Abstract
The mammalian kidney is a highly complex organ, composed of various cell types within a unique structural framework. Nonetheless, in recent years, giant leaps in our understanding of nephrogenesis and the origin of new cells in the adult kidney have resulted in novel routes to regenerate damaged nephrons. While several strategies can be envisioned to achieve this aim, one common theme is the reliance on renal lineage cells, as extrarenal cells, such as bone marrow–derived cells, have been shown to be devoid of renal differentiation capacity. Herein, we will present the main motivation for the pursuit for cell-based therapies, which is the ever growing problem of chronic kidney disease (CKD), and discuss different strategies toward replenishing the damaged renal parenchyma. These include transplantation of fetal kidney grafts or fetal kidney stem cells, directed differentiation of pluripotent stem cells into kidney epithelia, establishment of renal progenitors from the adult kidney, and genetic reprogramming of mature kidney cells into a progenitor state. Taken together with novel techniques recapitulating the three-dimensional developmental environment, these advances are expected to take the field into a new era, bringing us closer than ever to the day when kidney stem cell–based therapy becomes a viable therapeutic option.
Similar content being viewed by others
Chronic kidney disease: a growing problem
Chronic kidney disease (CKD), defined as abnormalities of kidney structure or function, present for more than 3 months, with implications for health (1), has become one of the major global health problems in recent decades, owing to constant aging of the population, as well as the increase in the frequency of its main etiologies, such as diabetes and hypertension. With an estimated global incidence of about 11–13% (2) and expensive and complicated treatments, CKD is not only associated with lower life expectancy and impaired quality of life but it also represents a significant burden on modern health systems (3). In addition, given the growing aging population, CKD rates are expected to continue to rise, particularly in the United States and other developed countries (4). CKD is a relentless disease, steadily deteriorating from its early stages into kidney failure, also called end-stage renal disease, defined as a glomerular filtration rate below 15 ml/min per 1.73 m2 (1). Unfortunately, the therapeutic options that can be offered to such patients have not evolved significantly for many years, currently including renal transplantation, which suffers from severe shortage of donor organs, and dialysis that significantly damages patients’ quality of life (5). Accordingly, with major developments in the past 20 years in the field of regenerative medicine, various cell-based approaches have begun to emerge, opening the path toward novel treatments for CKD. Notably, as CKD entails not only loss of renal parenchyma per se but also other pathogenetic mechanisms, such as renal fibrosis and capillary rarefication (6), it has been proposed that stem cell–based therapy is best initiated early in the course of CKD, when structural changes are still relatively mild (5). At the same time, strategies that can facilitate the regeneration of renal vasculature, in addition to renal nephrons, would be probably needed. Moreover, various diseases result in damage predominantly to specific cell types within the nephron, which could potentially be ameliorated via the generation of a single cellular phenotype, which could serve to form “cell patches” to replace damaged tubular areas. In this review, we will discuss the various strategies that have been developed to derive renal progenitors, which could potentially allow regeneration of damaged kidneys.
Fetal kidney–derived stem cells
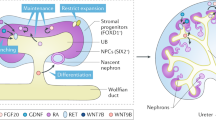
The most intuitive approach for obtaining bona fide renal stem cells is probably to look in the only place where new nephrons are formed during the lifetime of mammalians, the fetal kidney. Initially, to capture the activity of these cells, as well as to allow them to function within their physiological niche, whole kidney grafts were used (7, 8). Transplantation of early kidney precursors (7–14 weeks of gestation) into immunodeficient mice resulted in graft growth and differentiation into miniature kidneys, which demonstrated functional capacity, producing dilute urine (8). Despite the impressive results, it was evident that the whole grafts are not a feasible solution for patients, as the transplanted “miniorgans” fail to integrate fully into the host and harbor unwanted cell types, emphasizing the need to isolate and enrich only the desired population of fetal stem cells. A second strategy relying on fetal kidneys entailed the usage of fetal kidney–derived cells. While heterogeneous dissociated rat fetal kidney cells were shown to generate renal structures when injected under the kidney capsule, nontubular cell types were also formed in some instances (9, 10, 11), again underscoring the need to pinpoint specific sub-populations within the fetal kidney, which could function to regenerate kidneys. Such a population was recently identified in our lab, by using a unique strategy to identify the relevant marker (12, 13, 14, 15). This strategy entailed a search for markers that are: (i) overexpressed in progenitor-rich renal tissues (i.e. fetal kidneys and the embryonic renal Wilms’ tumor, known to arise from renal epithelial progenitors undergoing differentiation arrest); (ii) localize to the undifferentiated structures in fetal kidneys and (iii) select for cells with progenitor traits (e.g. clonogenic capacity and expression of renal stem cells markers). This resulted in the identification of neural cell adhesion molecule 1 (NCAM1) (CD56), which fulfilled, at least in part, all criteria. Consequently, Harari-Steinberg et al. (13) demonstrated that NCAM1+ human fetal kidney cells cultured in serum-free medium, harbor a mesenchymal phenotype, overexpress nephron progenitor genes (e.g. OSR1, SIX2, and CITED1) compared with the NCAM1− fraction, and possess multipotency toward different nephron segments. Importantly, NCAM1+ cells were shown to increase