Abstract
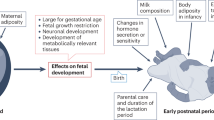
Obesity is reaching epidemic proportions and imposes major negative health crises and an economic burden in both high and low income countries. The multifaceted nature of obesity represents a major health challenge, with obesity affecting a variety of different organs and increases the risk of many other noncommunicable diseases, such as type 2 diabetes, fatty liver disease, dementia, cardiovascular diseases, and even cancer. The defining organ of obesity is the adipose tissue, highlighting the need to more comprehensively understand the development and biology of this tissue to understand the pathogenesis of obesity. Adipose tissue is a miscellaneous and highly plastic endocrine organ. It comes in many different sizes and shades and is distributed throughout many different locations in the body. Though its development begins prenatally, quite uniquely, it has the capacity for unlimited growth throughout adulthood. Adipose tissue is also a highly sexually dimorphic tissue, patterning men and women in different ways, which means the risks associated with obesity are also sexually dimorphic. Recent studies show that environmental factors during prenatal and early stages of postnatal development have the capacity to programme the structure and function of adipose tissue, with implications for the development of obesity. This review summarizes the evidence for a role for early environmental factors, such as maternal malnutrition, hypoxia, and exposure to excess hormones and endocrine disruptors during gestation in the programming of adipose tissue and obesity in the offspring. We will also discuss the complexity of studying adipose tissue biology and the importance of appreciating nuances in adipose tissue, such as sexual dimorphism and divergent responses to metabolic and endocrine stimuli. Given the rising levels of obesity worldwide, understanding how environmental conditions in early life affects adipose tissue phenotype and the subsequent development of obesity is of absolute importance.
Similar content being viewed by others
Introduction
Obesity is classed as a global epidemic by the World Health Organisation, with 13% of adults estimated to be obese in 2017 [1]. Although once only observed in high income countries, the rate of obesity is now increasing in prevalence in many middle and low income countries. The increased incidence of obesity represents a major health challenge as it increases the risk of developing other noncommunicable diseases, including type 2 diabetes [2], fatty liver disease, [3], cardiovascular diseases [4], and cancer [5]. In the UK, the cost to the national health service for obesity was £6.1 billion in 2015, and the wider economic impact was £27 billion [6]. Many factors contribute to the development of obesity. These include socio-economic factors and food marketing, which contribute to population risk, as well as genetic and lifestyle factors, which impact individual risk. However, environmental factors during early life development have the capacity to programme the structure and function of organs in the body, including the adipose tissue, the defining organ of obesity. Understanding how environmental conditions in early life impact adipose tissue and the subsequent development of obesity is of absolute importance.
The developmental origins of health and disease (DOHaD) encompasses a field of research that correlates long-term health and disease outcomes with early life conditions. This field was initiated by findings of an association between fetal malnutrition, and the development of metabolic diseases, like insulin resistance and obesity in adulthood [7, 8]. Extensions of this work using human population and experimental animal data have shown that exposure to a diverse array of suboptimal maternal environments, including nutritionally unbalanced diets, low oxygen availability, and high body mass index (BMI) during gestation, modifies the risk of the offspring developing obesity [9, 10]. The aim of this review is to present the findings of these studies. Particularly, the impact of a poor gestational environment on the development and function of the adipose and programming of obesity. It will first provide information on adipose tissue biology, including the differences and similarities between humans and the experimental animal species used in the DOHaD field, as well as describe sexual dimorphisms that exist in the adipose tissue within species. This background information is rarely considered and is of key importance when comparing findings to understand the developmental origins of obesity.
Adipose tissues
Adipose tissue is distributed throughout the body. Although initially thought of as inert storage sites for triacylglycerol, adipose tissue is now recognized as a dynamic endocrine organ vital to whole body homeostasis and metabolism. In mammals, adipose tissue is categorized as white adipose tissue (WAT) and brown adipose tissue (BAT). While both types are specialized to store lipids, they essentially have antagonistic functions. WAT acts as an energy reservoir, whereas BAT acts as an energy combustion site. Their functions are carried out by mature white and brown adipocytes, respectively. In addition to adipocytes, adipose contains other cell types, like leukocytes, pericytes, and endothelial cells. Moreover, both adipose types are vascularised and innervated [11], connecting the tissue to whole body metabolic regulation.
BAT was initially thought to be unique to neonates, hibernators, and small mammals, which is unsurprising as its primary function is to produce heat and these animals are predisposed to temperature loss [12]. However, work in the last decades has found that BAT is present in all mammals, including adult humans [13, 14].
Brown adipocytes are derived from a common progenitor with myocytes (Fig. 1) and are more closely related to myocytes than to white adipocytes. Mature brown adipocytes store lipids in multiple small vacuoles and have an abundance of mitochondria, which are vital to heat production. Breakdown of fatty acids in brown adipocytes leads to an escalation of proton ions in the intermembrane space of mitochondria. UCP1, an uncoupling protein that is a key characteristic of brown adipocytes, uncouples this proton gradient from the ATP production by increasing the permeability of the inner mitochondrial membrane, leading to heat production [15]. In addition to brown adipocytes [16], BAT has many precursor cells, known as adipoblasts or preadipocytes, present in the tissue.
Diagram depicts key stages in cell differentiation from mesenchymal stem cells to brown and white adipocytes, showing key cell markers at each cell stage. Brown and white adipocytes are derived from mesenchymal stem cells [156] and a number of factors control their differentiation. However, the GSK3β and β-catenin-WNT signaling pathways are particularly important for white adipocyte and brown adipocyte/myocyte lineage commitment, respectively [157]. A zinc finger transcriptional regulator, PRDM16 controls a bidirectional cell fate switch between skeletal myoblasts and brown adipocytes [158]. Differentiation of white adipocytes occurs down a separate lineage and adipogenesis of preadipocytes into mature adipocytes in WAT is controlled by transcriptional regulators, including peroxisome proliferator-activated receptor-γ (PPARγ) and CCAAT/enhancer-binding proteins (C/EBPs) [20,21,22,23,24]. C/EBPα and C/EBPβ are expressed early in the adipogenesis process along with certain zinc finger proteins, such as ZFP423, they are expressed shortly after commitment to the white adipocyte lineage and subsequently upregulate PPARγ [159]. These molecular factors then operate together to regulate the expression of other adipocyte-specific genes, resulting in the formation of mature adipocytes [159].
In contrast to brown adipocytes, white adipocytes have one large lipid droplet and few mitochondria. As the lipid droplet occupies the majority of the cell, white adipocytes have relatively little cytoplasm. White adipocytes are typically larger than brown (~100 µm in diameter versus 30 µm). White adipocytes differentiate from a separate lineage to brown adipocytes (Fig. 1 and [17]), although like BAT, WAT is full of progenitor cells. The presence of preadipocytes in WAT allows it to be highly plastic and capable of producing more mature white adipocytes when necessary [18]. For instance, when caloric intake is high, WAT increases its capacity for storing lipids, via both adipocyte hyperplasia and hypertrophy [19, 20]. WAT is also able to undergo a process known as “beiging”. Under certain conditions, such as cold exposure, some white preadipocytes can develop brown adipocyte characteristics, including multiple lipid droplets, increased mitochondrial density, and UCP1 expression [21]. These cells are known as brite or beige adipocytes and alter WAT physiology by affecting how it functions, and its hormonal output [22, 23].
Adipose depots, development, and differences between species
The quantity of BAT and WAT at birth and in adulthood differs greatly between species (Fig. 2). Human infants are born exceptionally fat [24], with fat making up ~15% of a neonate’s body weight. The closest experimental species to humans in terms of fatness at birth is the guinea pig, with ~11% fat at birth [25], (Fig. 2A). There is also a wide variation in the fat content of milk between species, with most small species containing more fat compared to larger species, like humans (Fig. 2A and [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]).
A Table comparing adipose development in different model species, showing average birth weight, average percentage fat at birth, timing of BAT and WAT development and average percentage fat in species milk composition. B Diagram depicting main adipose storage sites in different model species. VAT visceral adipose tissue, SAT subcutaneous adipose tissue.
Humans are born with WAT depots that develops during the final stages of gestation, with 90% being deposited during the last 10 weeks of gestation. Guinea pigs, rabbits, and sheep also accumulate WAT during the final third of gestation [39, 40]. During this time, there is an increased production of hormones, like insulin-like growth factors (IGFs) and leptin in the fetus that promote fetal adipose tissue growth [41,42,43]. However, mice and rats are born without WAT, which develops in the first 14 days post birth from progenitor cells in non-adipocyte structures [44]. Therefore, in terms of adipose development, the lactational period in rodents may be more equivalent to the third trimester of human pregnancy. Regardless, WAT depots tend to expand as age increases in the majority of species.
