Abstract
Objective
Umbilical arterial blood sampling (UABS) has been associated with cerebral oxygen saturation (CrSO2) decrements in very low birth weight (VLBW) neonates. We sought to determine patient- and UABS procedure-related factors contributing to this effect.
Study design
In this prospective cohort study, cerebral near-infrared spectroscopy was performed during UABS procedures in VLBW neonates. Analyses were conducted to determine subject- and procedure-related factors correlating with CrSO2 decrements.
Result
Thirty subjects (mean (±SD) 27 ± 2 week GA and 1058 ± 279 g BW) underwent 84 UABS procedures between 5 and 183 postnatal hours. Six (20%) experienced CrSO2 decrements, less than previously reported. Subjects with CrSO2 decrements had earlier GA and lower BW, though these were not statistically significant differences. CrSO2 decrements occurred with lower pre- and post-UABS pulse oximetry (p = 0.004; p < 0.001), lower arterial oxygen partial pressure (p < 0.001), lower baseline CrSO2 (p = 0.01), and faster “priming” blood reinfusion (p = 0.03) and saline flush (p = 0.02).
Conclusion
UABS procedures appear to be associated with CrSO2 decrements more commonly among VLBW neonates already experiencing disturbances in cerebral oxygen delivery–consumption balance. Shorter durations of UABS procedural components may contribute to CrSO2 decrements.
Similar content being viewed by others
Introduction
In very low birth weight (VLBW) neonates, umbilical arterial blood sampling (UABS) is a commonly performed procedure for serial laboratory monitoring [1, 2]. Previous reports, including our own, have demonstrated frequent decrements in cerebral tissue oxygen saturation (CrSO2) as measured utilizing near-infrared spectroscopy (NIRS) during UABS procedures [3,4,5,6,7]. Whether patient- or procedure-related factors contribute primarily to this phenomenon remains unknown.
Premature neonates have poorly developed cerebral autoregulatory mechanisms such that cerebral blood flow is more likely to vary with changes in cerebral perfusion pressure [8, 9]. As umbilical arterial (UA) catheters are most often placed in the thoracic aorta, it is plausible that UABS procedures may be associated with subtle variations in blood pressure transmitted to the cerebral circulation, with effects on cerebral blood flow [4, 6]. As previous studies have shown CrSO2 decrements with UABS procedures, the working mechanistic assumption is that transient changes in cerebral blood flow are resulting in increases in cerebral tissue oxygen extraction, evident as decreased CrSO2 during real-time NIRS monitoring.
Our previous work describing the effects of UABS procedures on CrSO2 was a post-hoc analysis of data collected during a normative survey of NIRS data in the VLBW population [4]. This prospective observational cohort study was designed a priori specifically to analyze the effects of UABS procedures on CrSO2 and determine whether patient- or procedure-related factors were most associated with CrSO2 decrements. We hypothesized that the smallest, most premature neonates would be most likely to demonstrate CrSO2 decrements during UABS procedures.
Subjects and methods
Study design
This prospective, single-center observational cohort study was approved by the Institutional Review Board of Stony Brook Children’s Hospital as a minimal risk study and written informed consent was obtained for all participants. Cerebral NIRS monitoring was performed to identify cerebral regional tissue oxygenation (rSO2) changes taking place during routine UABS procedures. All care decisions, including timing and indications for UABS blood draws, were solely made by neoantal intensive care unit (NICU) attending physicians according to standard NICU guidelines.
Patient population/sample size
All subjects were admitted to the 42-bed, Level IV Regional Perinatal Center at Stony Brook Children’s Hospital. Inclusion criteria consisted of VLBW status (<1500 g birth weight) with a thoracic UA catheter in situ. Exclusion criteria consisted of neonates with <500 g birth weight; severely immature, gelatinous skin precluding monitoring sensor placement; severe congenital and/or chromosomal anomalies; and neonates for whom survival was not expected for >48 postnatal hours.
For power analysis to determine sample size, CrSO2 decrements were defined as a 5% relative change from baseline, given a CrSO2 quiescent variability of 2–3% as determined in previous work in the VLBW patient population [10]. Based on our previous analysis, it was further assumed that CrSO2 decrements would occur in 90% of enrolled subjects and that each subject would undergo 3–4 UABS procedures throughout the study period. It was thus determined that 30 subjects would be adequate to demonstrate differences in the CrSO2 decrement occurrence rate based on patient-related factors, such as gestational age and birth weight, with 80% power and an alpha of 0.05. Furthermore, this sample size was confirmed as allowing for an adequate number of UABS procedures to analyze procedural factors potentially contributing to CrSO2 decrements as well.