creatinine clearance when administered to mice suffering from CKD induced by 5/6 nephrectomy chronic kidney injury model (13). Dekel and co-workers (16) went on to prospectively isolate human nephron progenitors. Since the previously identified markers (e.g. NCAM1 and FZD7) extend beyond the Cap Mesenchyme (CM) stage (in culture of mouse embryonic kidney rudiments, Ncam1 colocalizes with Six2 in the CM, and also extends into portions of differentiating nephron epithelia), a simple biomarker system was developed using positive and negative selection for nephron progenitor isolation. Accordingly, sorted NCAM1+CD133− cells cultured in serum-free media were shown to enrich for SIX2+ nephron progenitors. Nevertheless, in serum-free media, SIX2+ human nephron progenitors quickly differentiate and are lost within a week. Very recently, following implementation of a modified nephron progenitor media (17) in cultures of human fetal kidney and by prospective isolation of NCAM1+CD133− progenitor cells according to EpCAM expression (NCAM1+CD133−EpCAM−, NCAM1+CD133−EpCAMdim, NCAM1+CD133−EpCAMbright), preservation of uninduced and induced CM, as well as a transitioning mesenchymal–epithelial state in expanded cultures was shown at the single-cell resolution (18) (Figures 1). In summary, human fetal kidney–derived nephron progenitors can be prospectively isolated and expanded, and hence can potentially serve as off-the-shelf cells suitable for kidney regeneration. This strategy may prove useful in patients already on immunosuppressants, especially when considering the relatively low immunogenicity of fetal-derived cells. Alternatively, one can envision the formation of a tissue bank for fetal-derived renal progenitors, which could be implanted into patients requiring renal regeneration, much like donor kidneys are currently transplanted in CKD patients and successfully function as long as immunosuppressive agents are used.
Human fetal kidney populations can be defined and isolated using specific surface markers. (a) Schematic representation of the cellular phenotypes along the renal mesenchymal to epithelial transition (MET) axis in the human fetal kidney. Along this axis, neural cell adhesion molecule 1 (NCAM1) expression gradually subsides, while that of CD133 increases. Accordingly, several distinct fractions can be identified, including the CM progenitors, demarcated by an NCAM1+CD133− phenotype. (b) Successful preservation of this phenotype in vitro has been shown to be possible via culture in a modified nephron progenitor expansion medium (mNPEM). Using these unique culture conditions, heterogeneous cell morphology is seen during in vitro culturing on MG (Matrigel matrix)-coated plates showing cobblestone, spindle-shaped, and small ovoid-like cells. In addition, cells are organized in unique niches. Each niche included a center of small ovoid-like SIX2-expressing cells surrounded by larger cobblestone cells and crowned by an outer circle of spindle-shaped cells.
Pluripotent stem cell–derived stem cells and organoid technology
An alternative route to arrive at fetal stage renal stem cells is to mimic normal embryonic differentiation ex vivo, starting from a cell in a pluripotent state (i.e. capable of giving rise to all three germ layers) and gradually differentiating it into a renal progenitor state. Embryonic stem cells (ESCs), representing the in vitro equivalent of the inner cells mass, harbor the potential of giving rise to any of the cells in the three germ layers (19). Groundbreaking work by the Yamanaka group (20, 21) expanded the potential spectrum of using pluripotent stem cells (PSCs) by the generation of such cells, termed induced PSCs from fibroblasts. Beside the ability to derive autologous pluripotent cells, this finding revolutionized the field of regenerative medicine by introducing the notion that given the correct combination of transcription factors, each cell type can, in theory, be reprogrammed into a desired cell type (22). Early works were able to demonstrate the formation of tubule- and glomeruli-like structures with ESC–derived teratomas (23, 24), providing a glimpse at the vast potential of these cells. At the same time, to both achieve the desired cell type and to avoid potential tumorigenesis, an accurate differentiation process was in need, to direct PSCs (a collective term referring to both ESCs and induced pluripotent stem cells) into a renal end product. Concomitantly advances in the stem cell field resulted in the ability to generate complex in vitro three-dimensional (3D) structures resembling whole kidneys, which are termed “organoids”. These approaches apply culture systems that allow stem cell-derived cells to self-organize into 3D structures. This method has its roots, methodologically and conceptually, in classical developmental experiments, which demonstrated that dissociated embryonic kidney cells are capable of reaggregating and self-organizing into avascular fetal-like renal tissue (25). By applying this method to pluripotent stem cells, several groups were able to derive highly complex renal structures. Despite many attempts (26, 27, 28, 29, 30, 31), it was not until recently that such protocols were devised, as a result of major leaps in the understating of mammalian nephrogenesis, from the early gastrulation phase and up to the segmentation of the nephron. Initially, successful generation of intermediate mesoderm (IM), the precursor of the metanephric kidney, was reported. For instance, Mae et al. (32) established a two-step protocol for IM induction from PSCs, which entailed first the use of Activin A and the Wnt agonist CHIR99021, to promote mesoendoderm specification followed by bone morphogenetic protein 7 (BMP7) to robustly induce IM formation. Consistent with their IM identity, the cells were capable of integrating into mouse metanephric tissues to form renal structures (32). Nonetheless, the low rates of establishing such structures, coupled with the formation of other, non-desired phenotypes, such as cells expressing adrenal-related genes, underscored the need to generate bona fide renal progenitors. Accordingly, in an attempt to mimic normal nephrogenesis more fully, Taguchi et al. (33) applied a complex