The function and location of WAT is similar across most species. Most WAT depots can be regarded primarily as nutrient reserves and small amounts of fat are distributed throughout the body in association with different organs. Ectopic fat stores can vary in their secretory output and function based on their associated organ. In the majority of mammals, the bulk of WAT is contained in the superficial fascia between and within muscles, below the peritoneum and in the bone marrow. WAT depots are often also subdivided into either subcutaneous adipose tissue, which lies under the skin, or visceral adipose tissue, which lies inside the abdominal cavity in association with internal organs.
BAT, however, is much more restricted in its distribution and in most species the largest quantity of BAT is concentrated around the neck and spine (Fig. 2B). In contrast to WAT, most animals are born with BAT and therefore develop it prenatally. There are also some differences in BAT differentiation across the main species studied. In rabbits, guinea pigs, lambs, and humans, BAT is fully mature and functional at birth, whereas in mice and rats, although BAT is present at birth, it only matures 1–2 days after birth. Moreover, in mice, rats, and guinea pigs, BAT depots, including the interscapular BAT remain throughout life with little reduction in size. Contrastingly, larger species, like rabbits, sheep, cows, and humans are born with BAT stores, but most of these are gradually replaced by WAT after birth [13, 45, 46].
Sex differences in adipose tissue biology and obesity
Adipose tissue composition and location differs between sexes, with humans possibly displaying the most extreme example. The average human male body is comprised of 14% fat whereas the female body is comprised of 27% fat [47]. The disparity in adiposity can be observed as early as late gestation [48] and may indicate there are sex differences in mesenchymal cell fate decision towards the muscle versus adipocyte lineage.
There are also differences in localization of fat between sexes. Females typically have a gynoid fat distribution, where fat is stored around the hips and thighs, while males typically display an android form of fat distribution, where fat is stored around the waist [49, 50]. Increased adiposity in women produces a relatively benign form of obesity, whereas in males it is associated with higher risk for hypertension, insulin resistance, diabetes, dyslipidemia, and heart disease [51]. The higher risk associated with the android form of obesity is thought to be caused by leak of fatty acids from adipose into the portal vein, leading to accumulation of fat in ectopic organs and subsequent lipotoxicity. The differences in adipose location between sexes are thought to be largely caused by estrogen, as postmenopausal women develop a more android distribution.
Sexual dimorphism is far less documented in other species. Like humans, female rodents harbor greater fat mass compared to males [52, 53]. In addition, ovariectomized female mice demonstrate a similar patterning of adipose tissue to males [52]. Furthermore, unlike their male counterparts, several strains of female mice are relatively resistant to obesity on a high-fat diet [54, 55], but this protection can be removed by ovariectomy [56, 57]. Thus, fat patterning in adult females is influenced by reproductive hormones. There are also differences in BAT composition between sexes; in rodents and humans, females have a higher proportion of BAT compared to males. Thus, adipose tissue is a sexually dimorphic tissue, which is influenced by sex hormones and modifies the risk of obesity.
Maternal nutritional restriction and offspring adiposity
Maternal nutritional restriction was the first type of dietary manipulation to be studied in the context of DOHaD. The long-term consequences of the 1944 Dutch famine was the subject of the original epidemiological studies that led to the DOHaD hypothesis. This work found that exposure to famine particularly in the first trimester of pregnancy was associated with an increased risk of the offspring developing obesity in adulthood [58, 59].
Maternal nutrient and caloric restriction in a number of animal models has also been found to programme the offspring for increased adiposity ([60, 61] and Table 1). Moreover, there is evidence that this increase in adiposity is related to an increase in brown and beige adipose tissue in particular species, namely guinea pigs [62], as well as adipocyte hypertrophy in other species, like cows and baboons [61, 63]. Enhanced adiposity is also accompanied by changes in PPAR and UCP1/2 abundance in the adipose of offspring from undernourished mothers, however the timing of the maternal nutrient restriction seems to determine the nature of the molecular changes and level of adiposity [60, 63,64,65]. There are also programmed changes in the expression of mitochondrial-related genes, like PGC-1α and lipid handling genes, like FATP1 in the offspring adipose [61, 63]. Maternal nutrient restriction is often accompanied by decreased fetal growth or birth weight of the offspring [60, 62, 63, 65]. Thus, the greater propensity to become obese may reflect metabolic adaptations to optimize growth when nutrient supply is limited in utero, but may be mal-adaptive and lead to excess storage of nutrients when nutrient supply is not limited postnatally.
Symonds et al. found the timing of a nutrient restriction in sheep plays a role in determining the offspring outcome [26]. They found that exposure to maternal nutrient restriction up to day 110 of sheep gestation (term = 145 days) promoted adipose tissue deposition in the fetal lamb at term, whereas continuing the nutrient restriction after this time decreased adipose tissue mass. Bisham et al. [66] found that restoring maternal diets to the same as controls after day 80 of gestation further stimulated fat deposition in the fetus. Increased adiposity despite exposure to maternal nutrient restriction was accompanied by an increased abundance of IGF receptors, suggesting enhanced adipose tissue sensitivity to the anabolic effects of IGFs [67]. However, further information on the cellular, metabolic, and molecular changes governing alterations in offspring adiposity with maternal nutrient restriction, especially with regards to timing of insult, require investigation.
Maternal low protein diets and offspring adiposity
Studying the impact of gestational exposure to maternal low protein foods, may help to understand the recent rise in the rates of obesity and other metabolic diseases in many countries [68]. Observational studies in humans have shown that low protein consumption during pregnancy correlates with fetal growth restriction [69]. In addition, studies in areas, such as South India where many women are vegetarian, have shown a correlation between reduced milk protein intake and low birth weight [70].
In contrast to the human studies, there is a large volume of animal studies that have investigated the effects of a maternal low protein diet on offspring adipose tissue (Table 2). These involve examining the effects of a low protein diet for the entirety of pregnancy, at particular stages in gestation and continuing the low protein diet during lactation and across generations. Some studies also expose the offspring to different nutrient manipulations postnatally. Overall, the available data suggest that maternal low protein diets have a complex effect on adipose tissue biology, with studies finding divergent molecular changes [71, 72].
Across various species, maternal low protein diet appears to have a two stage impact on offspring adiposity (Table 2). Maternal low protein diets tend to reduce offspring birth weight and adiposity in early life [71, 73], and may even be protective against the development of obesity in juvenile offspring fed a high fat or obesogenic diet postnatally [74]. However, offspring from low protein fed mothers accumulate greater amounts of fat as they mature [75, 76] and often display insulin resistance and hyperglycemia in adulthood [77, 78]. Furthermore, as they get older, offspring from protein restricted mothers have a greater sensitivity to the obesogenic effect and metabolic dysfunction induced by chronic exposure to high-fat diet [79]. However, data from pigs has shown that calorically restricting offspring exposed to a maternal low protein diet during gestation prevents the programming of increased adiposity postnatally, although molecular changes are still evident in the adipose tissue [74].
Studies in rats have shown that the effect of a maternal low protein diet on offspring adiposity is still observed even when the offspring is cross-fostered [80]. Moreover, there are greater increases in fat mass in first and second generation males from mothers that had been subjected to protein restriction during just gestation rather than gestation and lactation [77]. These data highlight the importance of nutrient supply in utero in determining offspring adiposity, with possible consequences for the offspring in subsequent generations. Indeed in sheep, even maternal protein restriction during very late gestation can predispose offspring for enhanced visceral adiposity in postnatal life [73]. Work in cows also suggest that the timing of maternal protein intake imbalance is an important determinant of offspring adiposity [81].
Across the species surveyed, changes in offspring adiposity are accompanied by alterations in adipocyte size or number, insulin signaling and metabolic protein levels, including PPARγ, as well as inflammatory markers and leukocytes in the adipose tissue of the offspring born from low protein diet-fed mothers [71, 72, 78, 82]. Importantly there are changes in leptin, adiponectin, and other important hormones like IGFs in the adipose of offspring [81, 83], which may contribute to their enhanced adiposity postnatally. Finally, although less explored, offspring of low protein diet-fed mothers show increased BAT thermogenic activity and UCP1 expression [79], which may provide some explanation for their initial protection against high-fat-diet-induced obesity as young adults.
There is some evidence to suggest that adipose tissue biology and obesity in female and male offspring may be differentially programmed by exposure to a maternal low protein diet during development [73, 81, 84] however, further work is required.