UA catheter placement and blood sampling
Decisions concerning UA catheter placement and blood sampling were based solely on attending discretion with regard to clinical circumstances. All UA catheters used in this study were 3.5Fr and were connected to TruWave disposable pressure transducers (Edwards Lifesciences, Irvine, CA). Per unit practices, UA catheter tips were preferentially maintained in the “high” position and confirmed as between the sixth and ninth thoracic vertebrae on chest X-ray. For the sake of consistency, it was decided a priori to exclude low-lying UA catheters, though none were encountered during the course of this study.
Per unit protocol, all UABS procedures were performed utilizing a 3-way stopcock attached to the UA catheter system. Individual UABS procedures were performed by NICU bedside nurses and consisted of several phases according to standard procedure:
-
1.
Withdrawal of catheter dead space “priming” blood;
-
2.
Withdrawal of laboratory sample blood;
-
3.
Reinfusion of catheter dead space “priming” blood; and
-
4.
Normal saline flush.
Generally, UABS procedures were typically completed in approximately 1–2 min, though this was noted to vary depending on the volume of blood required for testing. As both blood drawing volumes and duration of procedural components could conceivably affect CrSO2, our study design purposely captured the patient-to-patient and procedure-to-procedure variability among volumes and durations to maintain equipoise.
Near-infrared spectroscopy (NIRS) monitoring
NIRS monitoring of CrSO2 was performed using the INVOS 5100 C Cerebral/Somatic Oximeter with OxyAlert NIRSensors – Infant Model IS [Neonatal] (Medtronic, Boulder, CO). This device measures the oxygenation status of regional venous, arterial, and capillary hemoglobin sources in a 75:20:5 ratio, with rSO2 displayed as a proportion of oxygenated hemoglobin to total hemoglobin [oxyhemoglobin/(oxyhemoglobin + deoxyhemoglobin)×100] [11,12,13,14,15]. For measurement of CrSO2, monitoring sensors were placed transversely across the subjects’ foreheads [4]. Standard bedside cardiopulmonary monitoring was performed using the GE Solar 8000i monitoring system (General Electric Healthcare Bio-Sciences, Pittsburgh, PA) and transcutaneous CO2 monitoring was performed using the SenTec Digital Monitoring System (SenTec, Therwil, Switzerland).
Data collection
Demographic, antenatal, and birth history data were collected. Continuous NIRS monitoring of cerebral rSO2 was performed to coincide with scheduled UABS procedures as follows:
-
1.
Fifteen minutes prior to UABS procedure (baseline);
-
2.
During UABS procedure; and
-
3.
Continuation of monitoring for 30 min post-UABS.
Once cerebral rSO2 was confirmed with adequate signal integrity and a cerebral saturation reported on the INVOS device, further NIRS data collection was performed throughout and following the UABS procedure in a blinded fashion. CrSO2 data sampling occurred every 6 s, the most frequent data setting available by the INVOS 5100C device.
Volumes of blood (both “priming” and laboratory sample) and normal saline flush were recorded during each UABS procedure. Durations of each component of the UABS procedure were obtained in real-time using a stopwatch.
The hour of life for each subject’s UABS procedures was noted. Vital sign data, including heart rate, upper extremity cuff systolic/diastolic blood pressure, pulse oximetry, and transcutaneous CO2 were collected prior to and following all UABS procedures. All ventilator parameters were additionally recorded. Finally, hemoglobin/hematocrit (either obtained during the current UABS procedure or most recently up to 12 h prior) and blood gas parameters (obtained during the current UABS procedure only) were recorded.
Data management/statistical analysis
Pre- and post-UABS CrSO2 data were analyzed in 15-min epochs. As UABS procedures were all expected to be <15 min, cerebral NIRS data collected during the performance of UABS procedures were analyzed in a separate averaging window. Based on a previous analysis of baseline variability, the 15-min epoch length was determined as providing an adequate signal-to-noise ratio with 2–3% quiescent variability while being a convenient data sampling time frame [10].