four-step differentiation protocol on human PSCs, meant to recapitulate kidney organogenesis and arrive at the end product of Metanephric Mesenchyme (MM) stage renal progenitors. First, for nascent mesoderm induction, Activin, BMP4, and high-dose Wnt agonists are used. Then, posteriorization into T+ posterior mesoderm is undertaken via the application of BMP4 and high-dose Wnt agonists, followed by specification into OSR1+ cells using a combination of BMP4, retinoic acid, Activin, and medium levels of Wnt agonists. Finally, to allow maturation into MM progenitors, fibroblast growth factor-9 and low-dose Wnt agonists are applied. This process resulted in the formation of renal epithelial progenitors, which upon exposure to spinal cord or Wnt-expressing feeder cells remarkably differentiate into 3D structures containing glomeruli and renal tubules of different segments. The renal progenitors generated via this protocol were later shown to generate highly developed glomeruli in vivo, successfully undergoing integration into the host vasculature, and harboring podocytes with numerous cell processes around fenestrated endothelial cells (34). Other groups similarly reported the use of complex differentiation protocols meant to guide PSCs into a nephron progenitor state, demonstrating that the end product is capable of giving rise to nephron-like structures, expressing markers of different tubular segments (35, 36). Interestingly, by applying the protocol to PSCs carrying a mutation in disease-causing genes (e.g. Podocalyxin or PKD1/2) generated via CRISPR/Cas9 technology, differentiation defects resembling the human phenotype to some extent could be detected within differentiating organoids (36). Concomitant with this work, Izpisua Belmonte and co-workers (37) reported a 4-day two-step protocol for derivation of ureteric bud (UB) progenitor-like cells from human PSCs, consisting of BMP4 and fibroblast growth factor-2 exposure, followed by retinoic acid, Activin A, and BMP2 (37). The UB identity was demonstrated using a 3D reaggregation assay, whereby the tested cells are mixed with dissociated mouse embryonic kidney cells. The cells were seen to contribute to the formation of the ureteric tree, but not to MM–derived structures, indicating their UB lineage (37). Applying a third protocol, Takasato et al. (38) sequentially directed human ESCs into posterior primitive streak, IM and finally both MM and ureteric epithelium. Using this protocol, while growing the cells in unique 3D structures termed “organoids” resulted in the formation of complex structures, containing both ureteric epithelium (GATA3+PAX2+RET+DBA+CDH1+) and MM (SIX2+WT1+PAX2+HOXD11+) cells (38). By better defining the signals regulating the induction of posterior vs. anterior IM the same group further refined the differentiation protocol so as to allow formation of kidney organoids containing collecting ducts and a large number of segmented nephrons (39). In addition, surrounding the latter were renal stromal population and a vascular network, which could even be identified with the nascent glomeruli on rare occasions (39). Notably, despite the remarkable progress in the ability to derive the lineages comprising the kidney parenchyma (i.e. nephrons), successful regeneration of an organ would most certainly require also the formation of supporting stroma, as well as fully developed vasculature. To tackle this issue, Takebe et al. (40) used a different strategy, combining organ-specific progenitors derived from human PSCs with endothelial cells and mesenchymal stem cells, establishing a protocol for generating in vitro 3D, transplantable organ buds of various tissues, including the kidney. Remarkably, upon transplantation into mice, the kidney bud underwent vascularization and generated glomerular- and tubular-like structures, which were shown to express markers of podocytes and proximal tubules (40). Importantly, while the past 4 years have seen a tremendous progress in our understanding of renal development and consequently in the ability to arrive at differentiation-competent renal progenitors, the presented papers clearly demonstrate a large variability in protocols and, not surprisingly, in the renal end products. In addition, the newly gained knowledge has not yet been translated into improved functional outcomes (41, 42). Nonetheless, the established models are already showing much promise as novel tools for disease modeling (36) and toxicity screening (43, 44). At the same time, despite the major progress obtained in recent years, organoid technologies still have a long way to go to allow generation of tissues that recapitulate the human kidney anatomically and physiologically. Some important problems are major anatomical deficiencies, such as a lack of collecting ducts or the random formation of multiple individual collecting ducts instead of a single unified collecting system. Consequently, the tree-like organization and cortical-medullary architecture of the kidney are missing from organoids made through self-organization processes, constituting a significant obstacle to producing functional organs. This problem is highlighted by lessons from embryonic kidney rudiment transplantation, which teaches us that in vivo maturation after grafting is not enough to generate a functional kidney, but rather only dilute urine is produced (8). At the same time, the avascular in vitro niche still impedes organoids from maturing into a state resembling that of adult organs (45). In addition, an important risk that needs to be fully addressed before the transition into clinical use is that of neoplastic transformation. For instance, recent data show that PSCs accumulate dominant-negative P53 mutations as well global loss of imprinting (46, 47). Finally, direct evaluation of the relevance of induced pluripotent stem cell–derived nephron progenitors as potential cell therapy is necessary, to determine whether these cells are capable of simulating regeneration observed with other nephrogenic cell types (13). In summary, organoid technologies will undoubtedly provide a methodological window, allowing us to study human development and disease in depth, and will make it possible to personalize treatment.
