Abstract
Zwitterions are a class of organic salts whose cation and anion are covalently tethered. This characteristic imparts unique physicochemical properties that differ from those of conventional organic salts. Recently, there has been increasing attention paid to the use of these zwitterions as building blocks for functional organic materials due to their unique properties. These materials have been applied to polymers, liquid crystals, dendrimers and nanoparticles. In this review, I focus on the development of nanostructured ionic materials through the organization of zwitterions. The strategies for such development can be roughly classified into two groups: the introduction of liquid-crystalline properties into zwitterions and the design of block copolymers with zwitterionic segments. These zwitterion derivatives show various organizational behaviors, such as self-organization, co-organization with acids or lithium salts and solvent-induced organizational behavior; they also function as unique ionic matrices. The potential applications of these ionic matrices are described.
Similar content being viewed by others
Introduction
Self-organization has been recognized as a significant strategy for creating nanostructured materials with well-ordered periodicity. A number of technologies that make use of self-organization have been developed in recent decades.1 Liquid crystals,2, 3 block copolymers,4 metal-organic-frameworks5 and supramolecules6 are representative self-organizing systems. By introducing functional groups into these self-organizing materials, it is possible to achieve novel functions and properties with these well-organized structures. For the development of new materials with innovative functions, it is important to select appropriate functional groups and align them in suitable structural geometries.
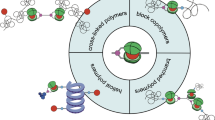
Zwitterions are a class of organic salts whose cation and anion are covalently tethered. This characteristic imparts these salts with higher crystallinity and higher melting temperature than conventional organic salts. Recently, there has been increasing attention paid to the use of zwitterions as alternatives to conventional ionic groups as building blocks for developing new functional materials; the tethered ion structures result in novel phenomena that are not produced in normal ionic groups. In the course of these studies, zwitterions have been organized via various materials design approaches, for example, using amphiphiles,7 polymeric betaines,8 polymer brushes,9 attachment on particles,10 introduction of liquid-crystalline (LC) properties11 and block copolymerization.12 In this review, I will focus on the design of liquid crystals and block copolymers containing zwitterions as molecular blocks and their potential as ionic matrices for various applications (Figure 1).
Studies on zwitterionic liquid crystals
Corresponding to the development of synthetic technologies, a variety of LC molecules have been designed in recent decades.2, 3 These liquid crystals can be classified from several viewpoints. For example, using dimensional geometry, they are classified into nematic, smectic (Sm), columnar (Col), bicontinuous cubic (Cubbi) and micellar cubic (Cubm) phases.13 In terms of molecular structures, rod-shaped-,14 disc-shaped-,15, 16 and wedge-shaped-structures16, 17 are representative. The liquid crystals may or may not contain ionic structures in their molecular structures and can be accordingly divided into two groups: ionic liquid crystals and others. A significant characteristic of ionic liquid crystals is that electrostatic interactions between the ionic parts have a major role in determining their self-organization behavior due to differences in strength between electrostatic interactions and other intermolecular interactions. Comprehensive reviews on ionic liquid crystals have been published.18, 19, 20, 21 In the present review, a class of ionic liquid crystals with zwitterionic moieties is highlighted, and their unique characteristics, which differ from normal ionic liquid crystals, are described.
Phospholipid is a representative amphiphilic biomolecule with a zwitterionic part, namely an ammonium betaine. It is well known that this molecule exhibits self-organization behavior in water and shows lyotropic LC behavior. Apart from this most well-known example, there have been artificial zwitterionic amphiphiles showing lyotropic and/or thermotropic LC behavior. Representative molecular structures are shown in Figure 2. An early report on amphiphilic zwitterions showing thermotropic LC behavior was published by Galin and colleagues in the 1990s.11 The authors reported that amphiphilic ammonium betaine 1n exhibits thermotropic LC behavior, which varies with alkyl chain length. For example, 114 exhibits the Cubbi phase from 78 to 114 °C, while 116 exhibits the Sm phase from 84 to 199 °C. Strong dipolar interactions between the zwitterionic moieties are an important driving force for self-organization.
In three decades of studies on ionic materials, an important turning point has been the growing interest in organic salts that exist in the liquid state at ambient temperature, that is, ‘ionic liquids’.22 The unique properties of ionic liquids, such as their negligible vapor pressure,23 flame retardancy24 and high ionic conductivity,25 have motivated applications as liquid electrolytes in batteries. A variety of ion designs have been exploited to develop functional ionic liquids.26 To increase the transference number of target ions, such as protons and lithium ions, zwitterions consisting of imidazolium-, pyridinium- and ammonium-betaines were prepared.27 These molecules form homogeneous mixtures with specific acids and/or lithium salts (Figure 3a) to provide ionic liquid-like media that selectively transport target ions such as protons and lithium ions.28, 29 Taking into account their drastically altered physicochemical properties on incorporation of these particular acids and lithium salts, it is expected that this ability can be used for morphological control of amphiphilic zwitterions and zwitterionic polymers. Compounds 2–9 are representative examples of zwitterionic liquid crystals whose phase behaviors are controlled by the addition of acids or lithium salts.30, 31, 32, 33, 34, 35, 36 For these zwitterionic liquid crystals, ion conduction behavior with ion-selectivity and anisotropy is expected. It is thus of interest whether they exhibit unique functions and properties that are more than a combination of the intrinsic properties of zwitterions and ionic liquid crystals. Lin et al.30 reported an early example of zwitterionic liquid crystals. They designed amphiphilic zwitterion 2 and examined the thermotropic LC behavior of their mixtures with certain lithium salts.30 In the following sections, I will describe in detail the phase behavior and function of compounds 3–9.31, 32, 33, 34, 35, 36
(a) Unique characteristic of zwitterion-forming ionic liquid-like ion pair through ion exchange with acids and/or lithium salts. (b) Formation mechanism of a saddle structure from a layered structure. (c) Phase change from a layered smectic phase to a bicontinuous cubic phase with a three-dimensional (3D) continuous gyroid minimal surface.
