the yield strain remains almost constant in a wide range of equilibrium shear moduli that cover two orders of magnitude.39
In contrast to the microgel assemblies in bulk solutions or pastes, our group has been studying the assembly at the air/water and oil/water interfaces. Suzuki and Kawaguchi et al. have developed Janus microgels, whose physicochemical properties can be tuned by external stimuli. Such Janus microgels, whose back and front surfaces exhibit different chemical structures, were fabricated at the oil/water interface, and the portion of the microgels in the aqueous phase was chemically modified.41, 42 This strategy is currently applied in our group to the synthesis of unique anisotropic microgel assemblies that are able to mimic the functions of biological cells and will be reported in the near future.
Beyond that we found that dilute aqueous dispersions that contain pNIPAm-based microgels assemble at the air/water interface during the evaporation of water.43 In general, when a droplet of a colloidal dispersion, such as conventional rigid polystyrene and silica particles, is dried on a solid substrate, ring-like, non-uniform films are obtained. However, in the case of our microgel, homogeneous thin films of a pNIPAm microgel monolayer were obtained on the solid substrate. Horigome and Suzuki have reported the formation mechanisms of the structures formed during the drying of dilute pNIPAm microgel dispersions (Figure 4).43 When a dilute dispersion (~1 μm pNIPAm microgels; 50 μl) was dried at 25±2 °C on a polystyrene substrate, the pNIPAm microgel was adsorbed at the air-water interface in an early stage (~3 min), and the microgels subsequently assembled at the interface. Finally, the assembled structure was transferred onto the solid substrate after all the water had been removed. We further extended this experiment by using oppositely charged microgels, that is, cationic and anionic microgels.44, 45 These microgels were also assembled at the air/water interface upon mixing in solution (Figure 5).44 The microgels were then assembled into linear and branched-chain structures at the air/water interface. We found that the total microgel concentration, the concentration ratio of cationic and anionic microgels, and the salt concentration were important factors to create the microgel chains.45 Furthermore, the addition of a small amount of electrolyte to the binary mixtures prevented flocculation, resulting in non-close-packed structures on planar substrates in the dry state.44 These findings should lead to new materials such as precisely controlled microgel coatings, and similar studies are currently pursued in our research group.
Schematic images of the drying mechanism of pNIPAm microgels in dilute dispersion. Reprinted with permission from43 Copyright (2012) American Chemical Society. A full color version of this figure is available at Polymer Journal online.
SEM images of binary mixtures of cationically and anionically charged microgels deposited on polystyrene substrates and dried at 25 °C. It should be noted that the SEM sample (right image) was prepared by drying the mixture in the presence of 1 mM NaCl; after drying, the NaCl was gently removed by repeated washing with deionized water. Reprinted with permission from44 Copyright (2011) American Chemical Society. A full color version of this figure is available at Polymer Journal online.
Controlling the temporal structures of microgels
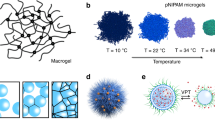
Living systems are usually based on periodic rhythms, such as the heartbeat or the assembling/disassembling of clock proteins. After graduating from Keio University in 2007, Suzuki joined Yoshida’s group at the University of Tokyo, who has developed self-oscillating gels46 that exhibit a swelling/deswelling in the absence of external stimuli, as a JSPS postdoctoral research fellow for two years. Learning from Yoshida’s previous work, Suzuki accomplished the preparation of microgels with temporal structures of uniform size by copolymerizing the catalyst for the Belousov–Zhabotinsky (BZ) reaction47 into pNIPAm-based microgels.48 These autonomously oscillating microgels also show repeated swelling/deswelling, which is evident from UV-vis spectroscopy.48, 49, 50 Moreover, Suzuki discovered that the microgels show dispersing/flocculating (or assembling/disassembling) oscillation near the volume-transition temperature of the pNIPAm-based microgels (Figures 6a and b).48, 51 Suzuki and Yoshida have also revealed that period, amplitude, and waveform of the swelling/deswelling and the dispersing/flocculating oscillation are controlled by the crosslinked network structures,49 as well as by the initial substrate concentration of the BZ reaction.50 Additionally, both the swelling/deswelling and the dispersing/flocculating oscillations of microgels can be detected as the changes in optical transmittance, and by the viscosity changes of the microgel dispersions (Figure 6c).51, 52 More recently, Matsui et al.53 have discovered changes in the internal microscopic structure of autonomously oscillating microgels using SWAXS. Nevertheless, microgels with temporal structures still represent a novel phenomenon, and the intrinsic details remain to be determined. Therefore, we will continue to examine such complex nonequilibrium phenomena as a model system to better understand living systems.
