Abstract
Extremely preterm infants are at high risk for morbidities including bronchopulmonary dysplasia, intraventricular hemorrhage, and retinopathy of prematurity likely related to their exposure to reactive oxygen and nitrogen species early in life. Selenium is a trace mineral contributes to the proper function of multiple systems including immunity, redox regulation, and inflammation via the “selenoenzymes” glutathione peroxidase, thioredoxin reductase, and selenoprotein P. Given that selenium accretion via the placenta occurs primarily during the third trimester, preterm neonates are born selenium deficient. While the role of selenium in animals and adults is better described, publications are lacking in the neonatal population regarding consequences of selenium deficiency or toxicity, accurate monitoring of levels, and proper enteral and parental dosages. This review highlights the role of selenium as it relates to the optimal function of antioxidant systems in extremely preterm infants in order to highlight the gaps in knowledge as it relates to the pathogenesis and prevention of morbidities in this population.
Similar content being viewed by others
Introduction
Extremely preterm neonates (<28 weeks gestation) are at high risk for morbidities including bronchopulmonary dysplasia (BPD), intraventricular hemorrhage (IVH), and retinopathy of prematurity (ROP). While the etiologies of these conditions are multifactorial, free radical generation and oxidative injury likely contribute toward pathogenesis. Exposure to supplemental oxygen, systemic inflammation, infection, ischemia, reperfusion, and aerobic metabolism lead to generation of high levels of reactive oxygen and nitrogen species (ROS, RNS) including superoxide (O2•−), nitric oxide (NO), and hydrogen peroxide (H2O2) [1,2,3,4,5]. There has been an increasing appreciation for the critical role of the trace mineral selenium (Se) for optimal function of endogenous antioxidant defense systems that mitigate the effects of oxidants. This review highlights the role of selenium as it relates to optimal function of antioxidant systems in extremely preterm infants in order to highlight the gaps in knowledge as it relates to the pathogenesis and prevention of morbidities in this population.
History of selenium
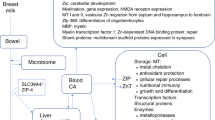
Se is an essential trace mineral that is acquired via the diet. Plasma concentrations vary geographically depending on soil Se levels, with lower plasma Se levels consistently reported in adults and infants from China, New Zealand, Australia, Iran, and parts of Europe when compared to countries such as the United States where soil levels are naturally higher [6,7,8,9]. While overt clinical manifestations of Se deficiency are rarely seen in otherwise healthy individuals, Se contributes to the proper function of multiple systems including immunity, redox regulation, inflammation, and thyroid metabolism [10,11,12]. More specifically, Se is required for the generation of the “selenoenzymes” glutathione peroxidases (GPx), thioredoxin reductases (TrxR), and selenoprotein P (SePP), so-named because they contain an active site selenocysteine (Sec) residue that is required for catalytic activity [3, 13,14,15]. The most abundant selenoproteins in the blood are SePP and GPx, which account for 50–80% and 10–30% of all selenoproteins in the blood, respectively [10, 11, 16].
Monitoring and measuring Se levels
Optimal methods to achieve accurate determination of Se status is an area of ongoing evolution as there are >35 selenoproteins in humans and only ~0.2 mg of plasma Se for every 20–40 mg Se in the body [3]. In adults, Se status is most commonly assessed by directly measuring Se concentration and/or GPx activity in plasma or erythrocytes [12]. While GPx activity and plasma Se tend to correlate in adults, studies have shown this is not the case for preterm infants [17]. Many theories have been proposed to explain this discrepancy including exposure to supplemental oxygen [12], antenatal steroids, immaturity of GPx, and preferential use of Se stores for GPx synthesis [13]. Se levels can be altered by normal acute phase responses, and it is therefore advised that Se levels should be measured at multiple time points and interpreted in the context of other markers of inflammation [18].
The role of Se in animals, adults, and children
The specific roles of Se and GPx, TrxR, and SePP in prematurity and oxidant injury are poorly understood. Animal models have illustrated the protective role of Se in the breakdown of hydroperoxidases in the lung following exposure to oxidative stress [19,20,21]. Similarly, in hyperoxia-exposed rats, Se deficiency exacerbates lung injury and is associated with increased susceptibility to oxidative lung injury [7, 12]. Clinically, Se deficiency in animals can present with a nutritional myopathy called white muscle disease that affects both skeletal and cardiac muscle. Seen in areas with low soil Se content, Se supplementation prevents the variety of problems associated with white muscle disease including failure to thrive, cardiovascular collapse, pulmonary edema, and progressive muscular weakness [22].