Molecular designs for three-dimensional gyroid structures
Nanosegregated phases formed by liquid crystals are classified into Sm, Col, Cubbi and Cubm depending on their structural geometry. Among these, our recent target is the Cubbi phase, which forms gyroid structures due to their unique three-dimensional structures.37, 38, 39, 40 In the Cubbi LC assembly, the end of an LC molecule is located in the center of the nanochannel, and the other end sits on an infinite periodic minimal surface that appears as a set of middle points between the two interwoven nanochannels. There are three types of infinite periodic minimal surface: gyroid, primitive and diamond. Among these, gyroid is observed most often. A variety of gyroid-structured materials have been produced, and various unique functions and properties derived from gyroid structures have been explored.41 For example, the three-dimensional nanochannels in Cubbi liquid crystals have been studied intensively for transport pathways because these nanochannels enable maintenance of macroscopic continuity without special alignment control of the liquid crystal domains.42, 43, 44, 45, 46
It has been mathematically revealed that a gyroid minimal surface is formed by the connection of saddle-like surfaces in three-dimension. Therefore, it is important to induce saddle structures to construct gyroid structures. When one imagines a red flat layer sandwiched by blue layers, a saddle structure is created by shrinking the blue layers in the cross direction or by expanding the red layer (Figure 3b). This model enables us to envision that the formation of a saddle structure at a molecular level leads to the creation of Cubbi LC assemblies. With this idea in mind, we have designed amphiphilic compound 3n, consisting of a pyridinium-based zwitterionic head group, an amide group and a long alkyl chain.31 This compound exhibits Sm phases consisting of alternate layers of ionic and non-ionic layers. To control the organizational behavior of 3n, bis(trifluoromethanesulfonyl)imide (HTf2N) was selected as an additive. An equimolar mixture of 3n and HTf2N was successfully prepared by slow evaporation of a solution of the two components in methanol. As expected, Cubbi phases are observed in the obtained mixtures. For example, the 314/HTf2N mixture shows a Cubbi phase from 10 to 91 °C (Figure 4a). The Cubbi phases were characterized by synchrotron X-ray diffraction measurements (Figure 4b). Interestingly, the induced LC phases show a clear dependence on alkyl chain length. Elongation of the alkyl chain leads to the induction of Sm phases, and their shortening results in the induction of Col phases. Polarizing optical microscopy images of the mixture upon phase transition from the Col to Cubbi phase are shown in Figure 4c. The alkyl chain dependence of the LC phases indicates that the volume balance between ionophilic and ionophobic domains is a significant factor governing the mesophase patterns. The change in thermotropic LC phases from Sm to Cubbi on the addition of HTf2N can be attributed to swelling of the ionic domains on the formation of ionic liquid-like ion pairs between the pyridinium-betaine moiety of 3n and HTf2N (Figure 3c). Ion exchange between the two components has been successfully revealed by Raman spectroscopy.47
(a) Thermotropic liquid-crystalline behavior of 3n/HTf2N mixtures.31 Cr, crystalline; Cubbi, bicontinuous cubic; Col, columnar; Sm, smectic; Iso, isotropic. (b) Synchrotron X-ray diffraction pattern of 314/HTf2N mixture in the Cubbi phase. (c) Polarizing optical microscopy of 312/HTf2N mixture upon phase transition from Col to Cubbi phases.31 Reproduced with permission from the reference. A full colour version of this figure is available at the Polymer Journal journal online.
A halogen bond is an interaction between an electron-rich group and an electron poor group. This interaction has been used to design LC materials.48, 49 For example, Bruce et al.48 designed complexes of alkoxystilbazoles and an iodopentafluorobenzene exhibiting LC behavior through the formation of supramolecular mesogens via halogen bonds. Apart from previous examples of halogen bond interaction for liquid crystal design, this interaction has been used for ionic liquid design.50, 51 In this context, I expect that the introduction of halogen bonds into zwitterionic amphiphiles can be used to control the interface curvature of molecular assemblies. Accordingly, amphiphilic zwitterion 4 with an iodine-substituted imidazolium cation has been designed.32 For comparison, an analogous compound 5 was also prepared. Although 4 and 5 exhibit only Sm phases, Cubbi and Col phases have been observed for a 4/HTf2N mixture and a 5/HTf2N mixture, respectively. To examine the interionic interactions between these mixtures, infrared (IR) spectroscopy has been performed. The C2H of the imidazolium ring of 4 was observed at 3108 cm–1, which shifts to 3129 cm–1 on the addition of HTf2N (Figure 5). Because the C2H of the imidazolium ring of 1-butyl-4,5-diiodo-3-methylimidazolium bis(trifluoromethane)sulfonimide, [BmimI2][Tf2N] is observed at 3139 cm–1, it is assumed that the Tf2N anion in a 4/HTf2N mixture is located near the imidazolium cation of 4. Although definitive evidence for the formation of a halogen bond between the ions has not been obtained, it is assumed that the iodine-substituted imidazolium cation can form halogen bonds with the electron-rich Tf2N anion present near the cation.
