Abstract
Background
Human milk has a high content of the antimicrobial compound hydrogen peroxide (H2O2). As opposed to healthy full-term infants, preterm neonates are fed previously expressed and stored maternal milk. These practices may favor H2O2 decomposition, thus limiting its potential benefit to preterm infants. The goal of this study was to evaluate the factors responsible for H2O2 generation and degradation in breastmilk.
Methods
Human donors’ and rats’ milk, along with rat mammary tissue were evaluated. The role of oxytocin and xanthine oxidase on H2O2 generation, its pH-dependent stability, as well as its degradation via lactoperoxidase and catalase was measured in milk.
Results
Breast tissue xanthine oxidase is responsible for the H2O2 generation and its milk content is dependent on oxytocin stimulation. Stability of the human milk H2O2 content is pH-dependent and greatest in the acidic range. Complete H2O2 degradation occurs when human milk is maintained, longer than 10 min, at room temperature and this process is suppressed by lactoperoxidase and catalase inhibition.
Conclusion
Fresh breastmilk H2O2 content is labile and quickly degrades at room temperature. Further investigation on breastmilk handling techniques to preserve its H2O2 content, when gavage-fed to preterm infants is warranted.
Similar content being viewed by others
Main
Aside from its superior nutritional content, when compared with formula, human milk provides significant neonatal protection against infections. Breastfed infants experience fewer and shorter bouts of infections (1) and have a lower morbidity rate, when compared with formula-fed children of a similar age (2, 3). Exclusive use of human milk lowers the incidence of diarrhea, urinary tract infections, and otitis media in older children (4).
Human milk contains an appreciable amount of hydrogen peroxide (H2O2) that peaks within days after birth, before declining toward the fourth postnatal week (5). H2O2 has been identified as a Jekyll and Hyde signaling molecule showing beneficial effects at physiologically relevant lower concentrations (6), but having biologically harmful properties at higher levels. Endogenously, H2O2 is mostly generated via mitochondrial oxidative phosphorylation (7) and phagocytic respiratory burst (8), but other enzymatic processes, such as lipoxygenase, xanthine oxidase, and myeloperoxidase, contribute to its formation. H2O2 is bactericidal and together with milk-containing thiocyanates (9) generates, via the lactoperoxidase system (LPS), hypothiocyanite, a non-stable molecule (10), that further enhances its microbicidal activity (11).
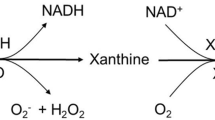
Human milk production is oxytocin-dependent (12). Its H2O2 content is regulated by the mammary epithelial cells’ xanthine oxidase (XO) expression and activity. XO availability, in turn, is dependent on the expression and activity of xanthine oxidoreductase (XOR), an enzyme that converts molybdoflavoprotein xanthine dehydrogenase (XDH) to XO via either oxidation of sulfhydryl residues, or proteolysis (13). XO, in the presence of the substrates hypoxanthine or xanthine, generates reactive oxygen species and microbicidal molecules such as superoxide, H2O2, thiocyanate and hypothiocyanite (Figure 1).
Cartoon depicting the oxytocin effect on the milk fat globule in the mammary gland and the conversion of xanthine dehydrogenase to the oxidase form. The generation of the reactive oxygen species superoxide, hydrogen peroxide (H2O2) and its conversion to hypothiocyanite by the milk-containing lactoperoxidase is also shown.
There is limited data on the regulatory factors responsible for H2O2 generation in the mammary glands and its stability in human milk. Addressing this knowledge gap is relevant to our understanding of human milk’s H2O2 anti-infectious properties when fed to preterm infants.
As preterm infants cannot coordinate the suck–swallow mechanism until reaching maturity, expressed human milk, or pasteurized donor milk is gavage-fed. The extent to which the maternal human milk collection, storage, and pasteurization preserves its H2O2 content has not been previously investigated. This issue is of clinical importance since LPS can induce H2O2 decomposition during milk handling and storage process resulting in reduction or complete degradation of its H2O2 content.
Therefore, the goal of this study was to evaluate the factors responsible for H2O2 generation and degradation of mother’s milk.
Methods
Design
Studies were conducted in a laboratory setting utilizing human milk donor samples and lactating rat mammary tissue and milk, as well as their pups’ milk curds.