(a) Schematic illustration of the temporal structures of autonomously oscillating microgels synchronized with the BZ reaction. (b) Oscillation profiles of the optical transmittance and redox potential changes in the cell for the microgel dispersions measured under varying conditions. Reprinted with permission from49 Copyright (2008) American Chemical Society. (c) Temperature-dependent oscillation profiles of the viscosity in the microgel dispersions. Reprinted with permission from51 Copyright (2009) American Chemical Society. A full color version of this figure is available at Polymer Journal online.
The aforementioned oscillating microgels do not only exert a function as a single component, but they can also act as an assembly. For example, in a living muscle, hierarchical structures amplify and transform the microscopic movements of actin–myosin into macroscopic displacements. Using such living muscles as inspiration, our group introduced a hierarchical structure into an artificial muscle in order to amplify the swelling/deswelling oscillation. Autonomously oscillating soft actuators have been successfully obtained by simple methods, for example, by assembling pre-existing microgels (Figure 7a).54 Such assemblies exhibit large displacements due to the cooperative dispersing/flocculating oscillation of the constituent microgels in the absence of external stimuli (on/off switch) under otherwise constant conditions. We expect that such autonomous soft materials could be used as micropumps that do not require an external device source. We will continue to develop microgels and microgel assemblies with temporal structures as model systems in order to better understand living systems, where small colloidal particles such as proteins show pre-programmed dynamic ordering to regulate vital functions. In order to progress in this research area, the design and construction of autonomously oscillating microgels with well-defined nanostructures, such as core/shell structures (Figure 7b)55 should be of paramount importance.
(a) Schematic illustration and optical microscopy images (scale bars: 0.5 mm) of an assembly of autonomously oscillating microgels. Reprinted with permission from54 Copyright (2012) Royal Society of Chemistry. (b) Different oscillation modes for autonomously oscillating core/shell microgels. Reprinted with permission from55 Copyright (2010) Nature Publishing Group.
Concluding remarks
In this review, we have summarized recent advances of microgel research and technology of mainly our group and our collaborators. The results presented herein should contribute to further the development of microgel research. We hope that our microgel research will not only support the advancement of pre-existing applications, for example, in coatings and cosmetics, but also allow the development of unprecedented applications.
References
Saunders, B. R. & Vincent, B. Microgel particles as model colloids: theory, properties and applications. Adv. Colloid Interf. Sci. 80, 1–25 (1999).
Pelton, R. Temperature-sensitive aqueous microgels. Adv. Colloid Interf. Sci. 85, 1–33 (2000).
Nayak, S. & Lyon, L. A. Soft nanotechnology with soft nanoparticles. Angew. Chem. Int. Ed. 44, 7686–7708 (2005).
Hoare, T. & Pelton, R. Characterizing charge and crosslinker distributions in polyelectrolyte microgels. Curr. Opin. Colloid Interf. Sci. 13, 413–428 (2008).
Pich, A. & Richtering, W. Microgels by precipitation polymerization: synthesis, characterization, and functionalization. Adv. Polym. Sci. 234, 1–37 (2010).
Lu, Y. & Ballauff, M. Thermosensitive core-shell microgels: from colloidal model systems to nanoreactors. Prog. Polym. Sci. 36, 767–792 (2011).
Lyon, L. A. & Fernandez-Nieves, A. The polymer/colloid duality of microgel suspensions. Annu. Rev. Phys. Chem. 63, 25–43 (2012).
Hellweg, T. Responsive core-shell microgels: synthesis, characterization, and possible applications. J. Polym. Sci. B Polym. Phys. 51, 1073–1083 (2013).
Kawaguchi, H. Thermoresponsive microhydrogels: preparation, properties and applications. Polym. Int. 63, 925–932 (2014).
Yunker, P. J., Chen, K., Gratale, M. D., Lohr, M. A., Still, T. & Yodh, A. G. Physics in ordered and disordered colloidal matter composed of poly(N-isopropylacrylamide) microgel particles. Rep. Prog. Phys. 77, 1–29 (2014).