harnessing the kidney’s intrinsic growth potential for renal cell therapy
It is widely accepted that the adult mammalian kidney is devoid of true regenerative potential, defined as the ability to generate new nephrons (5, 48). This results from the exhaustion of the fetal pool of renal stem cells, which is depleted soon after (mice) or several weeks before (humans) birth. Nonetheless, both during normal homeostasis and following injury, the kidney is capable of recovering from damage (49). This notion becomes evident when considering the baseline shedding of epithelial cells into the urine, estimated at about 70,000 per hour (50). Even more so, kidney function often returns to normal within a short period of time following various injurious insults, manifested as acute kidney injury. Although it is agreed that the epithelial lining of damaged tubules is repopulated by newly formed epithelial cells, the source of the latter has been a matter of much debate. In general, this source has been proposed to be either extrarenal (e.g. bone marrow (BM) derived cells (51)) or intrarenal (5). Indeed, a large body of work in the previous decade has ascribed significant differentiation capacity to various BM–derived cells, and in particular mesenchymal stem cells (MSCs) (52, 53). In addition, the kidney has been shown to harbor MSC–like cells (54, 55). However, it has been firmly proven that despite their potential role in both renal fibrosis (56) and tumorigenesis (57), these cells are unable to differentiate into renal lineages, with previous reports regarding such capacity probably related to artifacts (58). Validating this notion, an elegant lineage tracing model, used by Humphreys et al. (59), marked all renal tubular epithelial cells and demonstrated that following ischemic damage, all nascent epithelial cells originate from within the renal epithelium itself, and not from extrarenal sources. In an attempt to better define the source of new cells in the adult kidney during homeostasis or following damage, Rinkevich et al. (60) applied a transgenic mouse model, termed the “rainbow” mouse, in which every cell is randomly labeled with one of four colors. Interestingly, during both steady-state and following ischemic damage, new tubular cells were found to form via clonal expansion. Importantly, these clonal units were seen to form in a segment-restricted manner, with Wnt signaling regulating their expansion (60) (Figure 2). Interestingly, more clones were seen to form following injury. Thus, this study demonstrated that tubular homeostasis and repair are initiated by lineage-specific clone-forming cells that undergo local expansion. This suggests that ex vivo recapitulation of this process for regenerative purposes would probably require isolation and expansion of specific clones, which could repopulate their respective nephron segment. Of note, it remains to be determined whether any tubular cell under specific conditions is capable of giving rise to such clones, or only a predetermined fraction of cells within the nephron is endowed with this potential (stem state vs. stem cell). Notably, although it has been shown that no SIX2+ population exists in the postnatal kidney (59), this does not exclude the possibility of a progenitor population with a narrower differentiation potential, or alternatively with a different gene expression profile than the CM. Kusaba et al. (61), using a transgenic mouse model in which fully differentiated proximal tubule cells are labeled, demonstrated that following renal damage, all tubular cells remained labeled, indicating that the source of repopulating cells is the surviving, mature epithelial cells. Consistent with earlier observations (51), it was shown that upon damage, a certain degree of dedifferentiation takes place to facilitate repair, as indicated by the induction of renal progenitor markers Pax2 and Vimentin. Similarly, Berger et al. (62) demonstrated that postischemic tubular repair can originate from any tubular cell, thereby precluding the existence of a progenitor sub-population. These results affirmed the conclusions of earlier works, which demonstrated that both cycling and noncycling cells within renal tubules are in fact differentiated cells (63, 64). One approach to circumvent this problem has been the usage of specific culture conditions to promote acquisition of progenitor traits in adult kidney-derived cells. For instance, Buzhor et al. (65), through the use of human kidney epithelial cells expanded in 2D adherent culture and then transferred into growth as 3D spheroids, demonstrated the cells to upregulate specific markers of kidney function (transporter molecules) and acquisition of progenitor characteristics, as manifested by their ability to regenerate renal structures when grafted into the chick embryo. However, a large number of studies reported the identification of various cell types, harboring trait/s compatible with a progenitor identity, identified as such via the expression of specific surface marker expression (65, 66, 67), their label retention following a chase period (68, 69), or the propensity to expel cytotoxic substances via efflux pumps. Although some of these cell fractions demonstrated the capacity to improve kidney function in murine models (70, 71), while the exact mechanism of tubule cell replacement still awaits final confirmation, several explanations can be offered to account for discrepancies between different studies. First, the notion that each surviving epithelial cell is equally capable of dedifferentiating and proliferating to replace lost cells is based on staining for cell-cycle markers, such as Ki-67 or 5-bromo-2′-deoxyuridine uptake. Following renal injury, these markers are diffusely expressed and involve the large majority of tubular epithelial cells. Nonetheless, the expression of such cell cycle markers as a surrogate of cell division is questionable. Indeed, these markers may label start of DNA synthesis or cell cycle activation, but their presence does not differentiate between undergoing true division and cells that are merely undergoing compensatory hypertrophy. Although in both cases cell cycle activation takes place, the latter reflects functional compensation but not kidney regeneration. Furthermore, lineage tracing analyses consistently report the presence of a replication-competent tubular cell sub-population with enhanced resistance to death and limited self-renewal potential, alongside a less differentiated cell phenotype, in comparison with other tubular cells. While this tubular population undergoes clonogenic expansion upon injury, other tubular cells, like other epithelial cell types, seem unable to complete cell division, being able only to duplicate their DNA content, but not to complete mitosis by dividing their cytoplasm (cytokinesis). Finally, endoreplicating cells are highly transcriptionally active and therefore appear as dedifferentiated and proliferating based on immunostaining for embryonic and cell cycle markers, even if they are unable to generate a progeny. This notion is highly relevant from the translational perspective, suggesting that function recovery and tissue regeneration are two independent phenomena and that endoreplicating and truly dividing tubular cells may represent different targets for new possible treatments.
Generation of new tubular epithelial cells in the adult mammalian kidney. Transgenic “rainbow” mice harboring a random marker in each cell were followed during a chase period, representing either normal homeostasis (i.e. to compensate for the baseline shedding of ∼70 000 cells per hour) or following renal damage. Individual renal epithelial cells give rise to segment-specific clones in a lineage restricted manner. The clonal expansion is Wnt-dependent, whereby Wnt-responsive renal epithelial cells function as unipotent progenitors.
Lineage reprogramming towards renal stem cells
As mentioned previously, once it has become clear that ectopic expression of a small number of transcription factors in a given cell type can generate a different cell type, much effort was directed at establishing various cellular phenotypes by the use of this approach. In contrast to “pluripotent” reprogramming, whereby the initial cell type is reprogrammed into an ESC-like state, potentially allowing for directed differentiation into the desired cell type, “lineage” reprogramming has been suggested as a means to switch between two, nonpluripotent cell fates (22). In several tissues, such as the heart (72, 73, 74) and pancreas (75, 76, 77, 78), various protocols have been devised to derive either tissue-specific progenitors or functional cell types. In contrast, despite the major progress in understanding the molecular factors governing nephrogenesis, including the identification of the major transcription factors defining renal stem cells during kidney development, to date there have been only few reports of reprogramming cells into a kidney progenitor/epithelial state. Starting from human proximal tubule cells, Hendry et al. (79) ectopically expressed six transcription factors, which resulted in the establishment of cells expressing several renal developmental markers and capable of integrating into CM areas when mixed with reaggregating mouse embryonic kidney cells. Nonetheless, the cells were not shown to differentiate into tubular structures, which could be attributed to the continued ectopic expression of the reprogramming factors. Thus, other approaches would probably be necessary to both dedifferentiate mature renal cells into a progenitor state, while at the same time direct their differentiation once in the desired niche, for instance, via the use of transient expression of the reprogramming factors or conditional expression cassettes. Recently, via forced expression of Emx2, Hnf1b, Hnf4a, and Pax8 in mouse or human fibroblasts, Kaminski et al. (80) were able to generate cells that exhibited epithelial characteristics and marker expression, clustered with renal cells in terms of global gene expression profile, possessed some functional traits of mature tubular cells and were sensitive to nephrotoxic substances. In addition, the reprogrammed cells were able to contribute to tubular structures when mixed with dissociated embryonic kidney cells. While these studies prove that renal end products can be generated via ectopic transcription factor expression, more effective and robust protocols would have to be devised so as to generate cells that could effectively repair and boost kidney function.