Subjects were divided into groups based on the occurrence of at least one CrSO2 decrement of 5% relative to baseline with any UABS procedure vs. the absence of any CrSO2 decrements. CrSO2 decrement and no-decrement groups were compared for differences in demographic, birth-related factors, and clinical parameters present at the time of UABS procedures. Analyses of UABS procedural factors, including blood volumes and durations of procedural components, were additionally conducted to evaluate for effects on CrSO2 decrements.
Categorical variables were assessed via chi-square or Fisher exact test based on data tabulation. Continuous data were analyzed using parametric vs. nonparametric testing depending on data distributions. All tests of significance were two-sided and evaluated at the level of p < 0.05. Statistical analyses were performed using SPSS version 20 (IBM, Armonk, NY).
Results
Study population
Between February 2015 and November 2016, a total of 84 UABS procedures were performed between 5 and 183 postnatal hours in 30 VLBW neonates with gestational age 27 ± 2 weeks and birth weight 1058 ± 279 g (mean ± SD). Subject recruitment and enrollment is displayed in Fig. 1. Six subjects (20%) demonstrated at least one CrSO2 decrement, whereas 24 subjects (80%) demonstrated stable CrSO2 during UABS procedures. No statistical differences in gestational age or birth weight were observed between the CrSO2 decrement and no-decrement groups (GA 26 ± 2 vs. 28 ± 2 weeks, p = 0.18; BW 937 ± 297 vs. 1089 ± 272 g; p = 0.24). Additionally, no differences were noted between groups with regard to antenatal steroids, Apgar scores, treatment for patent ductus arteriosus, and presence of intraventricular hemorrhage.
Effects of UABS procedures on CrSO2
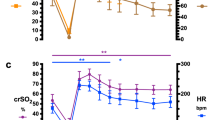
The effects of individual UABS procedures in both the CrSO2 decrement (n = 21 UABS procedures in 6 subjects) and no-decrement (n = 63 UABS procedures in 24 subjects) groups are presented in Fig. 2. Among subjects in the CrSO2 decrement group, decreases in CrSO2 were seen in 12 of the 21 (57%) UABS procedures; note assignment to the CrSO2 decrement group was based on observance of any single occurrence of CrSO2 >5% from baseline at the time of any UABS procedure. No effect of gestational age on baseline CrSO2 values or effects of UABS procedures was observed.
The effects of individual UABS procedures in both CrSO2 decrement (n = 21 UABS procedures in 6 subjects) and no-decrement (n = 63 UABS procedures in 24 subjects) groups. Boxplots display median ± interquartile range with outlying points. Single plots represent individual UABS procedures within each group. *p = 0.008 for CrSO2 pre- vs. post-UABS procedure within CrSO2 decrement group via paired t-test
Subjects in the CrSO2 decrement group had lower arterial oxygen saturation (SpO2) during both pre- and post-UABS procedures compared to the no-decrement group (pre-SpO2 p = 0.004 and post-SpO2 p < 0.001). Additionally, lower CrSO2 absolute values were noted in the CrSO2 decrement group prior to and following UABS procedures compared to the no-decrement group (pre-CrSO2 p = 0.01 and post-CrSO2 p < 0.001). No relationship was observed between hour of life and absolute CrSO2 values or the effects of UABS procedures on CrSO2 data, either for the full cohort or within each subject group. Pre- and post-UABS vital sign and CrSO2 data for both the decrement and no-decrement groups are shown in Table 1.
Laboratory data are displayed in Table 2. Hemoglobins and hematocrits obtained during UABS procedures or within the preceding 12 h are included. Arterial blood gas data, obtained only at the time of observed UABS procedures, demonstrated lower arterial oxygen partial pressure among neonates in the CrSO2 decrement group compared to the no-decrement group (54 ± 9 vs. 69 ± 19 mmHg, p < 0.001).
Volumes and durations of individual UABS procedural components are displayed in Table 3. A slightly lower volume of saline infusion was noted in the CrSO2 decrement group compared to the no-decrement group (1.0 ± 0.5 vs. 1.3 ± 0.6 mL, p = 0.02). Subjects in the CrSO2 decrement group underwent faster catheter dead space (“priming”) blood reinfusion and saline infusion compared to those in the no-decrement group (“priming” 13 ± 6 vs. 19 ± 11 s, p = 0.03; saline 8 ± 5 vs. 11 ± 7 s, p = 0.02).