IR spectra of 4, 4/HTf2N mixture and [BmimI2][Tf2N].32 Reproduced with permission from the reference.
The use of a gemini structure is an effective strategy for controlling the self-organization behavior of amphiphilic molecules.52 In particular, this strategy has been effective for decreasing the critical micellar concentration and lowering the Krafft temperature of amphiphiles in solvents. In the course of studies on gemini-amphiphiles for lyotropic liquid crystals, it was gradually found that gemini-amphiphiles are predisposed to form lyotropic Cubbi phases compared with monomeric-type ones.53, 54, 55 This insight leads us to hypothesize that the gemini design should also be effective for designing thermotropic Cubbi liquid crystals. To verify this hypothesis, gemini-type amphiphilic zwitterions 6m,n have been designed and synthesized.33 Notably, these zwitterions exhibit Cubbi phases over a wide temperature range. For example, 66,17 shows a Cubbi phase from 51 to 207 °C on heating. In contrast, 73,17, a monomeric-type compound, shows no mesomorphic behavior. It is also noteworthy that 66,17 shows a glass transition on cooling from the Cubbi LC state, whereas 73,17 shows crystallization at 191 °C on cooling from the isotropic state. The significant difference in crystallinity results from covalently tethered structures that prevent each long alkyl chain from aligning in a suitable position for crystallization. We assume that the strong incompatibility between the zwitterionic portion and the alkyl chain portion leads to minimization of the interface area, leading to Cubbi phases with a gyroid-structured interface. These results suggest that a combination of gemini amphiphile design and zwitterionic amphiphile design is a promising molecular strategy for developing thermotropic Cubbi liquid crystals.
Molecular designs for one-dimensional ion-conductive materials
Phosphonium-based zwitterions 8n have been designed and mixed with HTf2N to form ionic nanochannels surrounded by ionophobic sheath domains.34 Although the 8n/HTf2N mixtures show no mesomorphic behavior, the addition of water to the mixtures induces the formation of hexagonal columnar (Colh) phases with one-dimensional hydrophilic nanochannels. For example, an 86/HTf2N mixture with 20 wt% H2O exhibits a Colh phase from room temperature to 65 °C that can be identified by polarizing optical microscopy and X-ray diffraction measurements. The Colh liquid crystals have been successfully aligned using mechanical shearing (Figure 6, left), a technique to measure anisotropic ion conductivity in columnar liquid crystals.56, 57 Ionic conductivities of the 86/HTf2N mixture with 20 wt% H2O in the aligned Colh and polydomain states have been successfully measured using a cell equipped with comb-shaped gold electrodes. Notable improvements in conductivities have been observed on control of the alignment of the liquid crystal domain, while the conductivities converge after the phase transition to the isotropic phase (Figure 6, left). Similar ion onduction behavior has also been observed for other zwitterionic liquid crystals. For example, Soberats et al.35, 36 designed a series of wedge-shaped zwitterionic salts 9 and examined their applications to proton- and lithium ion-conductive materials. These results indicate that macroscopic alignment of one-dimensional nanochannels is required to obtain the maximum potential properties of these zwitterions to form anisotropic structures.
Lyotropic liquid-crystalline behavior of 8n/HTf2N mixtures using water and ionic liquid 10 as solvent. Using water leads to a columnar phase with effective ion conduction behavior in the aligned state.34 The use of ionic liquid 10 provides a nanobiphasic ionic liquid system dissolving various solvatochromic dyes into nanosegregated domains.58, 59 A full colour version of this figure is available at the Polymer Journal journal online.
The self-organization behavior of 86 can be controlled by the addition of certain ionic liquids as well as that of water.58, 59 For example, the addition of ionic liquid 10 to the 86/LiTf2N mixture leads to the formation of Col and Cubm phases, depending on the molar ratio of 10 in the mixture (Figure 6, right). As the obtained LC matrices are composed of high-polarity (10) and low-polarity (86/LiTf2N) ionic liquids, it is expected that these nanostructured matrices may function as nanobiphasic ionic liquids, where two ionic liquids co-exist on a macroscopic scale but exist separately on a nanometer scale. To prove the formation of the two incompatible ionic liquid-like domains, the solvatochromism of some dye molecules has been used. Certain dyes, such as naphthol yellow S (NYS) and 3,4′-dihexyl-2,2′-bithiophene (BTP), partition into one of the two domains (Figure 6, right). By examining the solvatochromic behavior of these dyes in the LC samples, we succeeded in clarifying the formation of high-polarity and low-polarity ionic liquid domains in the matrices. Recently, there has been increasing attention paid to the combination of ionic liquids and zwitterions.60, 61, 62 As the cation and anion of zwitterions are covalently tethered, they maintain ion pair topology even in the presence of other ions, such as ionic liquids, enabling the formation of novel ionic matrices that cannot be constructed using a combination of conventional organic salts.