Maternal obesity/obesogenic diets and offspring adiposity
With the incidence of obesity among women of child-bearing age rising, assessing the effect of maternal obesogenic diets on offspring metabolic outcomes is particularly relevant. In humans, maternal obesity is associated with an increased risk of their child having increased adiposity during infancy and obesity in adulthood [85]. Compelling evidence showing a role for developmental programming rather than genetic factors in the maternal transmission of obesity comes from studies of women who have undergone bariatric surgery to reduce weight; siblings born of women post surgery displayed a reduced prevalence of obesity compared to those born before surgery [86, 87].
Animal studies assessing the effect of maternal obesity/obesogenic diets on offspring adiposity broadly seem to support human association data and show consistent outcomes across species (Table 3). Offspring from obese/high-fat diet-fed mothers show increased adiposity [88,89,90] and adipocyte hypertrophy postnatally [89, 91]. They also often show increased inflammatory marker expression and a higher infiltration of macrophages and other immune cells in their adipose tissue [89, 91, 92], which may reflect lipotoxicity and likely contributed to the glucose intolerance seen in such offspring. Indeed, offspring from mothers fed obesogenic diet often also demonstrate decreased insulin signaling protein levels in their WAT [89, 93].
Work in mice suggests that the duration of maternal high fat diet consumption has little effect on the magnitude of changes in offspring adiposity, as long as the diet was consumed within 9 weeks of mating [89]. Other work has shown that offspring of mothers consuming an obesogenic diet during gestation may be more prone to dietary-induced changes postnatally. For instance, rat offspring exposed to maternal obesity show greater morphological changes, including enhanced adipocyte hyperplasia in response to a postnatal high-fat diet [94]. Finally, some research suggests that programmed changes in offspring adipose biology may be permanent, as an exercise intervention in mothers fed a high-fat diet did not prevent the increased adiposity or trigger beneficial changes in insulin signaling component expression in their fetuses [95]. However, whether there are beneficial impacts of maternal exercise in ameliorating the impacts of maternal obesity/obesogenic diets on offspring later in life warrants investigation.
No studies have explored whether maternal obesity/obesogenic diets programme changes in offspring BAT. Similarly, whether maternal obesity/obesogenic diets programme offspring adipose biology and obesity in a sex-dependent manner have been under investigated. Of the studies that have, there are differences in adipose tissue morphology and leukocyte influx, as well as metabolic phenotype between exposed male and female offspring [92, 96]. However, the mechanisms underlying sexually dimorphic alterations in programmed outcomes remains unexplored.
Hypoxia and offspring adiposity
Hypoxia can have many deleterious effects on a developing fetus and can be caused by a variety of situations, such as placental insufficiency, maternal anemia, smoking, and increased altitude [97, 98]. In humans, cigarette smoking in early pregnancy increased the likeliness of the infant being overweight at 3 years of age [99]. Experimentally, hypoxia can also be induced by placing animals in chambers where the availability of oxygen is reduced. The impact of hypoxia on the developing fetus depends on a variety of factors, including the severity of the hypoxic episode, duration, and stage of gestation, however these have not be studied in the context of programmed changes in offspring adipose biology and obesity risk. In the studies available (Table 4), exposure to hypoxia during gestation was linked to increased offspring adiposity in adulthood [100, 101]. This was related to an elevated expression of pro-inflammatory markers in the adipose tissue of hypoxia exposed rodent offspring compared to their normoxic counterparts [100]. Exposure to hypoxia during intrauterine development was also associated with molecular changes, including enhanced UCP1, DIO, and PPARγ expression in the adipose of sheep fetuses, suggesting the adipose may be programmed towards the beige phenotype [102]. However, further work is required to assess the impact of gestational hypoxia and influence of offspring sex.
Endocrine disruptors and offspring adiposity
In recent years, there has been an increasing body of research into the effects of endocrine disruptors on human health and development. The focus of these studies is on synthetic chemicals known as persistent organic pollutants due to their resistance to environmental and biological degradation and accumulation in the environment and biological tissues [103]. These include; bisphenol A (BPA), which is found in clear plastics, and phalates (such as di-2-ethylexyl phalate, DEHP), which are found in products, such as shampoos, moisturizers, and liquid soaps. Both these chemicals can disrupt biological processes by acting as endocrine disruptors. There is accumulating evidence from human and animal studies that shows associations between exposure to organic pollutants and other widely diffused toxins to an increased risk of metabolic diseases, including obesity [104]. These synthetic chemicals are thought to promote obesity in children and adults by increasing adipogenesis [105, 106]. There is evidence that BPA can traverse the placenta and reach the fetus in humans and other species [107, 108]. In humans, maternal urinary BPA has been associated with increased hip to waist ratios in the offspring, an affect that was more severe in females [109].
Animal studies of BPA and phalate exposure in utero have consistently shown to increase offspring adiposity in later life (Table 5 and [106, 110,111,112]). In part, these changes are associated with enhanced adipocyte size, infiltration of inflammatory leukocytes, and elevated expression of lipogenic markers, like PPAR-γ and SREBP [113]. Developmental exposure to BPA has also been linked to enhanced levels of oxidative stress in the adipose of exposed offspring however, such an effect was not prevented by exposure to a perinatal Mediterranean diet that is known to offer protection against oxidative stress [114]. There may also be a greater number of adipocytes in the BAT of adult mouse offspring exposed to phalate during gestational and lactational development [111]. Likely due to their endocrine disrupting effects, BPA and phalates have been shown to exert sex-dependent impacts on offspring adiposity [106, 110]. However, due to the low number of studies that have specifically looked at sex-specific effects, firm conclusive results are yet to be made. Given the available data and their persistence in tissues, organic pollutants, like BPA and phalates possibly play a key role in the rapid epidemiological growth of obesity. However, more studies are required to examine this.
Glucocorticoids and offspring adiposity
Glucocorticoids are a class of steroid hormones that are part of the hypothalamic pituitary adrenal axis and play an important role in fetal development, including the maturation of fetal organs prior to birth [115, 116]. However, excess exposure to glucocorticoids during gestation can slow growth and development of the fetus with negative effects on offspring health and developmental programming. Maternal stress, anxiety and depression during pregnancy have been shown to increase fetal exposure to glucocorticoids [117]. The use of synthetic glucocorticoids to advance lung maturation in pregnancies with threatened preterm birth also increases fetal exposure to glucocorticoids. In humans, maternal second trimester levels of corticotropin-releasing hormone, a hormone that stimulates the synthesis of glucocorticoids, are positively correlated with central adiposity in infants at 3 years of age [118]. In animal studies, excess glucocorticoid exposure in utero was consistently shown to programme the offspring for increased adiposity (Table 6 and [119,120,121,122,123]). This was coupled with alterations in adipocyte morphology, expression of lipogenic genes, insulin signaling proteins and inflammatory cytokine expression in the offspring WAT [124]. There were also cellular and molecular changes in the BAT of offspring over-exposed to glucocorticoids prenatally, namely decreased UCP1 expression, increased lipid droplet size, upregulated prolactin receptor and decreased mitochondria content, which would be expected to compromise tissue function [64, 125]. However, sex effects have not been explored and further work is required to identify the importance of length, timing and level of glucocorticoid over-exposure during gestation to the developmental programming of offspring adipose biology and obesity.
Androgens and offspring adiposity
Androgens are steroid hormones that typically regulate the development and maintenance of male characteristics. Possibly the most well-known and typically investigated androgen in developmental studies is testosterone. As a lipid-soluble hormone, testosterone is thought to be able to cross the placenta and this notion is supported by data showing that fetal plasma testosterone concentration is positively correlated with maternal testosterone [126]. For obvious reasons, in utero androgen exposure is particularly harmful to female offspring. There have been many studies showing that excess testosterone in utero contributes to the development of polycystic ovary syndrome (PCOS), which is estimated to affect 10–20% of women in the UK [127]. Therefore, androgen exposure in utero is thought to be both a cause and a consequence of PCOS, impacting the transmission of the disease [128]. PCOS increases the risk of type 2 diabetes and obesity in women, thus in utero androgen exposure may both directly and indirectly have an impact on adiposity in later life [129, 130]. However, PCOS is not the only cause of excess androgen exposure in utero; female dizygotic twins may also be exposed due to excess androgens from their male twin during gestation. Moreover, maternal testosterone concentration has been shown to negatively correlate with birth weight and BMI in female offspring [131].