Deficiency of Se in the human diet has been associated with a variety of disease states including a fatal cardiomyopathy called Keshan Disease, which is characterized by heart failure, cardiac enlargement, and cardiac shock [23]. While scientific literature has suggested a potential benefit of Se with regard to cancer and cardiovascular risk, more recent environmental and nutritional studies on the human health effects of Se have reported conflicting results. Recent studies have suggested that the toxic effects of Se overexposure may be more common than previously thought. These negative effects include endocrine system alterations, increased risk of type 2 diabetes, and increased the risk of some cancers including melanoma and lymphoid cancers [24]. That being said, studies in critically ill adults, including a prospective randomized study in patients with systemic inflammatory response syndrome and multiple organ failure, demonstrated that sodium selenite supplementation over 28 days was associated with a reduction in mortality rate from 40 to 15% [25]. In 2015 a prospective observational study in a Brazilian pediatric intensive care unit found that an increase in plasma Se concentrations from admission to day 5 of stay was independently associated with shorter duration of ventilation and total length of stay [3].
Selenium deficiency in pregnancy
Pregnancy is considered a time of increased oxidative stress [26] and neonates are dependent on maternal antioxidant status for protection against free radicals [26,27,28]. Pregnant women have been reported to have lower plasma Se concentrations and decreased GPx activity compared to non-pregnant women [28]. A prospective observational study in 2014 that involved 126 pregnant women between 28 and 32 weeks gestation revealed an association between lower maternal Se levels and delivery of small for gestational age infants suggesting Se deficiency as a possible risk factor for intrauterine growth retardation [29]. A meta-analysis published in 2015 reported an inverse relationship between Se levels and risk of preeclampsia. Further, Se supplementation reduced the incidence of preeclampsia in this same study [30].
Se accretion in utero and after birth
Fetal Se accretion occurs via placental transfer, primarily during the third trimester. Se accumulation occurs primarily in the fetal liver between the 20th and 40th week of gestation [31, 32]. Makhoul et al. [14] identified a linear relationship between umbilical cord Se levels and gestational age, birth weight, and 5 min Apgar score. Umbilical cord Se levels are ~60–75% of maternal plasma Se levels and in preterm infants, cord blood levels are significantly lower than term infants [9, 33].
Preterm infants are inherently Se deficient due to many reasons including those outlined above. To make matters worse, this deficiency is compounded postnatally because preterm neonates often require prolonged parenteral nutrition, have poor intestinal Se absorption when enterally fed, and have immature pathways for Se metabolism [34]. Multiple studies have demonstrated that parenteral nutrition and formula feeding in preterm neonates are associated with lower and declining indicators of Se status when compared with breastfeeding over the first weeks and months of life [11, 14, 17, 35,36,37,38]. This Se deficiency is likely to be physiologically relevant given that concentrations increase in healthy term breastfed infants after birth [36, 39].
Studies in preterm infants
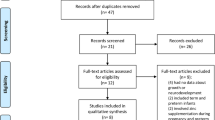
Most data regarding Se deficiency and supplementation in preterm infants have been extrapolated from animal models, adults, children, and full term healthy infants. Given the major physiologic differences between these groups, such extrapolations are likely inappropriate. Less than 10 publications regarding Se deficiency and treatment in preterm and extremely preterm populations were identified when performing a literature search for this review and the majority of the identified publications were prospective observational studies performed in countries with low plasma Se concentrations in the general population. Two studies included infants born in the United States [8], two included extremely premature infants [8, 40], and one reported data including extremely low birth weight infants [40]. By far the largest study was published by Darlow et al. [41] in 2000 and included 534 infants. The remaining studies involved sample sizes between 18 and 79 patients. All studies evaluated markers of Se status and included outcome measures confined to the first few days, weeks, or months of life. None of these studies included endpoints beyond 36 weeks’ postmenstrual age. A Cochrane review from 2003 explored the impact of Se supplementation in the prevention of short-term morbidity in preterm neonates. Three trials met inclusion criteria for this meta-analysis and 2 of these trials were performed in geographical areas with inherently low plasma Se concentrations. Overall, results of this meta-analysis, which included infants with a birth weight of <2000 grams, suggested low plasma Se was associated with increased complications of prematurity including BPD, increased days of oxygen dependency, and increased risk of adverse respiratory outcomes. Regarding Se supplementation, the meta-analysis found that Se supplementation reduced episodes of late onset sepsis, but not BPD, in very preterm infants (RR 0.73 (0.57 to 0.93); RD −0.10 (−0.17 to −0.02); NNT 10 (5.9 to 50)) [42]. Table 1 contains more detailed description of all studies identified and reviewed herein.