Animals
All procedures were conducted in accordance with the Canadian Animals for Research Act and Canadian Council on Animal Care regulations, and the Animal Care Committee of the Hospital approved the study protocol for Sick Children. Sprague-Dawley rats (Charles River, Montreal, QC, Canada) were studied. These included adult and younger animals at 1, 2, and 3 weeks of age. They were fed regular rodent pellets and were housed under standard lighting and temperature conditions. The animals were killed by pentobarbital sodium injection (60 mg/kg ip (adult)), and the gastric tissue was quickly excised.
Human Milk
Fresh human mother’s milk samples were obtained from healthy volunteer donors (N=5) following the informed consent and under a protocol approved by The Hospital for Sick Children Research Ethics Board. Following maternal expression, the human milk was immediately flash-frozen in liquid nitrogen, and was stored at −80 °C for further processing.
Rat Breast Tissue and Milk
Non-lactating (control) and lactating rats (N=3 per group) were killed, and the mammary gland tissue was quickly excised, flash-frozen in liquid nitrogen, and stored at −80 °C. Lactating rat mothers’ milk was collected by a non-invasive technique reported by others (14). To evaluate the oxytocin effect on the mammary gland enzymatic activity, lactating rats received 1.5 IU of oxytocin ip 5 min prior to the tissue harvesting.
To obtain newborn gastric milk samples, 1-week-old pups (N=6) were killed by pentobarbital sodium injection 3 h after maternal separation. The pups’ stomach milk curd was retrieved and immediately frozen for further processing. In order to evaluate freshly ingested mother’s milk, same age pups were separated for a 3-h period and then were returned to mothers for a 2-h period of breastfeeding. Pups were then killed, their gastric milk curd was retrieved and immediately frozen for further processing. All milk and curd samples were kept at −80 °C.
Measurements
All chemicals and reagents were obtained from Sigma-Aldrich (Oakville, ON, Canada), unless otherwise indicated.
Activity Assays and H2O2 Content Measurements
Tissue XD+XO and XO were assayed in the presence and absence of NAD+, respectively, as previously described (15). Briefly, rat mammary glands were homogenized in 50 mM K+ phosphate buffer (pH 7.8) containing 1.0 mM EDTA, 0.2 mM PMSF, and 0.5 mM DTT, and tissue protein content was evaluated by Bradford assay (Bio-Rad, Mississauga, ON, Canada). A volume of 5 μl of homogenate was mixed with 50 μM hypoxanthine (substrate) with or without 1.0 mM NAD+, and the increase in absorbance at 295 nm following the formation of uric acid was used as a measure of the activity of XD+XO or XO, and normalized to protein content. One unit of tissue XD+XO, or XO was defined as the amount of enzyme required to form 1 μmol of uric acid/min.
For organ explant studies, the rat mammary glands were maintained overnight in DMEM (Wisent, St-Bruno, QB, Canada) supplemented with fetal bovine serum (Wisent) and 10% antibiotics/antimycotics (Wisent) at 37 °C, 5% CO2. Control and oxytocin-treated (1 μM for 1, 7, or 30 min) tissue explants were incubated in culture medium and the H2O2 content was measured using Amplex Red, as described below.
For H2O2 content measurement, frozen milk samples were thawed and diluted 1:5 in PBS. Milk curds were homogenized in PBS. H2O2 content of mothers’ milk and pups’ milk-curd samples was determined by the Amplex Red hydrogen peroxide/peroxidase assay kit (Molecular Probes, Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Briefly, samples were incubated in the reaction mixture containing 50 μM Amplex Red reagent and 0.1 U/ml horseradish peroxidase (HRP), and fluorescence was measured at the excitation of 560 nm and emission detection at ~590 nm.
To measure the human milk LPS activity, the following procedure was performed. The human milk was thawed and was maintained at room temperature for 1 h to allow complete degradation of its H2O2 content. The milk was then aliquoted into four samples of similar volumes that were studied without and following HCl (6 N) addition at different concentrations to obtain the following pH values: 7, 6, 5, and 4. The milk was spiked with 100 μM of H2O2 and the H2O2 content changes continuously measured with Amplex Red over a 10 min period. Only human milk was utilized, since the expressed volume obtained from the rat dam was insufficient for repeated experiments.