As shown in Table 7, work in experimental animals has shown that excess androgen exposure in utero or in early neonatal life programmes the female offspring to have changes in adipose tissue biology [132,133,134,135]. There is a change in the size of WAT adipocytes [132, 136] and an increase in chemokine and pro-inflammatory cytokine expression and dysregulated adiponectin expression, depending on the timing of the androgen exposure and WAT depot in female offspring [132, 137]. Androgen exposed female offspring also seem to have an additional increase in pro-inflammatory cytokines when fed an obesogenic diet postnatally [137]. Exposure of female offspring to testosterone neonatally leads to increased BAT which is dysfunctional [136], although whether prenatal exposure also affects BAT phenotype postnatally has yet to be explored. There is also a greater sensitivity of androgen exposed female offspring to increase their body weight with an obesogenic diet postnatally [137]. However, further work is required to identify the importance of timing, duration, and level of androgen over-exposure during gestation, versus in the neonatal period to the programming of offspring adiposity, in relation to the pathogenesis of metabolic disorders like obesity and PCOS.
Programming mechanisms of adipose tissue
The programming mechanisms behind changes in adipose remain elusive. The studies which do explore this broadly involve evaluating epigenetic changes in the adipose tissue and studying the adipogenic potential of mesenchymal stem cells from exposed offspring. However, as WAT develops postnatally in some species like rats and mice, changes induced by maternal manipulations may also be the result of alterations in other systems, particularly the hypothalamic pituitary adrenal hormonal axis in the offspring during gestation [138]. Indeed, as described above, there are links between glucocorticoid over-exposure in utero and the programing of obesity in the offspring.
Epigenetic mechanisms
Suboptimal maternal environments, particularly those which occur during critical developmental windows may permanently alter gene expression in offspring tissues via epigenetic mechanisms. Such mechanisms include changes in DNA methylation, histone modifications, and the expression of noncoding RNAs. Certainly, a number of studies exploring fetal programming of adipose tissue have suggested that each of these epigenetic processes may contribute.
In the WAT of males from obese rats, there are alterations in DNA methylation of CpG sites proximal to C/EBP-β and Zfp423 genes, which encode key transcriptional factors initiating adipogenic commitment and is consistent with the increased expression of key adipogenic regulators, PPAR-γ, C/EBP-α, and C/EBP-β [139]. In another study, exposure to maternal obesity was also associated with lower Zfp423 promoter methylation levels and increased Zfp423 gene expression in offspring adipose tissue in alliance with enhanced adiposity [140]. In addition, a maternal low protein diet throughout gestation and lactation lead to alterations in the methylation of CpG sites proximal to the leptin gene in the adult offspring [141] and likely contributed to the abnormalities in adipose tissue biology seen in other work on the model. Some studies have also shown that changes in offspring adiposity with gestational hypoxia are coupled with changes in DNA methylation patterns in the adipose tissue [101, 142]. There was also increased DNA methylation of the Ppargc1a promoter in neonatal BAT and brown adipocyte progenitor cells, which was linked with attenuated BAT development in response to glucocorticoid over-exposure during gestation [125]. Finally, the methylation of multiple genes was aberrant in the visceral WAT of infant and adult offspring exposed to excess androgens, which likely contributed to the development of obesity and PCOS in the experimental model [133].
Maternal obesity and high-fat diets have also been associated with alterations in histone modifications in the adipose tissue of adult offspring. Of note, maternal obesity was associated with increased H3K4me1/H3K27ac histone modifications in enhancer sites upstream of the leptin gene, which correlated with enhanced leptin expression in the WAT of male offspring [143]. Moreover, a maternal high-fat diet during pregnancy lead to alterations in histone modifications at the promoter regions of the adiponectin and leptin gene in the WAT of offspring [144].
Noncoding RNAs have also been shown to play a role in the fetal programming of adipose tissue. For example, maternal obesity was associated with upregulated expression of miR-126, which negatively regulates insulin receptor susbtrate-1 (IRS1) and this was correlated to reduced IRS1 abundance and insulin sensitivity of the WAT in exposed offspring [93]. Furthermore, maternal low protein diets during gestation were associated with an increase in miRNA-483-3p levels, which is known to reduce adipose adipogenesis [145].
Mesenchymal studies
To inform on the programming of obesity, mesenchymal stem cells have been harvested from the umbilical cord of offspring exposed to different in utero/maternal environments. Previous work has shown there is a positive correlation between the preference of mesenchymal stem cell fate decisions towards the adipocyte lineage and infant adiposity [88, 146]. In addition, mesenchymal stem cells from infants of obese mothers showed a decrease in the abundance of β-catenin, which would favor adipocyte rather than myocyte differentiation [88]. Consistent with this, the differentiation of mesenchymal stem cells to adipocytes in vitro was increased and adiposity greater in 5-month-old infants from obese mothers [88]. Higher adiposity in infants of obese mothers was also linked to increased lipid species content and lipid transport gene expression in differentiated adipocytes and elevated oxidative stress, lower amino acid concentrations and expression of growth-promoting genes in their differentiated neonatal myocytes [146].
Summary and conclusions
Alterations to the in utero environment as a result of suboptimal maternal conditions are related to programmed changes in the development, structure, and function of adipose tissue in the offspring. A variety of nutritional manipulations and differing metabolic environments such as low protein, caloric/nutrient restriction, obesity/obesogenic diets have all been shown to impact offspring adipose structure and function. Non-nutritional changes, such as hypoxia and exposure to hormones and pollutants/synthetic chemicals have also been shown to affect adipose tissue in the offspring. The outcomes of these manipulations differ and are dependent on the manipulation itself, that is, its timing, duration, and severity. Across a variety of mammalian species, the impacts of certain manipulations, such as low protein diet remains relatively consistent increasing the reliability of this data. However, broadly speaking, while the majority of investigations available studied programmed changes in whole body metabolism, the adipose tissue was examined in a very limited capacity. The majority of studies also analyse only one fat depot and as discussed, these can vary greatly and are reflective of the organ they are associated with. In addition, certain adipose pads, particularly the subcutaneous adipose vary with offspring sex. The focus of investigations also tend to be on WAT so the effect of maternal manipulations on the programming of BAT development and function remains relatively unknown.
The impact of sex on the fetal programming of adipose tissue has been relatively under-studied. The majority of the studies only investigated male offspring and of the studies that do include female offspring, very few compare sexes. The studies which do compare sexes frequently find disparities in offspring outcomes. Given the multiple differences in adipose tissue between males and females previously discussed, this is unsurprising. However, the mechanisms underlying these disparities in outcome from the same in utero environment remain uninvestigated. There have been speculations that some sex differences may be partly due to sex-specific epigenetic modifications operating at the level of the placenta during development. The placenta is the functional interface between mother and fetus and the main determinant of maternal nutrient, oxygen, and hormonal supply to the fetus during gestation [9, 147]. Of note, Gallou-Kabani et al. showed that the global methylation pattern of the placenta was different between male and females, even though they were exposed to the same in utero environment [148]. However, whether a placenta-specific manipulation is sufficient to programme alterations in offspring adipose biology and obesity and do so, in a manner influenced by fetal sex, are yet to be studied. Indeed, previous work has shown that several of the maternal environmental manipulations surveyed here affect placental formation and function in association with changes in fetal growth and offspring phenotype [9, 147, 149,150,151,152]. The placenta likely plays a major role in mediating the programmed alterations in offspring adipose development and function, and hence, susceptibility to develop obesity.
While many studies examine if maternal environmental challenges are linked to changes in adiposity or adipocyte biology in the offspring, the mechanisms underlying these programming impacts remains largely unknown. Generally, the molecular analyses performed are targeted and focus on either adipose inflammation or insulin signaling pathways, with few studies undertaking wider capturing analyses, such as RNA sequencing, which would provide a greater understanding of underlying programming effects. In addition, mechanisms by which obesity may be programmed across generations is largely unexplored. Where there have been studies into transgenerational inheritance, some studies have found robust changes, suggesting a change in the offspring heritable epigenome. For example, in mice, female progeny of obese dams exposed to high-fat diet displayed obesity and high levels of WAT inflammation in association with hypomethylation at certain inflammatory genes for three generations [153]. Furthermore, investigations of the Dutch famine cohort found indications of transgenerational transmission of obesity in humans [154]. Thus, perhaps the most significant avenue for the future of this field, is designing studies to further elucidate the cellular and epigenetic mechanisms underlying fetal programming and transgenerational effects. This is important as the adipose tissue plays a fundamental role in whole body homeostasis and metabolism and the potential transgenerational inheritance of obesity maybe one of the factors contributing to its increase in many populations worldwide.