Discussion
Extremely preterm infants are at high risk for complications related to prematurity. The combination of early exposure to hyperoxia and inflammation, increased reactive oxygen species as a result of these exposures, and immature antioxidant defenses are all likely to contribute to the development of morbidities commonly associated with extreme prematurity. Data support Se as an important contributor to optimal function of antioxidant systems in preterm infants; however, mechanistic details for the role of Se in these processes are lacking. While clinical implications of Se deficiency have been more extensively investigated in animals and in human adults, recent studies contradicting previously identified benefits have led to controversy. Specifically, the toxic effects of Se exposure may be more significant than historically thought [24]. Significant knowledge gaps remain in the preterm neonatal population, especially in extremely preterm neonates. As discussed previously, few studies have been performed in this highly vulnerable population, and of those that have been reported, weaknesses including small sample size, and observational study design have made conclusions difficult to make. In addition, the majority of studies have been performed in areas with inherently low soil Se levels making generalizability of the findings to all populations.
No data currently exists for the ever-growing population of infants being resuscitated at 22–23 weeks gestation or with a birth weight of <500g. As of 2015, the American Society for Clinical Nutrition (ASCN) recommends parenteral Se intake of 2 µg/kg/day and the American Academy of Pediatrics Committee on Nutrition recommends enteral Se intake of 1.3–4.5 µg/kg/day in stable, growing preterm infants. These recommendations are based on trace mineral content in human milk for healthy full-term infants, accretion rates in utero, and enteral absorption rates; however, these recommendations have yet to be rigorously tested. Se supplementation is historically recommended in infants requiring parenteral for >2–4 weeks of life. Recent suggestions have supported the addition of Se at the onset of parenteral nutrition but again, data supporting this approach are incomplete at best [34]. Several studies have suggested that current parenteral dosing recommendations are insufficient and that a dose of 3 µg/kg/day is necessary to prevent the fall in Se following birth [36]. Furthermore, it is likely that 5–7 µg/kg/day is required to reach Se levels that are comparable to healthy breastfed infants [41]. Finally, infants fed Se supplemented formula vs unsupplemented formula had a rise in plasma Se and GPx values and supplementation prevented the fall in plasma Se from birth to 1 month of age that was seen in unsupplemented infants [43].
In recent years there have been issues with Se availability for parenteral nutrition. This has created a situation in which institutions are left to make decisions regarding supply rationing and supplementation in inpatient settings, including the neonatal intensive care unit. A 2015 survey of clinicians found that only 30% reported commercial availability of neonatal multi-trace element product at their institution. To compound matters, of those with product available, 31% of clinicians administered less than the recommended dose as a way to ration supply. There are currently 2 manufacturers of neonatal parenteral multi-trace products approved for use in the United States and only 1 preparation includes Se [34]. A U.S. based study by Mentro et al. revealed that standard feeding protocols led to insufficient Se supplementation and that mean Se intakes of 0.82 µg/kg/day at 1 week of life and 1.7 µg/kg/day by 4 weeks of life were standard, and far less than the recommended 2 µg/kg/day.
Conclusion
Greater numbers of extremely preterm neonates are being born at earlier gestational ages and lower birth weights. This has resulted in global increases in the numbers of ex-preterm infants with long-term morbidities. While a lack of Se is not in itself likely to completely account for free radical injury and oxidant stress in the preterm neonate, adequate Se levels theoretically enhance endogenous antioxidant defenses to mitigate the effects of oxidant stress and lessen the burden of morbidities associated with prematurity. Given the lack of standardized monitoring guidelines and deficiencies in Se availability for supplementation, it is reasonable to conclude that Se deficiency is in extremely preterm infants is likely widespread and underdiagnosed. There are no reported adverse effects associated with Se supplementation in preterm infants; therefore, the possible benefits of Se supplementation likely outweigh any theoretical or perceived risks of deficiency. In conclusion, the lack of data provides a compelling rationale for multi-center studies to adequately define Se status in extremely preterm infants, understand typical nutritional requirements, identify the clinical implications of Se deficiency and overexposure on neonatal outcomes, and to propose rational supplementation strategies targeted to this high-risk population.
References
Lunec J. Free radicals: their involvement in disease processes. Ann Clin Biochem. 1990;27:173–82.
Nassi N, Ponziani V, Becatti M, Galvan P, Donzelli G. Anti-oxidant enzymes and related elements in term and preterm newborns. Pediatr Int. 2009;51:183–7.
Leite H, Noguiera P, Iglesias S. Increased plasma selenium is associated with better outcomes in children with systemic inflammation. Nutrition. 2015;31:485–90.