Data Analysis
Data were first evaluated to determine Gaussian distribution by Skewness, Kurtosis, and Omnibus testing. Normally distributed data were analyzed by parametric data. Group differences were statistically evaluated by unpaired or paired Student’s t-test, one- or two-way analysis of variance (ANOVA) with multiple comparisons was obtained by the Dunnett’s test. Statistical significance was determined at P<0.05. All statistical analyses were performed with the Number Cruncher Statistical System software (NCSS, Kaysville, UT). Data are presented as mean±SEM.
Results
Milk H2O2 Generation
First, we looked at the activity of key enzymes involved in H2O2 generation in the rat breast tissue without and following oxytocin stimulation. Lactation was associated with a significant, but proportionally similar, increase in breast tissue XDH and XO activity (Figure 2a). Oxytocin administration to the lactating rats disproportionally increased the activity of both enzymes. The XO activity was two- to threefold greater, while the XDH values increased to a lesser extent, when compared with non-lactating animals. Expressed as a ratio, the breast tissue XO/XDH activity was significantly increased only for the oxytocin-treated lactating animals (Figure 2c).
Mammary gland tissue Xanthine dehydrogenase (XDH; a) and xanthine oxidase (XO; b) activity from non-lactating (Control; N=3 animals), lactating (N=3), and lactating animals that received 1.5 IU of oxytocin ip 5 min prior to tissue harvesting (Lact+oxytocin; N=3). The XO/XDH activity ratio is shown in (c). **P<0.01 by one-way analysis of variance (ANOVA) and Dunnett’s multiple-comparison testing.
In vitro exposure of rat breast tissue explants to oxytocin resulted in rapid and significant increase in the explant-media H2O2 content (Figure 3), suggesting a direct effect of this compound on XO activity. Interestingly, the effect of oxytocin on H2O2 generation was consistently higher in tissues derived from lactating animals, as compared with non-lactating rats (Figure 3).
Oxytocin-induced hydrogen peroxide (H2O2) generation in the media overtime in mammary gland explant from non-lactating (Control; N=3) and lactating rats (N=3). **P<0.01 when compared to time zero values by two-way analysis of variance (ANOVA) and Dunnett’s multiple-comparison testing. Except for time zero, the media H2O2 content of explants derived from lactating rats was significantly higher, when compared with Control rat tissue by two-way ANOVA.
Milk H2O2 Stability and Degradation
We proceeded to evaluate the H2O2 content of the rat milk and pups’ gastric milk curd. Milk curds obtained from pups that were first fasted to clear their stomach content and returned to the mother for a 2-h breast feeding session had measurable, but lower H2O2 content, when compared with the freshly ordained rats’ milk. In contrast, milk curds derived from breast-fed pups separated from their mothers for 3 h (old curds) showed an unmeasurable H2O2 content (Figure 4).
H2O2 content from collected milk from rat dams 1-week post partum (milk; N=3) and gastric milk curds from 1-week-old pups following either 2 h of breast-feeding (fresh curd; N=6) or 3 h after maternal separation (old curd; N=6). See text for details. **P<0.01, vs. rat dam milk by unpaired Student’s t-test. The H2O2 content in old curds was nil.
Lastly, we utilized human milk to measure the rate of H2O2 decomposition according to the milk pH, since XO activity is known to be pH-dependent and highest at pH of 7. At a pH of 7, the spiked human milk’s H2O2 content was completely degraded in 4 min (Figure 5). At a pH of 4, the milk H2O2 content minimally changed over the 10-min period, with intermediate results for the other tested pH values (Figure 5). To confirm that the spiked human milk H2O2 degradation was dependent on the enzyme activity of its constituents, we obtained measurements at pH of 7 in the absence and presence of the LPS and catalase inhibitor 3-AT (10−3 M) (16, 17). 3-AT completely abolished H2O2 degradation (Figure 6).
H2O2 content changes overtime in human milk at different pH values. The milk was spiked with H2O2 (100 μM) at time zero and the H2O2 content was continuously measured by Amplex Red over a 10 min period. N=3 for each pH. **P<0.01 when compared with the respective pH time zero values by two-way analysis of variance (ANOVA) and Dunnett’s multiple-comparison testing. pH 7 values at 10 min were significantly different, when compared with the results from the other 3 pH tested.