Given the current epidemic levels of metabolic diseases across the globe, the central focus of research in this area should be on understanding the mechanisms of fetal programming in a way to create an optimal in utero environment to prevent and alleviate disease transmission. This requires a better understanding of the impact of diet and hormone exposures on the developing adipose tissue in humans, as well as a greater knowledge of the influence these may have on critical events, such as mesenchymal stem cell fate decisions. It also requires a better understanding of the plasticity of adipose tissue across the life course, as this will help inform on what interventions may be beneficial in combatting the effects of a poor in utero environment on the developmental programming of offspring obesity risk. There have already been interesting studies in this area, including data showing that maternal dietary supplementation with methyl donors during gestation or lactation partially prevented the development of an obese phenotype in the offspring from high sucrose diet-fed mothers [155]. This “deprogramming” of offspring phenotype via such an approach may prove to be a promising strategy to overcome the transgenerational transmission of obesity and help curb the current obesity epidemic and the high costs to health services worldwide.
Change history
10 May 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41366-021-00828-z
References
Organisation WH. Obesity and overweight 2020. Updated 1 April 2020. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6.
Pugazhenthi S, Qin L, Reddy PH.Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim et Biophys Acta. 2017;1863:1037–45.
Van Gaal LF, Mertens IL, De, Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–80.
Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer—mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10:455–65.
Fenton K. Obesity and the food environment. Public Health England. Health Matters. GOV.UK. 2017. Accessed Date 31 March 2017. https://ublichealthmatters.blog.gov.uk/2017/03/31/health-matters-obesity-and-the-food-environment/.
Barker DJ, Clark PM. Fetal undernutrition and disease in later life. Rev Reprod. 1997;2:105–12.
Schoettl T, Fischer IP, Ussar S. Heterogeneity of adipose tissue in development and metabolic function. J Exp Biol. 2018;221:jeb162958.
Sferruzzi-Perri AN, Camm EJ. The programming power of the placenta. Front Physiol. 2016;7:33.
Christoforou ER, Sferruzzi-Perri AN. Molecular mechanisms governing offspring metabolic programming in rodent models of in utero stress. Cell Mol Life Sci. 2020;77:4861–98.
Fliers E, Kreier F, Voshol PJ, Havekes LM, Sauerwein HP, Kalsbeek A, et al. White adipose tissue: getting nervous. J Neuroendocrinol. 2003;15:1005–10.
Gessner K. Conradi Gesneri Medici Tigurine Historiae Animalium: Lib. I De Quadrupedibus Viviparis. 1551.
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–17.
Oberkofler H, Dallinger G, Liu YM, Hell E, Krempler F, Patsch W. Uncoupling protein gene: quantification of expression levels in adipose tissues of obese and non-obese humans. J Lipid Res. 1997;38:2125–33.
Matthias A, Ohlson KBE, Fredriksson JM, Jacobsson A, Nedergaard J, Cannon B. Thermogenic responses in brown fat cells are fully UCP1-dependent. J Biol Chem. 2000;275:25073–81.
Géloën A, Collet AJ, Guay G, Bukowiecki LJ. In vivo differentiation of brown adipocytes in adult mice: An electron microscopic study. Am J Anat. 1990;188:366–72.
Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Pnas. 2007;104:4401–6.
Prins JB, O’Rahilly S. Regulation of adipose cell number in man. Clin Sci. 1997;92:3–11.
Hirsch J, Batchelor B. Adipose tissue cellularity in human obesity. Clin Endocrinol Metabol. 1976;5:299–311.
Yang X, Heckmann BL, Zhang X, Smas CM, Liu J. Distinct mechanisms regulate ATGL-mediated adipocyte lipolysis by lipid droplet coat proteins. Mol Endocrinol. 2013;27:116–26.
Jeremic N, Chaturvedi P, Tyagi SC. Browning of white fat: novel insight into factors, mechanisms, and therapeutics. J Cell Physiol. 2017;232:61–8.
Villarroya F, Cereijo R, Villarroya J, Giralt M. Brown adipose tissue as a secretory organ. Nat Rev Endocrinol. 2017;13:26–35.
Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–39.
Widdowson EM. Chemical composition of newly born mammals. Nat. 1950;166:626–8.
Raffel M, Trillmich F, Houner A. Energy allocation in reproducing and non-reproducing guinea pig (Cavia porcellus) females and young underad libitumconditions. J Zool. 1996;239:437–52.
Symonds ME, Pearce S, Bispham J, Gardner DS, Stephenson T. Timing of nutrient restriction and programming of fetal adipose tissue development. Proc Nutr Soc. 2004;63:397–403.
Vernon RG, Flint DJ, Stock MJ, Cinti, S. Encyclopedia of Food Science and Nutrition. 2nd Edition. Caballero B, Finglas P, Toldra F, editors. Massachusetts: Academic Press; 2003.
Jenness R. The composition of human milk. Semin Perinatol. 1979;3:225–39.
Morrison JL, Botting KJ, Darby JRT, David AL, Dyson RM, Gatford KL, et al. Guinea pig models for translation of the developmental origins of health and disease hypothesis into the clinic. J Physiol. 2018;596:5535–69.
Yoxall AT, Hird JFR. Physiological basis of small animal medicine. 1st Edition. Yoxall AT, Hird JFR, editors. Boston: Blackwell Scientific Publications; 1980.
McCance RA, Widdowson EM. Fat. Pediatr Res. 1977;11:1081–3.
Lindmark-Månsson H, Fondén R, Pettersson H-E. Composition of Swedish dairy milk. Intl Dairy J. 2003;13:409–25.
Manners MJ, McCrea MR. Changes in the chemical composition of sow-reared piglets during the 1st month of life. Br J Nutr. 1963;17:495.
Gardner DS, Buttery PJ, Daniel Z, Symonds ME. Factors affecting birth weight in sheep: maternal environment. Reproduction. 2007;133:297–307.
Alexander G, Bennett JW, Gemmell RT. Brown adipose tissue in the new-born calf (Bos taurus). J Physiol. 1975;244:223–34.
Hudson DG, Hull D. Growth of adipose tissue in the fetal rabbit. Biol Neonate. 1975;27:71–9.
Zhang S, Chen F, Zhang Y, Lv Y, Heng J, Min T, et al. Recent progress of porcine milk components and mammary gland function. J Anim Sci Biotechnol. 2018;9:77.
Hausman GJ, Kauffman RG. The histology of developing porcine adipose tissue. J Anim Sci. 1986;63:642–58.
Symonds ME, Bryant MJ, Clarke L, Darby CJ, Lomax MA. Effect of maternal cold exposure on brown adipose tissue and thermogenesis in the neonatal lamb. J Physiol. 1992;455:487–502.
Symonds ME, Lomax MA. Maternal and environmental influences on thermoregulation in the neonate. Proc Nutr Soc. 1992;51:165–72.
Symonds ME, Bispham J, Dandrea J, Heasman L, Wilson V, Stephenson T. Maternal nutrition and endocrine programming of fetal adipose tissue development. Biochem Soc Transac. 1999;27:A7–A.
Lorenzo M. IGF-I is a mitogen involved in differentiation-related gene expression in fetal rat brown adipocytes. J Cell Biol. 1993;123:1567–75.
Yuen B. Abundance of leptin mRNA in fetal adipose tissue is related to fetal body weight. J Endocrinol. 1999;163:R11–R4.
Han J, Lee JE, Jin J, Lim JS, Oh N, Kim K, et al. The spatiotemporal development of adipose tissue. Development. 2011;138:5027–37.
Dawkins MJR, Hull D. Brown adipose tissue and the response of new-born rabbits to cold. J Physiol. 1964;172:216–38.
Hull D. The function of brown adipose tissue in the newborn. Biochem Soc Trans. 1976;4:226–8.
Jackson A, Stanforth P, Gagnon J, Rankinen T, Leon A, Rao D, et al. The effect of sex, age and race on estimating percentage body fat from body mass index: The Heritage Family Study. Int J Obes. 2002;26:789–96.
Fields DA, Krishnan S, Wisniewski AB. Sex differences in body composition early in life. Gender Medicine. 2009;6:369–75.
Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues—the biology of pear shape. Biol Sex Differ. 2012;3:13.
Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes. 2010;34:949–59.
Pi-Sunyer FX. The epidemiology of central fat distribution in relation to disease. Nutr Rev. 2004;62:S120–S6.
Grove KL, Fried SK, Greenberg AS, Xiao XQ, Clegg DJ. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int J Obes. 2010;34:989–1000.
Macotela Y, Boucher J, Tran TT, Kahn CR. Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes. 2009;58:803–12.
Nishikawa S, Yasoshima A, Doi K, Nakayama H, Uetsuka K. Involvement of sex, strain and age factors in high fat diet-induced obesity in C57BL/6J and BALB/cA mice. Exp Anim. 2007;56:263–72.
Hwang L-L, Wang C-H, Li T-L, Chang S-D, Lin L-C, Chen C-P, et al. Sex differences in high-fat diet-induced obesity, metabolic alterations and learning, and synaptic plasticity deficits in mice. Obesity. 2010;18:463–9.