Halliwell B. How to characterize a biological antioxidant. Free Radic Res Commun. 1990;9:1–32.
Sies H. Antioxidant activity in cells and organs. Am Rev Respir Dis. 1987;136:478–80.
Allingstrup M, Afshari A Selenium supplementation for critically ill adults. Cochrane Datab, Syst. Rev. CD003703 (2015).
Hawker FH, Ward HE, Stewart PM, Wynne LA, Snitch PJ. Selenium deficiency augments the pulmonary toxic effects of oxygen exposure in the rat. Eur Respir J. 1993;6:1317–23.
Mentro AM, Smith AM, Moyer-Mileur L. Plasma and erythrocyte selenium and glutathione peroxidase activity in preterm infants at risk for bronchopulmonary dysplasia. Biol Trace Elem Res. 2005;106:97–106.
Sluis KB, Darlow BA, George PM, Mogridge N, Dolamore BA, Winterbourn CC. Selenium and glutathione peroxidase levels in premature infants in a low selenium community (Christchurch, New Zealand). Pediatr Res. 1992;32:189–94.
Ashton K, Hooper L, Harvey LJ, Hurst R, Casgrain A, Fairweather-Tait SJ. Methods of assessment of selenium status in humans: a systematic review. Am J Clin Nutr. 2009;89:2025S–2039S.
Daniels L, Gibson RA, Simmer K, Van Dael P, Makrides M. Selenium status of term infants fed selenium-supplemented formula in a randomized dose-response trial. Am J Clin Nutr. 2008;88:70–76.
Domellof M. Nutritional Care of Premature Infants: Microminerals. In: Koletzko B, Poindexter B, Uauy R, (eds). Nutritional Care of Preterm Infants: Scientific Basis and Practical Guidelines. Basel: Karger; 2014. p. 129–30. vol. 110
Freitas R, Nogueira R, Antonio M. Selenium Deficiency and the effects of supplementation on preterm infants. Rev Paul De Pediatr. 2014;32:126–35.
Makhoul IR, Sammour RN, Diamond E, Shohat I, Tamir A, Shamir R. Selenium concentrations in maternal and umbilical cord blood at 24-42 weeks of gestation: basis for optimization of selenium supplementation to premature infants. Clin Nutr. 2004;23:373–81.
Nazemi L, Shariat M, Chamari M. Comparison of Maternal and Umbilical Cord Blood Selenium Levels in low and normal birth weight infants. J Fam Reprod Health. 2015;9:125–8.
Harrison I, Littlejohn D, Fell GS. Distribution of selenium in human blood plasma and serum. Analyst. 1996;121:189–94.
Daniels LA, Gibson RA, Simmer K. Glutathione peroxidase is not a functional marker of selenium status in the neonatal period. J Pediatr Gastroenterol Nutr. 1998;26:263–8.
Asci A, Surmeli-Onay O, Erkekoglu P, Yigit S, Yurdakok M, Kocer-Gumusel B. Oxidant and antioxidant status in neonatal proven and clinical sepsis according to selenium status. Pediatr Int. 2015;57:1131–7.
Bhandari V, Maulik N, Kresch M. Hyperoxia causes an increase in antioxidant enzyme activity in adult and fetal rat type II pneumocytes. Lung. 2000;178:53–60.
Kim HY, Picciano MF, Wallig MA. Postnatal selenium repletion protects lungs of neonatal rats from hyperoxia. J Nutr. 1992;122:1760–7.
Lavoie JC, Spalinger M, Chessex P. Glutathione synthetic activity in the lungs in newborn guinea pigs. Lung. 1999;177:1–7.
Delesalle C, de Bruijn M, Wilmink S, Vandendriessche H, Mol G, Boshuizen B, et al. White muscle disease in foals: focus on selenium soil content. A case series. BMC Vet Res. 2017;13:121.
Oropeza-Moe M, Wisløff H, Bernhoft A. Selenium deficiency associated porcine and human cardiomyopathies. J Trace Elem Med Biol. 2015;31:148–56.
Vinceti M, Filippini T, Cilloni S, Bargellini A, Vergoni AV, Tsatsakis A, et al. Health risk assessment of environmental selenium: emerging evidence and challenges (Review). Mol Med Rep. 2017;15:3323–35.
Zimmermann T, Albrecht S, Kühne H, Vogelsang U, Grützmann R, Kopprasch S. Selenium administration in patients with sepsis syndrome. A prospective randomized study. Med Klin. 1997;92:3–4.