H2O2 content changes overtime in human milk at pH 7 in the absence and presence of 3-AT (10−3 M), an LPS and catalase inhibitor. The milk was spiked with H2O2 (100 μM) at time zero and the H2O2 content was continuously measured by Amplex Red over a 10-min period; N=3. **P<0.01 when compared with time zero by one-way analysis of variance (ANOVA) and Dunnett’s multiple-comparison testing.
Discussion
In this study, we identified the key factors responsible for the milk H2O2 generation and degradation. Rat tissue and milk, as well as, human milk were utilized for these experiments in order to more clearly evaluate the H2O2 pathways during lactation and newborn consumption.
We documented that lactation in the rat is associated with increased breast tissue XO and XDH activity that is further enhanced by oxytocin administration indicating the importance of this transduction factor in the process. The pattern of changes is such that oxytocin increased XO activity to a further extent than documented for the XDH enzyme isoform.
We further showed that in newborn rats, H2O2 is present in freshly collected milk curds suggesting that its gastric biological activity is maintained. In contrast, the human milk H2O2 content was rapidly degraded by the LPS and the enzymatic activity and the rate of decomposition was highest at a pH 7.
The lactating mammary gland is composed of branching ducts ending in alveolar clusters where the milk is produced. A single layer of polarized secretory epithelial cells forms the alveolar walls that are surrounded by myoepithelial cells embedded in vascularized connective tissue stroma. Milk lipids, primarily triglycerides, are synthesized in the smooth endoplasmic reticulum, at the basal region of the lactating alveolar cell (18).
Newly synthesized lipid molecules are coated by protein membranes forming cytoplasmic lipid droplets that are then transported to the apical plasma membrane, where they are secreted as milk fat globules (19). Figure 1 illustrates this process.
XOR is distributed among an intra-membranous pool. XO and XDH are the two present isoforms but the content of the latter predominates (12)). Milk XO generation depends on membrane-bound sulfhydryl oxidase during the secretion process (20). Human sulfhydryl oxidase converts milk XDH to XO by oxidizing two vicinal thiols (21). Sulfhydryl oxidase is confined to the apical mammary gland epithelial membrane and milk product present in the alveolar lumen. This suggests that the XDH to XO conversion occurs when the fat droplet is released into the mammary gland lumen (22).
Ejection of milk is dependent on the hormone oxytocin. Stimulation of oxytocin receptors produces an increase in contractions of myoepithelial cells that are located on the surface of the alveoli and along the mammary gland ducts. When myoepithelial cells contract, their compression increases intra-alveolar pressure resulting in duct changes that facilitate the passage of milk (23).
Freshly ordained human milk contains reactive oxygen species molecules with antimicrobial activity, of which H2O2 plays an important role (24). The H2O2 generation in human milk is dependent on the sulfhydryl oxidase expression/activity (25). During the first postpartum week, the milk H2O2 concentration is on average threefold higher than at 4 weeks’ post partum (5).
It appears that the H2O2 generation at the time that the milk is ejected has an antibacterial effect. Whether such effect is solely to protect the mother against mastitis, as suggested by some (26), or has an equally important antimicrobial protective role for the neonate is unclear. The data from our study suggest that it may have both effects.
We demonstrated that, when compared with basal conditions, in vivo oxytocin stimulation increases the XO/XD ratio. We also showed that breast tissue in vitro exposure to oxytocin enhances H2O2 generation. We found a significant inverse linear interrelationship between LPS activity and H2O2 content, suggesting that this enzymatic pathway plays a major role in H2O2 degradation, as suggested by others (13). Catalase, an ubiquitous enzyme in milk, most likely accounts for the remaining H2O2 decomposition (13).
The average human newborn gastric pH is 5.4 at 15–60 min after birth, declining to 3.1 after 1 h of life (27). In the present study, we confirmed that at an acidic pH, H2O2 is not degraded by the enzymes contained in milk. This was based on documenting in newborn rats, the gastric presence of sufficient amounts of this molecule in fresh milk curds. Thus, it is reasonable to conclude that human milk-derived H2O2 of breastfed neonates has biological activity in the stomach.