Development of glassy bicontinuous cubic liquid crystals
Some liquid crystals are known to show glass transitions (instead of crystallization) on cooling.63, 64, 65, 66, 67 These liquid crystals are called glassy liquid crystals, and several examples of glassy liquid crystals among ionic liquid crystals have been reported.68, 69, 70 In the design of functional LC materials, the importance of molecular dynamics changes is dependent on their applications. For example, liquid crystals for displays should have high molecular dynamics to enable high response to external stimuli. In contrast, in the case of organic semiconductors, the presence of molecular dynamics impairs function; therefore, glassification is used to improve function.66, 67 Therefore, it is expected that there are various potential applications where glassy liquid crystals are more suitable than normal liquid crystals. In this context, I expect that glassy liquid crystals can be exploited to develop nanostructured scaffolds whose structures do not change on the ingress of guest molecules. To construct glassy ionic liquid crystals, it is important to understand why some ionic liquid crystals adopt glassy states while others undergo crystallization upon cooling.68, 69, 70 Our understanding is that when alkyl chains begin ordering before freezing of the ionic domains, they crystallize. In contrast, when ionic domains freeze before the ordering of the alkyl chains starts, the alkyl chains do not form well-aligned states, resulting in glassification. Taking this idea into account, ion design that increases the glass transition temperature of ionic domains is expected to be important for the development of glassy ionic liquid crystals. In the course of studies of the physicochemical properties of ionic liquids, the relationships between the ion structures and the glass transition temperature of ionic liquids have gradually been revealed. It has been reported that the introduction of aromatic rings into ion structures leads to an increase in the glass transition temperature of ionic liquids.71, 72 This insight led us to design the imide-type acids, HA-Ts, HA-TfBz, and HA-Oct, as additives for zwitterionic liquid crystals (Figure 7a).73 Compound 312 was selected as the amphiphilic zwitterion, and homogeneous mixtures of 314 and the designed acids were prepared. As expected, the mixtures were LC materials showing glass transition behavior. For example, 312/HA-TfBz exhibits a Cubbi phase that forms a glassy state near 0 °C while maintaining the Cubbi structure. Interestingly, these vitrified Cubbi LC materials absorb water while preserving their molecular assembled structures (Figure 7b). The absorption of water can be confirmed by IR spectroscopy and ion conduction measurement (Figures 7c and d). In contrast, the absorption of water in the LC state induces a change in mesophase pattern from the Cubbi to the Col phase. These results clearly suggest that glassification prevents a change in molecular arrangement upon water absorption. We are now exploring a way to use glassified Cubbi liquid crystals as scaffolds for various functional guest molecules.
(a) Design of imide-type acids HA-TS, HA-TfBz and HA-Oct with aromatic or aliphatic groups.73 (b) Absorption of water into glassy Cubbi LC materials. (c) Change in differential transmittance of IR spectra of 312/HA-TfBz mixture before and after addition of D2O at −15 °C. (d) Change in ionic conductivities of 312/HA-TfBz mixture on addition of H2O in the glassy Cubbi state at −15 °C.73 Reproduced with permission from the reference.
Design of block copolymers with zwtterionic segments
Block copolymers are also organized structural materials that can potentially be formed through the segregation of incompatible segments.4 There is some commonality in the structural geometry between nanostructures formed by liquid crystals and those formed by block copolymers, although there is a definite difference in the lattice length between these two materials. A variety of zwitterionic block copolymers have recently been designed. Yoshida and co-worker reported the thermoresponsivity and pH sensitivity of zwitterionic block copolymers.74 Erel-Goktepe and colleagues reported micelle formation of poly[3-dimethyl(methacryloyloxyethyl)ammonium propane sulfonate]-block-poly[2-(diisopropylamino)ethyl methacrylate] and its application to pH-responsive layer-by-layer films.75 In these studies, the ability of zwitterions to recognize acids has been successfully introduced into block copolymer systems. Employment of bio-compatible zwitterions, such as phosphorylcholine derivatives, enable the use of zwitterionic block copolymers for biotechnologies and biofriendly materials.76
To construct solid electrolytes having mechanical stability and high ionic conductivity, we designed block copolymers, PS- b -PZI, composed of polystyrene and polyzwitterionic segments (Figure 8).77 These molecules form micellar assemblies comprising polystyrene cores and polyzwitterion shells in polar solvents such as water. Slow evaporation of the solvent at 70 °C followed by vacuum drying results in the formation of a thin-film sample. The morphology of this film has been investigated using transmission electron microscopy, which reveals that these PS- b -PZI form aggregated micelles in the films. When forming the film in the presence of specific acids or lithium salts, nanosegregated electrolytes can be successfully prepared. For example, a mixture of PS- b -PZI and phosphoric acid formed a solid electrolyte with an ionic conductivity of 10–5 S cm–1 at 40 °C. We believe that further control of the morphology of these block copolymers can be achieved by the suitable selection of the two incompatible segments and control of the component ratio between these segments.
Molecular structure of block copolymer PS- b -PZI,77 which forms core-shell micelles in water. Casting of the colloidal solution on a substrate and elimination of water results in the formation of a nanostructured film sample. A full colour version of this figure is available at the Polymer Journal journal online.