Ludgero-Correia A, Aguila MB, Mandarim-De-Lacerda CA, Faria TS. Effects of high-fat diet on plasma lipids, adiposity, and inflammatory markers in ovariectomized C57BL/6 mice. Nutrition. 2012;28:316–23.
Blaustein JD, Gentry RT, Roy EJ, Wade GN. Effects of ovariectomy and estradiol on body weight and food intake in gold thioglucose-treated mice. Physiol Behav. 1976;17:1027–30.
Roseboom TJ, Van Der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Bleker OP. Plasma lipid profiles in adults after prenatal exposure to the Dutch famine. Am J Clin Nutr. 2000;72:1101–6.
Roseboom TJ. Coronary heart disease after prenatal exposure to the Dutch famine, 1944-45. Heart. 2000;84:595–8.
Bispham J, Gardner DS, Gnanalingham MG, Stephenson T, Symonds ME, Budge H. Maternal nutritional programming of fetal adipose tissue development: differential effects on messenger ribonucleic acid abundance for uncoupling proteins and peroxisome proliferator-activated and prolactin receptors. Endocrinology. 2005;146:3943–9.
Long NM, Tousley CB, Underwood KR, Paisley SI, Means WJ, Hess BW, et al. Effects of early- to mid-gestational undernutrition with or without protein supplementation on offspring growth, carcass characteristics, and adipocyte size in beef cattle1. J Anim Sci. 2012;90:197–206.
Ashwell M, Purkins L, Cowen T, Day KC. Pre- and postnatal development of adipose tissue at four sites in the guinea pig: effect of maternal diet restriction during the second half of pregnancy. Ann Nutr Metab. 1987;31:197–210.
Tchoukalova YD, Krishnapuram R, White UA, Burk D, Fang X, Nijland MJ, et al. Fetal baboon sex-specific outcomes in adipocyte differentiation at 0.9 gestation in response to moderate maternal nutrient reduction. Int J Obes. 2014;38:224–30.
Bispham Heasman, Clarke Ingleton. Stephenson, symonds. effect of maternal dexamethasone treatment and ambient temperature on prolactin receptor abundance in brown adipose and hepatic tissue in the foetus and new-born lamb. J Neuroendocrinol. 2001;11:849–56.
Budge H, Edwards LJ, McMillen IC, Bryce A, Warnes K, Pearce S, et al. Nutritional manipulation of fetal adipose tissue deposition and uncoupling protein 1 messenger RNA abundance in the sheep: differential effects of timing and duration. Biol Reprod. 2004;71:359–65.
Bispham J, Gopalakrishnan GS, Dandrea J, Wilson V, Budge H, Keisler DH, et al. Maternal endocrine adaptation throughout pregnancy to nutritional manipulation: consequences for maternal plasma leptin and cortisol and the programming of fetal adipose tissue. Development. 2003;144:3575–85.
Teruel T, Valverde AM, Benito M, Lorenzo M. Insulin-like growth factor I and insulin induce adipogenic-related gene expression in fetal brown adipocyteprimary cultures. Biochem J. 1996;319:627–32.
Branca DF. Malnutrition: it’s about more than hunger 2017. https://www.who.int/news-room/commentaries/detail/malnutrition-it-s-about-more-than-hunger.
Kramer MS, Kakuma R. Energy and protein intake in pregnancy. Cochrane Database Syst Rev. 2003:1:Cd000032.
Mukhopadhyay A, Dwarkanath P, Bhanji S, Devi S, Thomas A, Kurpad AV, et al. Maternal intake of milk and milk proteins is positively associated with birth weight: a prospective observational cohort study. Clin Nutr ESPEN. 2018;25:103–9.
Pan S, Jia Y, Yang X, Cai D, Liu Z, Song H, et al. Amino acid starvation-induced autophagy is involved in reduced subcutaneous fat deposition in weaning piglets derived from sows fed low-protein diet during gestation and lactation. Eur J Nutr. 2018;57:991–1001.
Tarry-Adkins JL, Aiken CE, Ashmore TJ, Ozanne SE. Insulin-signalling dysregulation and inflammation is programmed trans-generationally in a female rat model of poor maternal nutrition. Sci Rep. 2018;8:4014.
Nielsen MO, Kongsted AH, Thygesen MP, Strathe AB, Caddy S, Quistorff B, et al. Late gestation undernutrition can predispose for visceral adiposity by altering fat distribution patterns and increasing the preference for a high-fat diet in early postnatal life. Br J Nutr. 2013;109:2098–110.
DuPriest EA, Lin B, Kupfer P, Sekiguchi K, Bhusari A, Quackenbush A, et al. Effects of postweaning calorie restriction on accelerated growth and adiponectin in nutritionally programmed microswine offspring. Am J Physiol Regul Integr Comp Physiol. 2018;315:R354–r68.
Guan H, Arany E, van Beek JP, Chamson-Reig A, Thyssen S, Hill DJ, et al. Adipose tissue gene expression profiling reveals distinct molecular pathways that define visceral adiposity in offspring of maternal protein-restricted rats. Am J Physiol Endocrinol Metab. 2005;288:E663–73.
Zhang T, Guan H, Arany E, Hill DJ, Yang K. Maternal protein restriction permanently programs adipocyte growth and development in adult male rat offspring. J Cell Biochem. 2007;101:381–8.
Alessandra, Isadora, Marcia, Carlos. Protein restriction during gestation and/or lactation causes adverse transgenerational effects on biometry and glucose metabolism in F1 and F2 progenies of rats. Clin Sci. 2008;114:381–92.
Kim J, Choi A, Kwon YH. Maternal protein restriction altered insulin resistance and inflammation-associated gene expression in adipose tissue of young adult mouse offspring in response to a high-fat diet. Nutrients. 2020;12:1103.
Dumortier O, Roger E, Pisani DF, Casamento V, Gautier N, Lebrun P, et al. Age-dependent control of energy homeostasis by brown adipose tissue in progeny subjected to maternal diet–induced fetal programming. Diabetes. 2017;66:627–39.
Berends LM, Fernandez-Twinn DS, Martin-Gronert MS, Cripps RL, Ozanne SE. Catch-up growth following intra-uterine growth-restriction programmes an insulin-resistant phenotype in adipose tissue. Int J Obes. 2013;37:1051–7.
Micke GC, Sullivan TM, McMillen IC, Gentili S, Perry VEA. Heifer nutrient intake during early- and mid-gestation programs adult offspring adiposity and mRNA expression of growth-related genes in adipose depots. Reproduction. 2011;141:697–706.
Xie L, Zhang K, Rasmussen D, Wang J, Wu D, Roemmich JN, et al. Effects of prenatal low protein and postnatal high fat diets on visceral adipose tissue macrophage phenotypes and IL-6 expression in Sprague Dawley rat offspring. PLOS ONE. 2017;12:e0169581.
Claycombe KJ, Uthus EO, Roemmich JN, Johnson LK, Johnson WT. Prenatal low-protein and postnatal high-fat diets induce rapid adipose tissue growth by inducing Igf2 expression in Sprague Dawley rat offspring. J Nutr. 2013;143:1533–9.
Bellinger L, Sculley DV, Langley-Evans SC. Exposure to undernutrition in fetal life determines fat distribution, locomotor activity and food intake in ageing rats. Int J Obes. 2006;30:729–38.
Kaar JL, Crume T, Brinton JT, Bischoff KJ, McDuffie R, Dabelea D. Maternal obesity, gestational weight gain, and offspring adiposity: the exploring perinatal outcomes among children study. J Pediatrics. 2014;165:509–15.
Smith J, Cianflone K, Biron S, Hould FS, Lebel S, Marceau S, et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J Clin Endocrinol Metab. 2009;94:4275–83.
Guenard F, Deshaies Y, Cianflone K, Kral JG, Marceau P, Vohl MC. Differential methylation in glucoregulatory genes of offspring born before vs. after maternal gastrointestinal bypass surgery. Proc Natl Acad Sci. 2013;110:11439–44.
Boyle KE, Patinkin ZW, Shapiro ALB, Baker PR, Dabelea D, Friedman JE. Mesenchymal stem cells from infants born to obese mothers exhibit greater potential for adipogenesis: The Healthy Start BabyBUMP Project. Diab. 2016;65:647–59.
Summerfield M, Zhou Y, Zhou T, Wu C, Alpini G, Zhang KK, et al. A long-term maternal diet transition from high-fat diet to normal fat diet during pre-pregnancy avoids adipose tissue inflammation in next generation. PLOS ONE. 2018;13:e0209053.