Human milk containing H2O2 may contribute to the antimicrobial defense mechanism of the neonate even prior to reaching the gastric space. Al-Shehri et al. (5) reported that human milk, when mixed with neonatal saliva, generate up to 40 μM of H2O2, a concentration sufficient to inhibit growth of opportunistic pathogens such as Staphylococcus aureus and Salmonella spp. LPS also catalyzes the oxidation of thiocyanate (part of saliva) forming hypothiocyanate that has effective antimicrobial properties against both Gram positive and Gram negative bacteria (28).
The present study findings raise concerns as to whether the gavage-fed human milk provided to preterm infants has a H2O2 content comparable to the one obtained during breastfeeding. This is so, since preterm infants are commonly fed stored frozen human milk, or pasteurized donor milk (29). There is often a significant delay between pump-aided human milk collection and sample freezing. On the basis of the findings from the present study, such delays and exposure to room temperature promotes H2O2 degradation. In addition, human milk handling procedures such as pasteurization are also known to negatively impact on its anti-infective properties (29).
The present data also raise the question of whether therapeutic H2O2 breastmilk supplementation is clinically justified. Given the, above discussed, anti-infective properties, prior to gavage-feeding H2O2 addition to breastmilk samples with lower concentration of this metabolite is of potential benefit. Yet, we are not aware of any clinical studies evaluating such approach. At high H2O2 concentrations, enteral intake by adults was associated with embolic events and increased risk of death (30). As such, further studies are warranted to evaluate the risks and potential benefit of breastmilk H2O2 supplementation for neonates.
Limitations
Rat and human samples were used in this study. Utilizing animal samples to gain knowledge about human milk has limitations. Yet, to obtain similar measurements from human mammary glands would be challenging. Equally problematic is to evaluate the gavage milk intragastric H2O2 content in healthy preterm infants. Although informative to the human physiology, the animal data presented in this study require confirmation before the findings can be extrapolated to human milk.
Conclusion
By studying rat and human milk biology, we uncovered the process involved in H2O2 generation and post-expression degradation by its own containing enzymes. When neonates are breastfeed, the LPS- and catalase-dependent H2O2 degrading effect is minimized by delivering fresh human milk directly to the stomach. In contrast, preterm infants are gavage-fed previously expressed, stored, and manipulated human milk that facilitates significant H2O2 degradation. If gavage-fed preterm infants are to benefit from its potential antimicrobial properties, further investigation is warranted on better collection and storage methods to preserve the human milk-derived H2O2 content.
References
Lonnerdal B . Bioactive proteins in breast milk. J Paediatr Child Health 2013;49 (Suppl 1): 1–7.
Van Gysel M, Cossey V, Fieuws S, Schuermans A . Impact of pasteurization on the antibacterial properties of human milk. Eur J Pediatr 2012;171:1231–1237.
Good M, Sodhi CP, Egan CE et al. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol 2015;8:1166–1179.
Tudehope DI . Human milk and the nutritional needs of preterm infants. J Pediatr 2013;162:S17–S25.
Al-Shehri SS, Knox CL, Liley HG et al. Breastmilk-saliva interactions boost innate immunity by regulating the oral microbiome in early infancy. PLoS ONE 2015;10:e0135047.
Gough DR, Cotter TG . Hydrogen peroxide: a Jekyll and Hyde signalling molecule. Cell Death Dis 2011;2:e213.
Wong H-S, Dighe PA, Mezera V, Monternier P-A, Brand MD . Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J Biol Chem 2017;292:16804–16809.
El‐Benna J, Hurtado‐Nedelec M, Marzaioli V, Marie JC, Gougerot‐Pocidalo MA, Dang PMC . Priming of the neutrophil respiratory burst: role in host defense and inflammation. Immunol Rev 2016;273:180–193.
Laurberg P, Andersen S, Knudsen N, Ovesen L, Nohr SB, Bulow Pedersen I . Thiocyanate in food and iodine in milk: from domestic animal feeding to improved understanding of cretinism. Thyroid 2002;12:897–902.
Bafort F, Parisi O, Perraudin JP, Jijakli MH . Mode of action of lactoperoxidase as related to its antimicrobial activity: a review. Enzyme Res 2014;2014:517164.
Almehdar HA, El-Fakharany EM, Uversky VN, Redwan EM . Disorder in milk proteins: structure, functional disorder, and biocidal potentials of lactoperoxidase. Curr Protein Peptide Sci 2015;16:352–365.