Interest in zwitterions has not been limited to their availability as bulk materials. For example, there is increasing interest in the use of zwitterions as surface-coating materials78 and interface-stabilizers,79 among other uses. In the course of these studies, a variety of novel functions and properties unique to zwitterions have gradually been found. I believe the accumulation of insight into these zwitterions will pave the way to develop next-generation technologies.
Conclusions
In this review, the potential utility of zwitterions as building blocks for functional soft materials such as liquid crystals and block copolymers has been described. These zwitterion-derived materials show diverse organizational behaviors, including self-organization, co-organization with acids and/or lithium salts and solvent-induced organization. In particular, co-organization with some acids and/or lithium salts is unique, as it varies significantly with the selected additives. This is attributed to the tendency of these zwitterions to form ionic liquid-like ion pairs with the acids and lithium salts. The selection of additives is important for obtaining the desired functions for the resultant materials. In particular, the application of these materials as ion transport materials has been the main focus. However, as some recent papers have reported the availability of zwitterions for the design of bio-compatible matrices,80 it is expected that the ionic materials described in this review will be used in a wide variety of fields ranging from materials chemistry to biotechnology.
References
Ariga, K., Hill, J. P., Lee, M. V., Vinu, A., Charvet, R. & Acharya, S. Challenges and breakthroughs in recent research on self-assembly. Sci. Technol. Adv. Mater. 9, 014109 (2008).
Kato, T., Mizoshita, N. & Kishimoto, K. Functional liquid-crystalline assemblies: self-organized soft materials. Angew. Chem. Int. Ed. 45, 38–68 (2006).
Tschierske, C. Development of structural complexity by liquid-crystal self-assembly. Angew. Chem. Int. Ed. 52, 8828–8878 (2013).
Bates, F. S. & Fredrickson, G. H. Block copolymer thermodynamics: theory and experiment. Annu. Rev. Phys. Chem. 41, 525–557 (1990).
Kitagawa, S., Kitaura, R. & Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 43, 2334–2375 (2004).
Lehn, J.-M. Supramolecular chemistry—scope and perspectives molecules, supermolecules, and molecular devices. Angew. Chem. Int. Ed. 27, 89–112 (1988).
Chevalier, Y., Germanaud, L. & Le Perchec, P. Micellar properties of zwitterionic phosphobetaine amphiphiles in aqueous solution: influence of the intercharge distance. Colloid Polym. Sci. 266, 441–448 (1988).
Kudaibergenov, S., Jaeger, W. & Laschewsky, A. Polymeric betaines: synthesis, characterization, and application. Adv. Polym. Sci. 201, 157–224 (2006).
Kobayashi, M., Terayama, Y., Kikuchi, M. & Takahara, A. Chain dimensions and surface characterization of superhydrophilic polymer brushes with zwitterion side groups. Soft Matter 9, 5138–5148 (2013).
Wei, H., Insin, N., Lee, J., Han, H.-S., Cordero, J. M., Liu, W. & Bawendi, M. G. Compact zwitterion-coated iron oxide nanoparticles for biological applications. Nano Lett. 12, 22–25 (2012).
Mathis, A., Galin, M., Galin, J. C., Heinrich, B. & Bazuin, C. G. Long alkyl chain dimethylammonioalkoxydicyanoethenolates as new zwitterionic thermotropic liquid crystals. Liq. Cryst. 26, 973–984 (1999).
Yusan, P., Tuncel, I., Bütün, V., Demirelc, A. L. & Erel-Goktepe, I. pH-responsive layer-by-layer films of zwitterionic block copolymer micelles. Polym. Chem. 5, 3777–3787 (2014).
Goodby, J. W., Collings, P. J., Kato, T., Tschierske, C., Gleeson, H. & Raynes, P . (eds). Handbook of Liquid Crystals, 2nd edn (Wiley-VCH, Weinheim, 2014).
Goodby, J. W., Saez, I. M., Cowling, S. J., Görtz, V., Draper, M., Hall, A. W., Sia, S., Cosquer, G., Lee, S.-E. & Raynes, E. P. Transmission and amplification of information and properties in nanostructured liquid crystals. Angew. Chem. Int. Ed. 47, 2754–2787 (2008).
Laschat, S., Baro, A., Steinke, N., Giesselmann, F., Hägele, C., Scalia, G., Judele, R., Kapatsina, E., Sauer, S., Schreivogel, A. & Tosoni, M. Discotic liquid crystals: from tailor-made synthesis to plastic electronics. Angew. Chem. Int. Ed. 46, 4832–4887 (2007).
Kato, T., Yasuda, T., Kamikawa, Y. & Yoshio, M. Self-assembly of functional columnar liquid crystals. Chem. Commun. 729–739 (2009).
Rosen, B. M., Wilson, C. J., Wilson, D. A., Peterca, M., Imam, M. R. & Percec, V. Dendron-mediated self-assembly, disassembly, and self-organization of complex systems. Chem. Rev. 109, 6275–6540 (2009).
Binnemans, K. Ionic liquid crystals. Chem. Rev. 105, 4148–4204 (2005).
Goossens, K., Lava, K., Bielawski, C. W. & Binnemans, K. Ionic liquid crystals: versatile materials. Chem. Rev. 116, 4643–4807 (2016).