Plamper, F. A. & Richtering, W. Functional microgels and microgel systems. Acc. Chem. Res. 50, 131–140 (2017).
Saxena, S., Hansen, C. E. & Lyon, L. A. Microgel mechanics in biomaterial design. Acc. Chem. Res. 47, 2426–2434 (2014).
Anselmo, A. C. & Mitragotri, S. Impact of particle elasticity on particle-based drug delivery systems. Adv. Drug Deliv. Rev. 108, 51–67 (2017).
Pelton, R. H. & Chibante, P. Preparation of aqueous latices with N-isopropylacrylamide. Colloid. Surf. 20, 247–256 (1986).
Jones, C. D. & Lyon, L. A. Shell-Restricted swelling and core compression in poly(N-isopropylacrylamide) core-shell microgels. Macromolecules 36, 1988–1993 (2003).
Dubbert, J., Nothdurft, K., Karg, M. & Richtering, W. Core-shell-shell and hollow double-shell microgels with advanced temperature responsiveness. Macromol. Rapid Commun. 36, 159–164 (2015).
Suzuki, D. & Kawaguchi, H. Modification of gold nanoparticle composite nanostructures using thermosensitive core-shell particles as a template. Langmuir 21, 8175–8179 (2005).
Suzuki, D. & Kawaguchi, H. Gold nanoparticle localization at the core surface by using thermosensitive core-shell particles as a template. Langmuir 21, 12016–12024 (2005).
Suzuki, D. & Kawaguchi, H. Hybrid microgels with reversibly changeable multiple brilliant color. Langmuir 22, 3818–3822 (2006).
Suzuki, D. & Kawaguchi Stimuli-sensitive core/shell template particles for immobilizing inorganic nanoparticles in the core. Colloid Polym. Sci. 284, 1443–1451 (2006).
Suzuki, D. & Yamakawa, S. Hydrogel particles as a particulate stabilizer for dispersion polymerization. Langmuir 28, 10629–10634 (2012).
Suzuki, D., Yamagata, T. & Murai, M. Multilayered composite microgels synthesized by surfactant-free seeded polymerization. Langmuir 29, 10579–10585 (2013).
Suzuki, D. & Kobayashi, C. Raspberry-shaped composite microgel synthesis by seeded emulsion polymerization with hydrogel particles. Langmuir 30, 7085–7092 (2014).
Kobayashi, C., Watanabe, T., Murata, K., Kureha, T. & Suzuki, D. Localization of polystyrene particles on the surface of poly(n-isopropylacrylamide-co-methacrylic acid) microgels prepared by seeded emulsion polymerization of styrene. Langmuir 32, 1429–1439 (2016).
Watanabe, T., Kobayashi, C., Song, C., Murata, K., Kureha, T. & Suzuki, D. Impact of spatial distribution of charged groups in core poly(N-isopropyl acrylamide)-based microgels on the resultant composite structures prepared by seeded emulsion polymerization of styrene. Langmuir 32, 12760–12773 (2016).
Hoare, T. & Pelton, R. Characterizing charge and crosslinker distributions in polyelectrolyte microgels. Curr. Opin. Colloid Interf. Sci. 13, 413–428 (2008).
Cohin, Y., Fisson, M., Jourde, K., Fuller, G. G., Sanson, N., Talini, L. & Monteux, C. Tracking the interfacial dynamics of PNiPAM soft microgels particles adsorbed at the air-water interface and in thin liquid films. Rheol. Acta. 52, 445–454 (2013).
Gelissen, A. P. H., Oppermann, A., Caumanns, T., Hebbeker, P., Turnhoff, S. K., Tiwari, R., Eisold, S., Simon, U., Lu, Y., Mayer, J., Richtering, W., Walther, A. & Wöll, 3D structures of responsive nanocompartmentalized microgels. Nano Lett 16, 7295–7301 (2016).
Horigome, K., Ueki, T. & Suzuki, D. Direct visualization of swollen microgels by scanning electron microscopy using ionic liquids. Polym. J. 48, 273–279 (2016).
Fernández-Barbero, A., Fernández-Nieves, A., Grillo, I. & López-Cabarcos, E. Structural modifications in the swelling of inhomogeneous microgels by light and neutron scattering. Phys. Rev. E 66, 051803 (2002).