Lima MS, Perez GS, Morais GL, Santos LS, Cordeiro GS, Couto RD, et al. Effects of maternal high fat intake during pregnancy and lactation on total cholesterol and adipose tissue in neonatal rats. Braz J Biol. 2018;78:615–8.
Murabayashi N, Sugiyama T, Zhang L, Kamimoto Y, Umekawa T, Ma N, et al. Maternal high-fat diets cause insulin resistance through inflammatory changes in fetal adipose tissue. Eur J Obstet Gynaecol Reprod Biol. 2013;169:39–44.
Chang E, Hafner H, Varghese M, Griffin C, Clemente J, Islam M, et al. Programming effects of maternal and gestational obesity on offspring metabolism and metabolic inflammation. Sci Rep. 2019;9:16027.
Fernandez-Twinn DS, Alfaradhi MZ, Martin-Gronert MS, Duque-Guimaraes DE, Piekarz A, Ferland-Mccollough D, et al. Downregulation of IRS-1 in adipose tissue of offspring of obese mice is programmed cell-autonomously through post-transcriptional mechanisms. Mol Metab. 2014;3:325–33.
Šnajder D, Perić Kačarević Ž, Grgić A, Bijelić N, Fenrich M, Belovari T, et al. Effect of different combination of maternal and postnatal diet on adipose tissue morphology in male rat offspring. J Matern Fetal Neonatal Med. 2019;32:1838–46.
Raipuria M, Bahari H, Morris MJ. Effects of maternal diet and exercise during pregnancy on glucose metabolism in skeletal muscle and fat of weanling rats. PLOS ONE. 2015;10:e0120980.
Lemonnier D. Effect of age, sex, and site on the cellularity of the adipose tissue in mice and rats rendered obese by a high-fat diet. J Clin Investig. 1972;51:2907–15.
Fajersztajn L, Veras MM. Hypoxia: from placental development to fetal programming. birth defects. Research. 2017;109:1377–85.
Aljunaidy MM, Morton JS, Cooke C-LM, Davidge ST. Prenatal hypoxia and placental oxidative stress: linkages to developmental origins of cardiovascular disease. Am J Physiol Regul Integr Comp Physiol. 2017;313:R395–R9.
Oken E, Huh SY, Taveras EM, Rich-Edwards JW, Gillman MW. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res. 2005;13:2021–8.
Vargas VE, Gurung S, Grant B, Hyatt K, Singleton K, Myers SM, et al. Gestational hypoxia disrupts the neonatal leptin surge and programs hyperphagia and obesity in male offspring in the Sprague-Dawley rat. PLOS ONE. 2017;12:e0185272.
Khalyfa A, Cortese R, Qiao Z, Ye H, Bao R, Andrade J, et al. Late gestational intermittent hypoxia induces metabolic and epigenetic changes in male adult offspring mice. J Physiol. 2017;595:2551–68.
Myers DA, Hanson K, Mlynarczyk M, Kaushal KM, Ducsay CA. Long-term hypoxia modulates expression of key genes regulating adipose function in the late-gestation ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1312–R8.
Mallozzi M, Bordi G, Garo C, Caserta D. The effect of maternal exposure to endocrine disrupting chemicals on fetal and neonatal development: A review on the major concerns. Birth Defects Res Part C. 2016;108:224–42.
Bansal A, Henao-Mejia J, Simmons RA. Immune system: an emerging player in mediating effects of endocrine disruptors on metabolic health. Endocrinology. 2018;159:32–45.
Sargis RM, Johnson DN, Choudhury RA, Brady MJ. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity. 2010;18:1283–8.
Desai M, Ferrini MG, Jellyman JK, Han G, Ross MG. In vivo and in vitro bisphenol A exposure effects on adiposity. J Dev Origins Health Dis. 2018;9:678–87.
Balakrishnan B, Henare K, Thorstensen EB, Ponnampalam AP, Mitchell MD. Transfer of bisphenol A across the human placenta. Am J Obstet Gynecol. 2010;202:393.e1–e7.
Takahashi O, Oishi S. Disposition of orally administered 2,2-Bis(4-hydroxyphenyl)propane (Bisphenol A) in pregnant rats and the placental transfer to fetuses. Environ Health Perspect. 2000;108:931–5.
Braun JM, Li N, Arbuckle TE, Dodds L, Massarelli I, Fraser WD, et al. Association between gestational urinary bisphenol a concentrations and adiposity in young children: The MIREC study. Environ Res. 2019;172:454–61.
Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S, et al. Perinatal Exposure to Bisphenol A Alters Early Adipogenesis in the Rat. Environ Health Perspect. 2009;117:1549–55.
Lee K-I, Chiang C-W, Lin H-C, Zhao J-F, Li C-T, Shyue S-K, et al. Maternal exposure to di-(2-ethylhexyl) phthalate exposure deregulates blood pressure, adiposity, cholesterol metabolism and social interaction in mouse offspring. Arch Toxicol. 2016;90:1211–24.
Hunt BG, Wang Y-L, Chen M-S, Wang S-C, Waltz SE. Maternal diethylhexyl phthalate exposure affects adiposity and insulin tolerance in offspring in a PCNA-dependent manner. Environ Res. 2017;159:588–94.
Malaisé Y, Menard S, Cartier C, Gaultier E, Lasserre F, Lencina C, et al. Gut dysbiosis and impairment of immune system homeostasis in perinatally-exposed mice to Bisphenol A precede obese phenotype development. Sci Rep. 2017;7:14472.
Neier K, Marchlewicz EM, Bedrosian LD, Dolinoy DC, Harris C. Characterization of the mouse white adipose tissue redox environment and associations with perinatal environmental exposures to bisphenol A and high-fat diets. J Nutr Biochem. 2019;66:86–97.
Funkhouser JD, Hughes ER. Glucocorticoids and fetal lung development. Paediatr Pulmonol. 1977;8:519–24.
Fowden AL, Li J, Forhead AJ. Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance? Proc Nutr Soc. 1998;57:113–22.
Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. 2009;3:19.
Gillman MW, Rich-Edwards JW, Huh S, Majzoub JA, Oken E, Taveras EM, et al. Maternal corticotropin-releasing hormone levels during pregnancy and offspring adiposity*. Obesity. 2006;14:1647–53.
Long NM, Smith DT, Ford SP, Nathanielsz PW. Elevated glucocorticoids during ovine pregnancy increase appetite and produce glucose dysregulation and adiposity in their granddaughters in response to ad libitum feeding at 1 year of age. Am J Obstet Gynecol. 2013;209:353.e1–e9.
De Blasio MJ, Dodic M, Jefferies AJ, Moritz KM, Wintour EM, Owens JA. Maternal exposure to dexamethasone or cortisol in early pregnancy differentially alters insulin secretion and glucose homeostasis in adult male sheep offspring. Am J Physiol Endocrinol Metab. 2007;293:E75–E82.
Zulkafli IS, Waddell BJ, Mark PJ. Postnatal dietary omega-3 fatty acid supplementation rescues glucocorticoid-programmed adiposity, hypertension, and hyperlipidemia in male rat offspring raised on a high-fat diet. Endocrinology. 2013;154:3110–7.
Dahlgren J, Nilsson C, Jennische E, Ho H-P, Eriksson E, Niklasson A, et al. Prenatal cytokine exposure results in obesity and gender-specific programming. Am J Physiol Endocrinol Metab. 2001;281:E326–E34.
Weiler HA, Kovacs H, Murdock C, Adolphe J, Wong SF. Leptin Predicts Bone and Fat Mass after Accounting for the Effects of Diet and Glucocorticoid Treatment in Piglets1. Exp Biol Med. 2002;227:639–44.
Mark PJ, Wyrwoll CS, Zulkafli IS, Mori TA, Waddell BJ. Rescue of glucocorticoid-programmed adipocyte inflammation by omega-3 fatty acid supplementation in the rat. Reprod Biol Endocrinol. 2014;12:39.
Chen Y-T, Hu Y, Yang Q-Y, Son JS, Liu X-D, De Avila JM, et al. Excessive glucocorticoids during pregnancy impair fetal brown fat development and predispose offspring to metabolic dysfunctions. Diabetes. 2020;69:1662–74.
Gitau R. Fetal plasma testosterone correlates positively with cortisol. Arch Dis Child. 2005;90:F166–F9.
Hart R, Hickey M, Franks S. Definitions, prevalence and symptoms of polycystic ovaries and polycystic ovary syndrome. Best Prac Res Clin Obstet Gynaecol. 2004;18:671–83.