Axenov, K. V. & Laschat, S. Thermotropic ionic liquid crystals. Materials 4, 206–259 (2011).
Yoshio, M. & Kato, T in Handbook of Liquid Crystals Vol. 8 (eds Goodby, J., Collings, P. J, Kato, T., Tschierske, C., Gleeson, H. & Raynes, P.) Ch. 23 (Wiley-VCH Verlag GmbH & Co. KGaA, 2014).
Dieter, K. M., Dymek, C. J., Heimer, N. E., Rovang, J. W. & Wilkes, J. S. Ionic structure and interactions in 1-methyl-3-ethylimidazolium chloride-aluminum chloride molten salts. J. Am. Chem. Soc. 110, 2722–2726 (1988).
Earle, M. J., Esperanca, J. M. S. S., Gilea, M. A., Lopes, J. N. C., Rebelo, L. P. N., Magee, J. W., Seddon, K. R. & Widegren, J. A. The distillation and volatility of ionic liquids. Nature 439, 831–834 (2006).
Kim, H.-T., Kang, J., Mun, J., Oh, S. M., Yim, T. & Kim, Y. G. Pyrrolinium-based ionic liquid as a flame retardant for binary electrolytes of lithium ion batteries. ACS Sustainable Chem. Eng. 4, 497–505 (2016).
Bonhôte, P., Dias, A.-P., Papageorgiou, N., Kalyanasundaram, K. & Grätzel, M. Hydrophobic, highly conductive ambient-temperature molten salts. Inorg. Chem. 35, 1168–1178 (1996).
Ohno, H. Functional design of ionic liquids. Bull. Chem. Soc. Jpn 79, 1665–1680 (2006).
Yoshizawa, M., Narita, A. & Ohno, H. Design of ionic liquids for electrochemical applications. Aust. J. Chem. 57, 139–144 (2004).
Yoshizawa, M. & Ohno, H. Anhydrous proton transport system based on zwitterionic liquid and HTFSI. Chem. Commun. 1828–1829 (2004).
Tiyapiboonchaiya, C., Pringle, J. M., Sun, J., Byrne, N., Howlett, P. C., Macfarlane, D. R. & Forsyth, M. The zwitterion effect in high-conductivity polyelectrolyte materials. Nat. Mater. 3, 29–32 (2004).
Lin, J. C. Y., Huang, C.-J., Lee, Y.-T., Lee, K.-M. & Lin, I. J. B. Carboxylic acid functionalized imidazolium salts: sequential formation of ionic, zwitterionic, acid-zwitterionic and lithium salt-zwitterionic liquid crystals. J. Mater. Chem. 21, 8110–8121 (2011).
Ichikawa, T., Kato, T. & Ohno, H. 3D continuous water nanosheet as a gyroid minimal surface formed by bicontinuous cubic liquid-crystalline zwitterions. J. Am. Chem. Soc. 134, 11354–11357 (2012).
Ichikawa, T., Okafuji, A., Kato, T. & Ohno, H. Induction of an infinite periodic minimal surface by endowing an amphiphilic zwitterion with halogen-bond ability. ChemistryOpen 5, 439–444 (2016).
Matsumoto, T., Ono, A., Ichikawa, T., Kato, T. & Ohno, H. Construction of gyroid-structured matrices through the design of geminized amphiphilic zwitterions and their self-organization. Chem. Commun. 52, 12167–12170 (2016).
Ueda, S., Kagimoto, J., Ichikawa, T., Kato, T. & Ohno, H. Anisotropic proton-conductive materials formed by the self-organization of phosphonium-type zwitterions. Adv. Mater. 23, 3071–3074 (2011).
Soberats, B., Yoshio, M., Ichikawa, T., Taguchi, S., Ohno, H. & Kato, T. 3D anhydrous proton-transporting nanochannels formed by self-assembly of liquid crystals composed of a sulfobetaine and a sulfonic acid. J. Am. Chem. Soc. 135, 15286–15289 (2013).
Soberats, B., Yoshio, M., Ichikawa, T., Ohno, H. & Kato, T. Zwitterionic liquid crystals as 1D and 3D lithium ion transport media. J. Mater. Chem. A 3, 11232–11238 (2015).
Impéror-Clerc, M. Thermotropic cubic mesophases. Curr. Opin. Colloid Interface Sci. 9, 370–376 (2005).
Kutsumizu, S. The thermotropic cubic phase: a curious mesophase. Curr. Opin. Solid State Mater. Sci. 6, 537–543 (2002).
Diele, S. On thermotropic cubic mesophases. Curr. Opin. Colloid Interface Sci. 7, 333–342 (2002).
Zeng, X., Ungar, G. & Impéror-Clerc, M. A triple-network tricontinuous cubic liquid crystal. Nat. Mater. 4, 562–567 (2005).
Wu, L., Zhang, W. & Zhang, D. Engineering gyroid-structured functional materials via templates discovered in nature and in the lab. Small 11, 5004–5022 (2015).
Cho, B. K., Jain, A., Gruner, S. M. & Wiesner, U. Mesophase structure-mechanical and ionic transport correlations in extended amphiphilic dendrons. Science 305, 1598–1601 (2004).