Sato, T., Sakai, H., Sou, K., Medebach, M., Glatter, O. & Tsuchida, E. Static structures and dynamics of hemoglobin vesicle (hbv) developed as a transfusion alternative. J. Phys. Chem. B 113, 8418–8428 (2009).
Kureha, T., Sato, T. & Suzuki, D. Relationship between temperature-induced changes in internal microscopic structures of poly(N-isopropylacrylamide) microgels and organic dye uptake behavior. Langmuir 30, 8717–8725 (2014).
Kureha, T., Shibamoto, T., Matsui, S., Sato, T. & Suzuki, D. Investigation of changes in the microscopic structure of anionic poly(N-isopropylacrylamide-co-acrylic acid) microgels in the presence of cationic organic dyes toward precisely controlled uptake/release of low-molecular-weight chemical compound. Langmuir 32, 4575–4585 (2016).
Weissman, J. M., Sunkara, H. B., Tse, A. S. & Asher, S. A. Thermally switchable periodicities and diffraction from novel mesocopically ordered materials. Science 274, 959–960 (1996).
Debord, J. D. & Lyon, L. A. Thermoresponsive photonic crystals. J. Phys. Chem. B 104, 6327–6331 (2000).
Suzuki, D., McGrath, J. G., Kawaguchi, H. & Lyon, L. A. Colloidal crystals of thermosensitive, core/shell hybrid microgels. J. Phys. Chem. C 111, 5667–5672 (2007).
Okubo, T., Suzuki, D., Yamagata, T., Katsuno, A., Sakurai, M., Kimura, H. & Tsuchida, A. Colloidal crystallization of thermo-sensitive gel spheres of poly(N-isopropyl acrylamide). Colloid. Polym. Sci. 289, 291–299 (2011).
Suzuki, D., Horigome, K., Yamagata, T., Shibata, K., Tsuchida, A. & Okubo, T. Colloidal crystallization of thermo-sensitive gel spheres of poly (N-isopropyl acrylamide). Influence of degree of cross-linking of the gels. Colloid. Polym. Sci. 289, 1799–1808 (2011).
Urayama, K., Cong, S., Saeki, T., Uratani, S., Takigawa, T., Murai, M. & Suzuki, D. A simple feature of yielding behavior of highly dense suspensions of soft micro-hydrogel particles. Soft Matter 10, 9486–9495 (2014).
Minami, S., Watanabe, T., Suzuki, D. & Urayama, K. Rheological properties of suspensions of thermo-responsive poly (N-isopropylacrylamide) microgels undergoing volume phase transition. Polym. J. 48, 1079–1086 (2016).
Suzuki, D., Tsuji, S. & Kawaguchi, H. Janus microgels prepared by surfactant free pickering emulsion-based modification and their self-assembly. J. Am. Chem. Soc. 129, 8088–8089 (2007).
Umeda, Y., Kobayashi, T., Hirai, T. & Suzuki, D. Effects of pH and temperature on assembly of multiresponsive janus microgels. Colloid. Polym. Sci. 289, 729–737 (2011).
Horigome, K. & Suzuki, D. Drying mechanism of poly(N-isopropylacrylamide) microgel dispersions. Langmuir 28, 12962–12970 (2012).
Suzuki, D. & Horigome, K. Binary mixtures of cationic and anionic microgels. Langmuir 27, 12368–12374 (2011).
Suzuki, D. & Horigome, K. Assembly of oppositely charged microgels at the air/water interface. J. Phys. Chem. B 117, 9073–9082 (2013).
Yoshida, R., Takahashi, T., Yamaguchi, T. & Ichijo, H. Self-oscillating gel. J. Am. Chem. Soc. 118, 5134–5135 (1996).
Zaikin, A. N. & Zhabotinsky, A. M. Concentration wave propagation in two-dimensional liquid-phase self-oscillating system. Nature 225, 535–537 (1970).
Suzuki, D., Sakai, T. & Yoshida, R. Self-flocculating/self-dispersing oscillation of microgels. Angew. Chem. Int. Ed. 47, 917–920 (2008).
Suzuki, D. & Yoshida, R. Temporal control of self-oscillation for microgels by crosslinking network structure. Macromolecules 41, 5830–5838 (2008).