Conclusion
The idea of applying cell-based therapies as a means to regenerate damaged kidneys and treat both acute kidney injury and CKD has been around for several decades. However, significant gaps in our understanding of the processes taking place during both renal homeostasis and following damage have set a high barrier in the way to translation. In recent years, the basic mechanisms underlying these processes have been unraveled, thereby opening a path for recreating renal parenchyma with the use of cells from the renal lineage. Despite the inherent complexity of the kidney, meticulous studies involving, among others, transgenic mouse models and novel differentiation protocols of PSCs, have shed light on the factors and processes involved in the formation of renal cells, thereby providing a roadmap to successful renal regeneration. Hence, the main challenge currently facing renal regenerative medicine is the implementation of these insights in a precise and safe manner so as to arrive at the desired cell types and establish ways of carrying out these processes in the clinic.
References
2013 Chapter 1: definition and classification of CKD. Kidney Int Suppl 2011;3:19–62.
Hill NR, Fatoba ST, Oke JL et al. Global prevalence of chronic kidney disease—a systematic review and meta-Analysis. PLoS ONE 2016;11:e0158765.
Levin A, Tonelli M, Bonventre J et al. participants ISNGKHS 2017 Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet. The PMID is: 28434650.
Hickson LJ, Eirin A, Lerman LO . Challenges and opportunities for stem cell therapy in patients with chronic kidney disease. Kidney Int 2016;89:767–778.
Pleniceanu O, Harari-Steinberg O, Dekel B . Concise review: kidney stem/progenitor cells: differentiate, sort out, or reprogram? Stem Cells 2010;28:1649–1660.
Nangaku M . Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol 2006;17:17–25.
Hammerman MR . Transplantation of renal precursor cells: a new therapeutic approach. Pediatr Nephrol 2000;14:513–517.
Dekel B, Burakova T, Arditti FD et al. Human and porcine early kidney precursors as a new source for transplantation. Nat Med 2003;9:53–60.
Kim SS, Park HJ, Han J et al. Improvement of kidney failure with fetal kidney precursor cell transplantation. Transplantation 2007;83:1249–1258.
Kim SS, Gwak SJ, Han J et al. Kidney tissue reconstruction by fetal kidney cell transplantation: effect of gestation stage of fetal kidney cells. Stem Cells 2007;25:1393–1401.
Kim SS, Gwak SJ, Han J, Park MH, Song KW, Kim BS . Regeneration of kidney tissue using in vitro cultured fetal kidney cells. Exp Mol Med 2008;40:361–369.
Dekel B, Metsuyanim S, Schmidt-Ott KM et al. Multiple imprinted and stemness genes provide a link between normal and tumor progenitor cells of the developing human kidney. Cancer Res 2006;66:6040–6049.
Harari-Steinberg O, Metsuyanim S, Omer D et al. Identification of human nephron progenitors capable of generation of kidney structures and functional repair of chronic renal disease. EMBO Mol Med 2013;5:1556–1568.
Metsuyanim S, Harari-Steinberg O, Buzhor E et al. Expression of stem cell markers in the human fetal kidney. PLoS ONE 2009;4:e6709.
Metsuyanim S, Pode-Shakked N, Schmidt-Ott KM et al. Accumulation of malignant renal stem cells is associated with epigenetic changes in normal renal progenitor genes. Stem Cells 2008;26:1808–1817.
Pode-Shakked N, Pleniceanu O, Gershon R et al. Dissecting stages of human kidney development and tumorigenesis with surface markers affords simple prospective purification of nephron stem cells. Sci Rep 2016;6:23562.
Brown AC, Muthukrishnan SD, Oxburgh L . A synthetic niche for nephron progenitor cells. Dev Cell 2015;34:229–241.
Pode-Shakked N, Gershon R, Tam G et al. Evidence of in vitro preservation of human nephrogenesis at the single-cell level. Stem Cell Rep 2017;9:279–291.
De Los Angeles A, Ferrari F, Xi R et al. Hallmarks of pluripotency. Nature 2015;525:469–478.
Takahashi K, Yamanaka S . Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676.
Takahashi K, Tanabe K, Ohnuki M et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–872.
Slack JM . Turning one cell type into another. Curr Top Dev Biol 2016;117:339–358.
Thomson JA, Itskovitz-Eldor J, Shapiro SS et al. Embryonic stem cell lines derived from human blastocysts. Science 1998;282:1145–1147.
Yamamoto M, Cui L, Johkura K et al. Branching ducts similar to mesonephric ducts or ureteric buds in teratomas originating from mouse embryonic stem cells. Am J Physiol Renal Physiol 2006;290:F52–F60.
Davies JA, Unbekandt M, Ineson J, Lusis M, Little MH . Dissociation of embryonic kidney followed by re-aggregation as a method for chimeric analysis. Methods Mol Biol 2012;886:135–146.
Kim D, Dressler GR . Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J Am Soc Nephrol 2005;16:3527–3534.
Vigneau C, Polgar K, Striker G et al. Mouse embryonic stem cell-derived embryoid bodies generate progenitors that integrate long term into renal proximal tubules in vivo. J Am Soc Nephrol 2007;18:1709–1720.