Silanikove N, Shapiro F, Leitner G . Posttranslational ruling of xanthine oxidase activity in bovine milk by its substrates. Biochem Biophys Res Commun 2007;363:561–565.
Silanikove N, Shapiro F, Shamay A, Leitner G . Role of xanthine oxidase, lactoperoxidase, and NO in the innate immune system of mammary secretion during active involution in dairy cows: manipulation with casein hydrolyzates. Free Radic Biol Med 2005;38:1139–1151.
Hock A, Miyake H, Li B et al. Breast milk-derived exosomes promote intestinal epithelial cell growth. J Pediatr Surg 2017;52:755–759.
Hashimoto S . A new spectrophotometric assay method of xanthine oxidase in crude tissue homogenate. Anal Biochem 1974;62:426–435.
Darr D, Fridovich I . Irreversible inactivation of catalase by 3-amino-1,2,4-triazole. Biochem Pharmacol 1986;35:3642.
Doerge DR, Niemczura WP . Suicide inactivation of lactoperoxidase by 3-amino-1,2,4-triazole. Chem Res Toxicol 1989;2:100–103.
Riskin A, Mond Y . Prolactin-induced Subcellular Targeting of GLUT1 Glucose Transporter in Living Mammary Epithelial Cells. Rambam Maimonides Medical Journal 2015;6:e0038 doi:10.5041/RMMJ.10223.
Pisanu S, Ghisaura S, Pagnozzi D et al. The sheep milk fat globule membrane proteome. J Proteomics 2011;74:350–358.
McManaman JL, Bain DL . Structural and conformational analysis of the oxidase to dehydrogenase conversion of xanthine oxidoreductase. J Biol Chem 2002;277:21261–21268.
Isaacs CE, Pascal T, Wright CE, Gaull GE . Sulfhydryl oxidase in human milk: stability of milk enzymes in the gastrointestinal tract. Pediatr Res 1984;18:532–535.
Blakistone BA, Aurand LW, Swaisgood HE . Association of sulfhydryl oxidase and xanthine oxidase in bovine mammary tissue. J Dairy Sci 1986;69:2803–2809.
Crowley WR . Neuroendocrine regulation of lactation and milk production. Compr Physiol 2015;5:255–291.
Hettinga K, van Valenberg H, de Vries S et al. The host defense proteome of human and bovine milk. PLoS ONE 2011;6:e19433.
Kurosaki M, Zanotta S, Li Calzi M, Garattini E, Terao M . Expression of xanthine oxidoreductase in mouse mammary epithelium during pregnancy and lactation: regulation of gene expression by glucocorticoids and prolactin. Biochem J 1996;319 (Pt 3): 801–810.
Silanikove N, Rauch-Cohen A, Shapiro F, Arieli A, Merin U, Leitner G . Lipopolysaccharide challenge of the mammary gland in cows induces nitrosative stress that impairs milk oxidative stability. Animal 2012;6:1451–1459.
Miclat NN, Hodgkinson R, Marx GF . Neonatal gastric pH. Anesth Analg 1978;57:98–101.
Lonnerdal B . Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr 2003;77:1537S–1543S.
Akinbi H, Meinzen-Derr J, Auer C et al. Alterations in the host defense properties of human milk following prolonged storage or pasteurization. J Pediatr Gastroenterol Nutr 2010;51:347–352.
Hatten BW, French LK, Horowitz BZ, Hendrickson RG . Outcomes after high-concentration peroxide ingestions. Ann Emerg Med 2017;69:726–736.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
STATEMENT OF FINANCIAL SUPPORT
Funding: This study was supported by a grant from the Canadian Institutes of Health Research (MOP 133664).
Rights and permissions
About this article
Cite this article
Cieslak, M., Ferreira, C., Shifrin, Y. et al. Human milk H2O2 content: does it benefit preterm infants?. Pediatr Res 83, 687–692 (2018). https://doi.org/10.1038/pr.2017.303
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2017.303
This article is cited by
-
Nitrite in breast milk: roles in neonatal pathophysiology
Pediatric Research (2021)
-
Hydrogen peroxide promotes gastric motility in the newborn rat
Pediatric Research (2018)
-
The effect of breastmilk and saliva combinations on the in vitro growth of oral pathogenic and commensal microorganisms
Scientific Reports (2018)