Boskabadi H, Maamouri G, Omran F. Effect of Prenatal Selenium Supplementation on Cord Blood Selenium and Lipid Profile. Pediatr Neonatol. 2012;53:334–9.
Chao HC. Impact of maternal selenium supplementation on neonates. Pediatr Neonatol. 2012;53:327–8.
Tara F, Rayman MP, Boskabadi H, Ghayour-Mobarhan M, Sahebkar A, Alamdari DH, et al. Prooxidant-antioxidant balance in pregnancy: a randomized double-blind placebo-controlled trial of selenium supplementation. J Perinat Med. 2010;38:473–8.
Mistry H, Kurlak L, Young S. Maternal selenium, copper, and zinc concentrations in pregnancy associated with small-for-gestational-age infants. Matern Child Nutr. 2014;10:327–34.
Xu M, Guo D, Gu H, Zhang L, Lv S. Selenium and preeclampsia: a systematic review and meta-analysis. Biol. Trace Element Res.; 2015;171;283–292.
Bayliss PA, Buchanan BE, Hancock RG, Zlotkin SH. Tissue selenium accretion in premature and full-term human infants and children. Biol Trace Elem Res. 1985;7:55–61.
Peirovifar A, Gharehbaghi MM, Abdulmohammad-Zadeh H, Sadegi GH, Jouyban A. Serum selenium levels of the very low birth weight premature newborn infants with bronchopulmonary dysplasia. J Trace Elem Med Biol. 2013;27:317–21.
Galinier A, Périquet B, Lambert W, Garcia J, Assouline C, Rolland M, et al. Reference range for micronutrients and nutritional marker proteins in cord blood of neonates appropriated for gestational ages. Early Hum Dev. 2005;81:583–93.
Finch C. Review of trace mineral requirements for preterm infants: what are the current recommendations for clinical practice? Nutr Clin Pract. 2015;30:44–58.
Sievers E, Arpe T, Schleyerbach U, Garbe-Schönberg D, Schaub J. Plasma selenium in preterm and term infants during the first 12 months of life. J Trace Elem Med Biol. 2001;14:218–22.
Daniels L, Gibson R, Simmer K. Randomised clinical trial of parenteral selenium supplementation in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1996;74:F158–164.
Huston RK, Jelen BJ, Vidgoff J. Selenium supplementation in low-birthweight premature infants: relationship to trace metals and antioxidant enzymes. JPEN J Parenter Enter Nutr. 1991;15:556–9.
Smith AM, Chan GM, Moyer-Mileur LJ, Johnson CE, Gardner BR. Selenium status of preterm infants fed human milk, preterm formula, or selenium-supplemented preterm formula. J Pediatr. 1991;119:429–33.
Kumpulainen J, Salmenperä L, Siimes MA, Koivistoinen P, Perheentupa J. Selenium status of exclusively breast-fed infants as influenced by maternal organic or inorganic selenium supplementation. Am J Clin Nutr. 1985;42:829–35.
Klinger G, Shamir R, Singer P, Diamond EM, Josefsberg Z, Sirota L. Parenteral selenium supplementation in extremely low birth weight infants: inadequate dosage but no correlation with hypothyroidism. J Perinatol. 1999;19:568–72.
Darlow BA, Winterbourn CC, Inder TE, Graham PJ, Harding JE, Weston PJ, et al. The effect of selenium supplementation on outcome in very low birth weight infants: a randomized controlled trial. The New Zealand Neonatal Study Group. J Pediatr. 2000;136:473–80.
Darlow BA, Austin NC. Selenium supplementation to prevent short-term morbidity in preterm neonates. Cochrane Database Syst Rev. 2003: CD003312.
Darlow BA, Inder TE, Sluis KB, Nuthall G, Mogridge N, Winterbourn CC. Selenium status of New Zealand infants fed either a selenium supplemented or a standard formula. J Paediatr Child Health. 1995;31:339–44.
Darlow BA, Inder TE, Graham PJ, Sluis KB, Malpas TJ, Taylor BJ, et al. The relationship of selenium status to respiratory outcome in the very low birth weight infant. Pediatrics. 1995;96:314–9.
Merz U, Peschgens T, Dott W, Hörnchen H. Selenium status and bronchopulmonary dysplasia in premature infants <1500 g. Z Geburtshilfe Neonatol. 1998;202:203–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Tindell, R., Tipple, T. Selenium: implications for outcomes in extremely preterm infants. J Perinatol 38, 197–202 (2018). https://doi.org/10.1038/s41372-017-0033-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-017-0033-3
This article is cited by
-
Selenium and neonatal outcomes
Journal of Perinatology (2018)