Bruce SJ, Rea RW, Steptoe AL, Busslinger M, Bertram JF, Perkins AC . In vitro differentiation of murine embryonic stem cells toward a renal lineage. Differentiation 2007;75:337–349.
Kobayashi T, Tanaka H, Kuwana H et al. Wnt4-transformed mouse embryonic stem cells differentiate into renal tubular cells. Biochem Biophys Res Commun 2005;336:585–595.
Batchelder CA, Lee CC, Matsell DG, Yoder MC, Tarantal AF . Renal ontogeny in the rhesus monkey (Macaca mulatta) and directed differentiation of human embryonic stem cells towards kidney precursors. Differentiation 2009;78:45–56.
Nakane A, Kojima Y, Hayashi Y, Kohri K, Masui S, Nishinakamura R . Pax2 overexpression in embryoid bodies induces upregulation of integrin alpha8 and aquaporin-1. In Vitro Cell Dev Biol Anim 2009;45:62–68.
Mae S, Shono A, Shiota F et al. Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat Commun 2013;4:1367.
Taguchi A, Kaku Y, Ohmori T et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 2014;14:53–67.
Sharmin S, Taguchi A, Kaku Y et al. Human induced pluripotent stem cell-derived podocytes mature into vascularized glomeruli upon experimental transplantation. J Am Soc Nephrol 2016;27:1778–1791.
Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV . Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 2015;33:1193–1200.
Freedman BS, Brooks CR, Lam AQ et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun 2015;6:8715.
Xia Y, Nivet E, Sancho-Martinez I et al. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat Cell Biol 2013;15:1507–1515.
Takasato M, Er PX, Becroft M et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol 2014;16:118–126.
Takasato M, Er PX, Chiu HS et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 2015;526:564–568.
Takebe T, Enomura M, Yoshizawa E et al. Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell 2015;16:556–565.
Toyohara T, Mae S, Sueta S et al. Cell therapy using human induced pluripotent stem cell-derived renal progenitors ameliorates acute kidney injury in mice. Stem Cells Transl Med 2015;4:980–992.
Islam S, Kjallquist U, Moliner A et al. Highly multiplexed and strand-specific single-cell RNA 5′ end sequencing. Nat Protoc 2012;7:813–828.
Narayanan K, Schumacher KM, Tasnim F et al. Human embryonic stem cells differentiate into functional renal proximal tubular-like cells. Kidney Int 2013;83:593–603.
Li Y, Kandasamy K, Chuah JK et al. Identification of nephrotoxic compounds with embryonic stem-cell-derived human renal proximal tubular-like cells. Mol Pharmacol 2014;11:1982–1990.
Dekel B . The ever-expanding kidney repair shop. J Am Soc Nephrol. 2015;27:1579–1581.
Merkle FT, Ghosh S, Kamitaki N et al. Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations. Nature 2017;545:229–233.
Bar S, Schachter M, Eldar-Geva T, Benvenisty N . Large-scale analysis of loss of imprinting in human pluripotent stem cells. Cell Rep 2017;19:957–968.
Dziedzic K, Pleniceanu O, Dekel B . Kidney stem cells in development, regeneration and cancer. Semin Cell Dev Biol 2014;36:57–65.
Harari-Steinberg O, Pleniceanu O, Dekel B . Selecting the optimal cell for kidney regeneration: fetal, adult or reprogrammed stem cells. Organogenesis 2011;7:123–134.
Prescott LF . The normal urinary excretion rates of renal tubular cells, leucocytes and red blood cells. Clin Sci 1966;31:425–435.
Abbate M, Brown D, Bonventre JV . Expression of NCAM recapitulates tubulogenic development in kidneys recovering from acute ischemia. Am J Physiol 1999;277:F454–F463.
Behr L, Hekmati M, Fromont G et al. Intra renal arterial injection of autologous mesenchymal stem cells in an ovine model in the postischemic kidney. Nephron Physiol 2007;107:65–76.
Wong CY, Cheong SK, Mok PL, Leong CF . Differentiation of human mesenchymal stem cells into mesangial cells in post-glomerular injury murine model. Pathology 2008;40:52–57.
Dekel B, Zangi L, Shezen E et al. Isolation and characterization of nontubular sca-1+lin− multipotent stem/progenitor cells from adult mouse kidney. J Am Soc Nephrol 2006;17:3300–3314.
Kramann R, Goettsch C, Wongboonsin J et al. Adventitial MSC-like cells are progenitors of vascular smooth muscle cells and drive vascular calcification in chronic kidney disease. Cell Stem Cell 2016;19:628–642.
Kramann R, Schneider RK, DiRocco DP et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell 2015;16:51–66.
Pleniceanu O, Shukrun R, Omer D et al. PPARG is central to the initiation and propagation of human angiomyolipoma, suggesting its potential as a therapeutic target. EMBO Mol Med 2017;9:508–530.