Suzuki, D. & Yoshida, R. Effect of initial substrate concentration of the Belousov-Zhabotinsky reaction on self-oscillation for microgel system. J. Phys. Chem. B 40, 12618–12624 (2008).
Suzuki, D., Taniguchi, H. & Yoshida, R. Autonomously oscillating viscosity in microgel dispersions. J. Am. Chem. Soc. 131, 12058–12059 (2009).
Taniguchi, H., Suzuki, D. & Yoshida, R. Characterization of autonomously oscillating viscosity induced by swelling/deswelling oscillation of the microgels. J. Phys. Chem. B 114, 2405–2410 (2010).
Matsui, S., Kureha, T., Nagase, Y., Okeyoshi, K., Yoshida, R., Sato, T. & Suzuki, D. Small-angle X-ray scattering study on internal microscopic structures of poly(N-isopropylacrylamide-co-tris(2,2′-bipyridyl))ruthenium(II) complex microgels. Langmuir 31, 7228–7237 (2015).
Suzuki, D., Kobayashi, T., Yoshida, R. & Hirai, T. Soft actuators of organized self-oscillating microgels. Soft Matter 8, 11447–11449 (2012).
Suzuki, D. & Yoshida, R. Self-oscillating core/shell microgels: effect of a crosslinked nanoshell on autonomous oscillation of the core. Polymer J. 42, 501–508 (2010).
Acknowledgements
DS acknowledge the following academics: Emeritus Prof H Kawaguchi (Keio Univ.) for introducing him to the world of polymer colloid chemistry; Prof L A Lyon (Georgia Institute of Technology) for accepting me in his laboratory as a visiting researcher (01-06 2006) and for collaborating on hybrid microgels and colloidal crystallizations; Prof R Yoshida (Univ. of Tokyo) for accepting me as a JSPS research fellow in his laboratory, and for continued research on autonomously oscillating microgels; Prof K Urayama (Kyoto Institute of Technology Univ.) for his collaboration regarding rheological experiments on microgels; Emeritus Prof T Okubo (Gifu Univ.) for his collaboration on colloidal crystals formed after deionization with resins; Prof K Murata and Dr S Chihon (NIPS) for Cryo-TEM measurements on composite microgels. DS, TK, and SM thank Prof T Sato (Shinshu Univ.) for his kind teaching of the details of SWAXS experiments and analysis. DS and KH thank Dr T Ueki (NIMS) for providing ionic liquids and for the kind discussions. We kindly acknowledge all other contributors, who are listed in the review. For financial support, DS acknowledge a Grant-in-Aid from the Japan Society for the Promotion of Science (JSPS) (05J08147), a Grant-in-Aid for the 21st Century COE program ‘KEIO Life-Conjugated Chemistry’, the JSPS for Young Scientists for Research fellowships (08J09189 and JP21850014), a Grant-in-Aid for Young Scientists (A) (JP22685024), a Grant-in-Aid for Challenging Exploratory Research (JP26620177), and a Grant-in-Aid for Scientific Research on Innovative Areas (JP26102517 and 16H00760) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT). KH acknowledges a Grant-in-Aid for JSPS Research Fellows (13J10547). TK acknowledges a Grant-in-Aid for JSPS Research Fellows (15J11533). SM acknowledges financial support from a Sasakawa Scientific Research Grant from The Japan Science Society (28–318).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Suzuki, D., Horigome, K., Kureha, T. et al. Polymeric hydrogel microspheres: design, synthesis, characterization, assembly and applications. Polym J 49, 695–702 (2017). https://doi.org/10.1038/pj.2017.39
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2017.39
This article is cited by
-
Recent advances in preparation of polymer hydrogel composites and their applications in enzyme immobilization
Polymer Bulletin (2023)
-
Controlling the shell structure of hard core/hydrogel shell microspheres
Colloid and Polymer Science (2022)
-
Preparation of poly (acrylic acid) microgels by alcohol type cross-linkers and a comparison with other cross-linking methods
Polymer Bulletin (2022)
-
Thermoresponsive structural changes of single poly(N-isopropyl acrylamide) hydrogel microspheres under densely packed conditions on a solid substrate
Polymer Journal (2020)
-
Recent advances in stimuli-responsive core-shell microgel particles: synthesis, characterisation, and applications
Colloid and Polymer Science (2020)