Lu, X., Nguyen, V., Zhou, M., Zeng, X., Jin, J., Elliott, B. J. & Gin, D. L. Crosslinked bicontinuous cubic lyotropic liquid-crystal/butyl-rubber composites: highly selective, breathable barrier materials for chemical agent protection. Adv. Mater. 18, 3294–3298 (2006).
Ichikawa, T., Yoshio, M., Hamasaki, A., Mukai, T., Ohno, H. & Kato, T. Self-organization of room temperature ionic liquids exhibiting liquid crystalline bicontinuous cubic phases: formation of nano-ion channel networks. J. Am. Chem. Soc. 129, 10662–10663 (2007).
Ichikawa, T., Yoshio, M., Hamasaki, A., Kagimoto, J., Ohno, H. & Kato, T. 3D interconnected ionic nano-channels formed in polymer films: self-organization and polymerization of thermotropic bicontinuous cubic liquid crystals. J. Am. Chem. Soc. 133, 2163–2169 (2011).
Ichikawa, T., Yoshio, M., Taguchi, S., Kagimoto, J., Ohno, H. & Kato, T. Co-organisation of ionic liquids with amphiphilic diethanolamines: construction of 3D continuous ionic nanochannels through the induction of liquid-crystalline bicontinuous cubic phases. Chem. Sci. 3, 2001–2008 (2012).
Matsumoto, T., Ichikawa, T., Sakuda, J., Kato, T. & Ohno, H. Design of amphiphilic zwitterions forming liquid-crystalline phases and effects of lithium salt addition on their phase behavior. Bull. Chem. Soc. Jpn 87, 792–796 (2014).
Nguyen, H. L., Horton, P. N., Hursthouse, M. B., Legon, A. C. & Bruce, D. W. Halogen bonding: a new interaction for liquid crystal formation. J. Am. Chem. Soc. 126, 16–17 (2004).
Metrangolo, P., Prasang, C., Resnati, G., Liantonio, R., Whitwood, A. C. & Bruce, D. W. Fluorinated liquid crystals formed by halogen bonding. Chem. Commun. 3290–3292 (2006).
Mukai, T. & Nishikawa, K. Halogen-bonded and hydrogen-bonded network structures in crystals of 1-propyl- and 1-butyl-4,5-dibromo-3-methylimidazolium bromides. Chem. Lett. 38, 402–403 (2009).
Kayama, Y., Ichikawa, T. & Ohno, H. Transparent and colourless room temperature ionic liquids having high refractive index over 1.60. Chem. Commun. 50, 14790–14792 (2014).
Menger, F. M. & Keiper, J. S. Gemini surfactants. Angew. Chem. Int. Ed. 39, 1906–1920 (2000).
Zhou, M., Nemade, P. R., Lu, X., Zeng, X., Hatakeyama, E. S., Noble, R. D. & Gin, D. L. New type of membrane material for water desalination based on a cross-linked bicontinuous cubic lyotropic liquid crystal assembly. J. Am. Chem. Soc. 129, 9574–9575 (2007).
Bara, J. E., Hatakeyama, E. S., Wiesenauer, B. R., Zeng, X., Noble, R. D. & Gin, D. L. Thermotropic liquid crystal behaviour of gemini imidazolium-based ionic amphiphiles. Liq. Cryst. 37, 1587–1599 (2010).
Perroni, D. V. & Mahanthappa, M. K. Inverse Pm3m cubic micellar lyotropic phases from zwitterionic triazolium gemini surfactants. Soft Matter 9, 7919–7922 (2013).
Yoshio, M., Mukai, T., Ohno, H. & Kato, T. One-dimensional ion transport in self-organized columnar ionic liquids. J. Am. Chem. Soc. 126, 994–995 (2004).
Yoshio, M., Kagata, T., Hoshino, K., Mukai, T., Ohno, H. & Kato, T. One-dimensional ion-conductive polymer films: alignment and fixation of ionic channels formed by self-organization of polymerizable columnar liquid crystals. J. Am. Chem. Soc. 128, 5570–5577 (2006).
Taguchi, S., Ichikawa, T., Kato, T. & Ohno, H. Nano-biphasic ionic liquid systems composed of hydrophobic phosphonium salts and a hydrophilic ammonium salt. Chem. Commun. 48, 5271–5273 (2012).
Taguchi, S., Ichikawa, T., Kato, T. & Ohno, H. Design and evaluation of nano-biphasic ionic liquid systems having highly polar and low polar domains. RSC Adv. 3, 23222–23227 (2013).
Taguchi, S., Matsumoto, T., Ichikawa, T., Kato, T. & Ohno, H. Gelation of an amino acid ionic liquid by the addition of a phosphonium-type zwitterion. Chem. Commun. 47, 11342–11344 (2011).
Byrne, N., Howlett, P. C., Macfarlane, D. R., Smith, M. E., Howes, A. P., Hollenkamp, A. F., Bastow, T. J., Hale, P. & Forsyth, M. Effect of zwitterion on the lithium solid electrolyte interphase in ionic liquid electrolytes. J. Power Sources 184, 288–296 (2008).
Ito, Y., Kohno, Y., Nakamura, N. & Ohno, H. Addition of suitably-designed zwitterions improves the saturated water content of hydrophobic ionic liquids. Chem. Commun. 48, 11220–11222 (2012).
Kessler, J. O. & Raynes, E. P. Glassy liquid crystals observation of a quenched twisted nematic. Phys. Lett. A 50, 335–336 (1974).