Abbott DH, Kraynak M, Dumesic DA, Levine JE. In utero androgen excess: a developmental commonality preceding polycystic ovary syndrome? Front Horm Res. 2019;53:1–17.
Moran C, Arriaga M, Rodriguez G, Moran S. Obesity differentially affects phenotypes of polycystic ovary syndrome. Int J Endocrinol. 2012;2012:1–7.
Wang ET, Calderon-Margalit R, Cedars MI, Daviglus ML, Merkin SS, Schreiner PJ, et al. Polycystic ovary syndrome and risk for long-term diabetes and dyslipidemia. Obstet Gynecol. 2011;117:6–13.
Huang G, Aroner SA, Bay CP, Gilman SE, Ghassabian A, Loucks EB, et al. Sex-dependent associations of maternal androgen levels with offspring BMI and weight trajectory from birth to early childhood. J Endocrinol Investig. 2020. https://doi.org/10.1007/s40618-020-01385-4.
Puttabyatappa M, Andriessen V, Mesquitta M, Zeng L, Pennathur S, Padmanabhan V. Developmental programming: impact of gestational steroid and metabolic milieus on mediators of insulin sensitivity in prenatal testosterone–treated female sheep. Endocrinology. 2017;158:2783–98.
Xu N, Kwon S, Abbott DH, Geller DH, Dumesic DA, Azziz R, et al. Epigenetic mechanism underlying the development of polycystic ovary syndrome (PCOS)-Like phenotypes in prenatally androgenized rhesus monkeys. PLoS ONE. 2011;6:e27286.
Nilsson C, Niklasson M, Eriksson E, Björntorp P, Holmäng A. Imprinting of female offspring with testosterone results in insulin resistance and changes in body fat distribution at adult age in rats. J Clin Investig. 1998;101:74–8.
Lu C, Cardoso RC, Puttabyatappa M, Padmanabhan V. Developmental programming: prenatal testosterone excess and insulin signaling disruptions in female sheep. Biol Reprod. 2016;94:113.
Nohara K, Waraich RS, Liu S, Ferron M, Waget A, Meyers MS, et al. Developmental androgen excess programs sympathetic tone and adipose tissue dysfunction and predisposes to a cardiometabolic syndrome in female mice. Am J Physiol Endocrinol Metab. 2013;304:E1321–30.
Gulan T, Yeernuer T, Sui S, Mayinuer N. A rat model of maternal polycystic ovary syndrome shows that exposure to androgens in utero results in dysbiosis of the intestinal microbiota and metabolic disorders of the newborn rat. Med Sci Monit. 2019;25:9377–91.
Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006;572:31–44.
Borengasser SJ, Zhong Y, Kang P, Lindsey F, Ronis MJJ, Badger TM, et al. Maternal obesity enhances white adipose tissue differentiation and alters genome-scale DNA methylation in male rat offspring. 2013;154:4113–25.
Yang QY, Liang JF, Rogers CJ, Zhao JX, Zhu MJ, Du M. Maternal obesity induces epigenetic modifications to facilitate Zfp423 expression and enhance adipogenic differentiation in fetal mice. Diabetes. 2013;62:3727–35.
Jousse C, Parry L, Lambert-Langlais S, Maurin AC, Averous J, Bruhat A, et al. Perinatal undernutrition affects the methylation and expression of the leptin gene in adults: implication for the understanding of metabolic syndrome. Faseb J. 2011;25:3271–8.
Badran M, Yassin BA, Lin DTS, Kobor MS, Ayas N, Laher I. Gestational intermittent hypoxia induces endothelial dysfunction, reduces perivascular adiponectin and causes epigenetic changes in adult male offspring. J Physiol. 2019;597:5349–64.
Lecoutre S, Oger F, Pourpe C, Butruille L, Marousez L, Dickes-Coopman A, et al. Maternal obesity programs increased leptin gene expression in rat male offspring via epigenetic modifications in a depot-specific manner. Mol Metabol. 2017;6:922–30.
Masuyama H, Hiramatsu Y. Effects of a high-fat diet exposurein uteroon the metabolic syndrome-like phenomenon in mouse offspring through epigenetic changes in adipocytokine gene expression. Endocrinology. 2012;153:2823–30.
Ferland-Mccollough D, Fernandez-Twinn DS, Cannell IG, David H, Warner M, Vaag AA, et al. Programming of adipose tissue miR-483-3p and GDF-3 expression by maternal diet in type 2 diabetes. Cell Death Differ. 2012;19:1003–12.
Baker PR 2nd, Patinkin ZW, Shapiro ALB, de la Houssaye BA, Janssen RC, Vanderlinden LA, et al. Altered gene expression and metabolism in fetal umbilical cord mesenchymal stem cells correspond with differences in 5-month-old infant adiposity gain. Sci Rep. 2017;7:18095.
Sferruzzi-Perri AN, Lopez-Tello J, Napso T, Yong HEJ. Exploring the causes and consequences of maternal metabolic maladaptations during pregnancy: Lessons from animal models. Placenta. 2020;98:43–51.
Gallou-Kabani C, Gabory A, Tost J, Karimi M, Mayeur S, Lesage J, et al. Sex- and diet-specific changes of imprinted gene expression and DNA Methylation inmouse placenta under a high-fat diet. PLoS ONE. 2010;5:e14398.
Fowden AL, Camm EJ, Sferruzzi-Perri AN. Effects of maternal obesity on placental phenotype. Curr Vasc Pharmacol. 2021;19:113–31.
Burton GJ, Fowden AL, Thornburg KL. Placental origins of chronic disease. Physiol Rev. 2016;96:1509–65.
Vaughan OR, Sferruzzi-Perri AN, Coan PM, Fowden AL. Environmental regulation of placental phenotype: implications for fetal growth. Reprod Fertil Dev. 2011;24:80–96.
Lu M, Sferruzzi-Perri AN. Placental mitochondrial function in response to gestational exposures. Placenta. 2020;104:124–37.
Ding Y, Li J, Liu S, Zhang L, Xiao H, LI J, et al. DNA hypomethylation of inflammation-associated genes in adipose tissue of female mice after multigenerational high fat diet feeding. Int J Obes. 2014;38:198–204.
Painter R, Osmond C, Gluckman P, Hanson M, Phillips D, Roseboom T. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG. 2008;115:1243–9.
Cordero P, Milagro FI, Campion J, Martinez JA. Supplementation with methyl donors during lactation to high-fat-sucrose-fed dams protects offspring against liver fat accumulation when consuming an obesogenic diet. J Dev Orig Health Dis. 2014;5:385–95.
Merrick D, Sakers A, Irgebay Z, Okada C, Calvert C, Morley MP, et al. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science. 2019;364:eaav2501.
Atit R, Sgaier SK, Mohamed OA, Taketo MM, Dufort D, Joyner AL, et al. β-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol. 2006;296:164–76.
Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–7.
Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Na Rev Mol Cell Biol. 2011;12:722–34.
Holness MJ, Sugden MC. Dexamethasone during late gestation exacerbates peripheral insulin resistance and selectively targets glucose-sensitive functions in β cell and liver. Endocrinology. 2001;142:3742–8.
Wyrwoll CS, Mark PJ, Waddell BJ. Developmental programming of renal glucocorticoid sensitivity and the renin-angiotensin system. Hypertension. 2007;50:579–84.
Acknowledgements
AR is supported by a PhD stipend covered by a Royal Society Fellows Enhancement Award to ANSP. This work was further supported by a Dorothy Hodgkin Research Fellowship, MRC New Investigator Grant and Lister Institute of Preventative Medicine Research Prize to ANSP. Figures were drawn with the use of Biorender.
Author information
Authors and Affiliations
Contributions
AR wrote the first draft of the paper which was edited by ANSP. Both authors approve the submitted version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rodgers, A., Sferruzzi-Perri, A.N. Developmental programming of offspring adipose tissue biology and obesity risk. Int J Obes 45, 1170–1192 (2021). https://doi.org/10.1038/s41366-021-00790-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-021-00790-w
This article is cited by
-
Epigenetics of the non-coding RNA nc886 across blood, adipose tissue and skeletal muscle in offspring exposed to diabetes in pregnancy
Clinical Epigenetics (2024)
-
Maternal choline supplementation mitigates premature foetal weight gain induced by an obesogenic diet, potentially linked to increased amniotic fluid leptin levels in rats
Scientific Reports (2024)
-
Relationships between intrauterine fetal growth trajectories and markers of adiposity and inflammation in young adults
International Journal of Obesity (2022)
-
Maternal childhood trauma is associated with offspring body size during the first year of life
Scientific Reports (2022)
-
Leptin as a key regulator of the adipose organ
Reviews in Endocrine and Metabolic Disorders (2022)