Discussion
In this report, UABS procedures were associated with CrSO2 decrements in 20% of enrolled subjects. These subjects tended to be of slightly lower gestational age and birth weight than subjects not experiencing CrSO2 decrements, though this difference was not statistically significant. Neonates in the CrSO2 decrement group had lower SpO2 and CrSO2 during both pre- and post-UABS procedures compared to the no-decrement group. Additionally, arterial partial pressure of oxygen at the time of UABS procedures was lower in the CrSO2 decrement group. Finally, faster reinfusion of arterial umbilical catheter dead space (“priming”) blood volume and faster normal saline flushes were performed in the CrSO2 decrement group compared to the no-decrement group.
Interestingly, CrSO2 decrements occurred less commonly than expected compared to previously published results [4,5,6,7]. While our former study on this subject was a non-a priori retrospective review of prospective data collected in a normative survey among VLBW neonates during their first 10 postnatal days, the current study represents a prospective cohort solely enrolled for the purpose of assessing the effects of UABS procedures. The primary strength of this report thus lies in its consistent approach to studying UABS procedures in VLBW neonates, with true equipoise in capturing the range of variability (e.g., blood volumes, durations of procedural components) seen with this common procedure.
Interestingly, neonates in the CrSO2 decrement group had lower pre- and post-SpO2 and lower PaO2 values than those in the no-decrement group. While changes in cuff blood pressure were not observed in response to UABS procedures in this report, it seems feasible that subtle variations in cerebral perfusion pressure due to UABS procedures may have resulted in temporary increases in cerebral oxygen extraction, thus producing the result of decreased CrSO2 via NIRS monitoring. Furthermore, impaired cerebral autoregulation, or the ability to maintain cerebral blood flow over a range of blood pressures, has been well-described in preterm neonates [8, 9, 16], and especially in the context of hypoxic stress [17]. As cerebral autoregulatory capacity itself was not evaluated in this study, future research may aim to address this potential factor in UABS-related CrSO2 decrements.
In contrast to previous reports, no differences in blood volumes drawn were observed for either “priming” or laboratory sample volumes between the CrSO2 decrement and no-decrement groups [5, 6]. Moreover, while our report demonstrated increased saline flush volume in the no-decrement group (Table 3), this effect does not appear clinically relevant especially given the miniscule volumes of saline being administered (1.0 ± 0.5 vs. 1.3 ± 0.6 mL). However, more rapid “priming” blood reinfusion and saline flush were observed in the CrSO2 decrement group compared to the no-decrement group [7]. Again considering the small volumes of blood or saline being infused over these varied intervals, it remains uncertain whether changes in CrSO2 could be attributed to this factor from a mechanistic perspective.
The NIRS monitoring technique is becoming increasingly common for the assessment of tissue oxygenation adequacy among premature neonates in both clinical and research settings [11, 15, 18]. As changes in tissue oxygen extraction often correlate with clinical sequelae, responses to interventions, and/or effects of bedside procedures, this noninvasive monitoring strategy will likely continue to become more widespread to study the balance of oxygen delivery and consumption across a range of clinical scenarios [11, 15, 19].
One limitation of this prospective study concerns whether events occurring between observed UABS procedures may have contributed to changes in CrSO2. As the NIRS monitor was reapplied for each observed UABS procedure, it is also possible that technical aspects of the NIRS monitoring technique may have contributed to variability from one blood draw to the next. Additionally, given this is a single-center study conducted in a small group of neonates, whether the results can be generalized remains to be proven in a larger study.
UA blood sampling procedures appear to be associated with CrSO2 decrements more commonly among premature neonates already experiencing disturbances in cerebral oxygen delivery–consumption balance. A larger study is warranted to evaluate whether certain neonates distinctly sensitive to UABS procedures may be identified utilizing cerebral NIRS monitoring. Finally, whether CrSO2 decrements occurring over short intervals are associated with short- or long-term neurodevelopmental outcomes remains to be determined.
References
Oelberg DG, Baker A, Quast D, Worley L. Impact of umbilical catheterization on morbidity and mortality in extremely premature newborns. J Neonatal Perinat Med. 2014;7:13–9.
Shahid S, Dutta S, Symington A, Shivananda S. Standardizing umbilical catheter usage in preterm infants. Pediatrics. 2014;133:e1742–52.