Tokita, M., Osada, K. & Watanabe, J. Thermotropic liquid crystals of main-chain polyesters having a mesogenic 4,4′-biphenyldicarboxylate unit XI. Smectic liquid crystalline glass. Polym. J. 30, 589–595 (1998).
Chen, S. H., Chen, H. M. P., Geng, Y., Jacobs, S. D., Marshall, K. L. & Blanton, T. N. Novel glassy nematic liquid crystals for non-destructive rewritable optical memory and photonic switching. Adv. Mater. 15, 1061–1065 (2003).
Adam, D., Schuhmacher, P., Simmerer, J., Häuβling, L., Paulus, W., Siemensmeyer, K., Etzbach, K. H., Ringsdorf, H. & Haarer, D. Photoconductivity in the columnar phases of a glassy discotic twin. Adv. Mater. 7, 276–280 (1995).
Wu, J., Usui, T. & Hanna, J. Synthesis of a novel smectic liquid crystalline glass and characterization of its charge carrier transport properties. J. Mater. Chem. 21, 8045–8051 (2011).
Bruce, D. W., Donnio, B., Guillon, D., Heinrich, B. & Ibn-elhaj, M. The synthesis and mesomorphism of a new series of silver(I) complexes showing glassy mesophases. Liq. Cryst. 19, 537–539 (1995).
Ujiie, S., Furukawa, H., Yano, Y. & Mori, A. Oriented glass states formed by ionic liquid–liquid crystals. Thin Solid Films 509, 185–188 (2006).
Kouwer, P. H. J. & Swager, T. M. Synthesis and mesomorphic properties of rigid-core ionic liquid crystals. J. Am. Chem. Soc. 129, 14042–14052 (2007).
Tao, R., Tamas, G., Xue, L. J., Simon, S. L. & Quitevis, E. L. Thermophysical properties of imidazolium-based ionic liquids: the effect of aliphatic versus aromatic functionality. J. Chem. Eng. Data 59, 2717–2724 (2014).
Fujiwara, S., Ichikawa, T. & Ohno, H. Cation-π interactions within aromatic amino acid ionic liquids: a new tool for designing functional ionic liquids. J. Mol. Liq. 222, 214–217 (2016).
Kobayashi, T., Ichikawa, T., Kato, T. & Ohno, H. Development of glassy bicontinuous cubic liquid crystals for solid proton conductive materials. Adv. Mater. 29, 1604429 (2017).
Jiménez, Z. A. & Yoshida, R. Temperature driven self-assembly of a zwitterionic block copolymer that exhibits triple thermoresponsivity and pH sensitivity. Macromolecules 48, 4599–4606 (2015).
Yusan, P., Tuncel, I., Bütün, V., Demirel, A. L. & Erel-Goktepe, I. pH-responsive layer-by-layer films of zwitterionic block copolymer micelles. Polym. Chem. 5, 3777–3787 (2014).
Du, J. & Armes, S. P. Preparation of biocompatible zwitterionic block copolymer vesicles by direct dissolution in water and subsequent silicification within their membranes. Langmuir 25, 9564–9570 (2009).
Matsumoto, T., Ichikawa, T. & Ohno, H. Design of ionic liquid-based polyelectrolytes by combining ‘nanostructurisation’ and ‘zwitterionisation’. Polym. Chem. 7, 1230–1233 (2016).
Schlenoff, J. B. Zwitteration: coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir 30, 9625–9636 (2014).
Guidetti, G., Atifi, S., Vignolini, S. & Hamad, W. Y. Flexible photonic cellulose nanocrystal films. Adv. Mater. 28, 10042–10047 (2016).
Cheng, G., Xue, H., Zhang, Z., Chen, S. & Jiang, S. A switchable biocompatible polymer surface with self-sterilizing and nonfouling capabilities. Angew. Chem. Int. Ed. 47, 8831–8834 (2008).
Acknowledgements
I thank all my co-workers for their contributions, with special thanks to Professor Hiroyuki Ohno (Tokyo University of Agriculture and Technology, Tokyo, Japan) and Professor Takashi Kato (The University of Tokyo, Tokyo, Japan) for their encouragement and strong support. This work was financially supported by the Japan Society for the Promotion of Science, Grant-in-Aid for Young Scientists (B) (No. 23750147), Grant-in-Aid for Young Scientists (A) (No. 25708014) and the Precursory Research for Embryonic Science and Technology (PRESTO) from the Japan Science and Technology Corporation (JST).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Ichikawa, T. Zwitterions as building blocks for functional liquid crystals and block copolymers. Polym J 49, 413–421 (2017). https://doi.org/10.1038/pj.2017.6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2017.6
This article is cited by
-
Zwitterionic materials with disorder and plasticity and their application as non-volatile solid or liquid electrolytes
Nature Materials (2022)
-
Gyroid nanostructured soft membranes formed by controlling the degree of crosslinking polymerization of bicontinuous cubic liquid-crystalline monomers
Polymer Journal (2021)
-
Functional liquid-crystalline polymers and supramolecular liquid crystals
Polymer Journal (2018)
-
Liquid-crystalline behavior and ion transport properties of block-structured molecules containing a perfluorinated ethylene oxide moiety complexed with a lithium salt
Polymer Journal (2018)