Humphreys BD, Bonventre JV . Mesenchymal stem cells in acute kidney injury. Annu Rev Med 2008;59:311–325.
Humphreys BD, Valerius MT, Kobayashi A et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2008;2:284–291.
Rinkevich Y, Montoro DT, Contreras-Trujillo H et al. In vivo clonal analysis reveals lineage-restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Rep 2014;7:1270–1283.
Kusaba T, Lalli M, Kramann R, Kobayashi A, Humphreys BD . Differentiated kidney epithelial cells repair injured proximal tubule. Proc Natl Acad Sci USA 2014;111:1527–1532.
Berger K, Bangen JM, Hammerich L et al. Origin of regenerating tubular cells after acute kidney injury. Proc Natl Acad Sci USA 2014;111:1533–1538.
Vogetseder A, Palan T, Bacic D, Kaissling B, Le Hir M . Proximal tubular epithelial cells are generated by division of differentiated cells in the healthy kidney. Am J Physiol Cell Physiol 2007;292:C807–C813.
Vogetseder A, Picard N, Gaspert A, Walch M, Kaissling B, Le Hir M . Proliferation capacity of the renal proximal tubule involves the bulk of differentiated epithelial cells. Am J Physiol Cell Physiol 2008;294:C22–C28.
Buzhor E, Omer D, Harari-Steinberg O et al. Reactivation of NCAM1 defines a subpopulation of human adult kidney epithelial cells with clonogenic and stem/progenitor properties. Am J Pathol 2013;183:1621–1633.
Sagrinati C, Netti GS, Mazzinghi B et al. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J Am Soc Nephrol 2006;17:2443–2456.
Appel D, Kershaw DB, Smeets B et al. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol 2009;20:333–343.
Maeshima A, Yamashita S, Nojima Y . Identification of renal progenitor-like tubular cells that participate in the regeneration processes of the kidney. J Am Soc Nephrol 2003;14:3138–3146.
Oliver JA, Maarouf O, Cheema FH, Martens TP, Al-Awqati Q . The renal papilla is a niche for adult kidney stem cells. J Clin Invest 2004;114:795–804.
Ronconi E, Sagrinati C, Angelotti ML et al. Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 2009;20:322–332.
Angelotti ML, Ronconi E, Ballerini L et al. Characterization of renal progenitors committed toward tubular lineage and their regenerative potential in renal tubular injury. Stem Cells 2012;30:1714–1725.
Lalit PA, Salick MR, Nelson DO et al. Lineage reprogramming of fibroblasts into proliferative induced cardiac progenitor cells by defined factors. Cell Stem Cell 2016;18:354–367.
Lalit PA, Rodriguez AM, Downs KM, Kamp TJ . Generation of multipotent induced cardiac progenitor cells from mouse fibroblasts and potency testing in ex vivo mouse embryos. Nat Protoc 2017;12:1029–1054.
Zhang Y, Cao N, Huang Y et al. Expandable cardiovascular progenitor cells reprogrammed from fibroblasts. Cell Stem Cell 2016;18:368–381.
Chera S, Baronnier D, Ghila L et al. Diabetes recovery by age-dependent conversion of pancreatic delta-cells into insulin producers. Nature 2014;514:503–507.
Li K, Zhu S, Russ HA et al. Small molecules facilitate the reprogramming of mouse fibroblasts into pancreatic lineages. Cell Stem Cell 2014;14:228–236.
Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA . In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008;455:627–632.
Ariyachet C, Tovaglieri A, Xiang G et al. Reprogrammed stomach tissue as a renewable source of functional beta cells for blood glucose regulation. Cell Stem Cell 2016;18:410–421.
Hendry CE, Vanslambrouck JM, Ineson J et al. Direct transcriptional reprogramming of adult cells to embryonic nephron progenitors. J Am Soc Nephrol 2013;24:1424–1434.
Kaminski MM, Tosic J, Kresbach C et al. Direct reprogramming of fibroblasts into renal tubular epithelial cells by defined transcription factors. Nat Cell Biol 2016;18:1269–1280.
Acknowledgements
This work was supported by the ICRF (grants 15731 and 15450), the Israel Cancer Association (grant 20150916), The Ziering Foundation (grant 45124), ISF (grant number ISF 2071/17) and NIH DIACOMP grant (14GHSU1381).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pleniceanu, O., Omer, D., Harari-Steinberg, O. et al. Renal lineage cells as a source for renal regeneration. Pediatr Res 83, 267–274 (2018). https://doi.org/10.1038/pr.2017.255
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2017.255
This article is cited by
-
The therapeutic potential of Camel Wharton jelly mesenchymal stem cells (CWJ-MSCs) in canine chronic kidney disease model
Stem Cell Research & Therapy (2022)
-
The genetic basis of congenital anomalies of the kidney and urinary tract
Pediatric Nephrology (2022)