Huning BM, Horsch S, Roll C. Blood sampling via umbilical vein catheters decreases cerebral oxygenation and blood volume in preterm infants. Acta Paediatr. 2007;96:1617–21.
Mintzer JP, Parvez B, La Gamma EF. Umbilical arterial blood sampling alters cerebral tissue oxygenation in very low birth weight neonates. J Pediatr. 2015;167:1013–7.
Roll C, Huning B, Kaunicke M, Krug J, Horsch S. Umbilical artery catheter blood sampling decreases cerebral blood volume and oxygenation in very low birthweight infants. Acta Paediatr. 2000;89:862–6.
Roll C, Huning B, Kaunicke M, Krug J, Horsch S. Umbilical artery catheter blood sampling volume and velocity: impact on cerebral blood volume and oxygenation in very-low-birthweight infants. Acta Paediatr. 2006;95:68–73.
Schulz G, Keller E, Haensse D, Arlettaz R, Bucher HU, Fauchere JC. Slow blood sampling from an umbilical artery catheter prevents a decrease in cerebral oxygenation in the preterm newborn. Pediatrics. 2003;111:e73–6.
Verhagen EA, Hummel LA, Bos AF, Kooi EM. Near-infrared spectroscopy to detect absence of cerebrovascular autoregulation in preterm infants. Clin Neurophysiol. 2014;125:47–52.
Vesoulis ZA, Liao SM, Trivedi SB, Ters NE, Mathur AM. A novel method for assessing cerebral autoregulation in preterm infants using transfer function analysis. Pediatr Res. 2016;79:453–9.
Mintzer JP, Parvez B, Chelala M, Alpan G, LaGamma EF. Quiescent variability of cerebral, renal, and splanchnic regional tissue oxygenation in very low birth weight neonates. J Neonatal Perinat Med. 2014;7:199–206.
Andersen CC, Hodyl NA, Kirpalani HM, Stark MJ. A theoretical and practical approach to defining “adequate oxygenation” in the preterm newborn. Pediatrics. 2017;139:e20161117.
Hyttel-Sorensen S, Pellicer A, Alderliesten T, Austin T, van Bel F, Benders M, et al. Cerebral near infrared spectroscopy oximetry in extremely preterm infants: phase II randomised clinical trial. BMJ. 2015;350:g7635.
Mintzer JP, Parvez B, Alpan G, LaGamma EF. Effects of sodium bicarbonate correction of metabolic acidosis on regional tissue oxygenation in very low birth weight neonates. J Perinatol. 2015;35:601–6.
Mintzer JP, Parvez B, Chelala M, Alpan G, LaGamma EF. Monitoring regional tissue oxygen extraction in neonates <1250 g helps identify transfusion thresholds independent of hematocrit. J Neonatal Perinat Med. 2014;7:89–100.
Sood BG, McLaughlin K, Cortez J. Near-infrared spectroscopy: applications in neonates. Semin Fetal Neonatal Med. 2015;20:164–72.
Rhee CJ, Fraser CD, Kibler K, Easley RB, Andropoulos DB, Czosnyka M, et al. The ontogeny of cerebrovascular pressure autoregulation in premature infants. Acta Neurochir Suppl. 2016;122:151–5.
Vesoulis ZA, Mathur AM. Cerebral autoregulation, brain injury, and the transitioning premature infant. Front Pediatr. 2017;5:64.
Alderliesten T, Dix L, Baerts W, Caicedo A, van Huffel S, Naulaers G, et al. Reference values of regional cerebral oxygen saturation during the first 3 days of life in preterm neonates. Pediatr Res. 2016;79:55–64.
Dix LM, van Bel F, Lemmers PM. Monitoring cerebral oxygenation in neonates: an update. Front Pediatr. 2017;5:46.
Acknowledgements
We would like to express our sincere thanks to the families of our patients for allowing us to perform NIRS monitoring on their VLBW neonates. We also extend our gratitude to the Stony Brook Children’s Hospital Neonatal Intensive Care Unit nursing and medical staff, without whose help this project could not have been conducted.
Funding
This work was supported by Stony Brook University School of Medicine Targeted Research Opportunity Clinical Research Award (2014/2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mintzer, J.P., Messina, C. Cerebral oxygenation during umbilical arterial blood sampling in very low birth weight neonates. J Perinatol 38, 368–373 (2018). https://doi.org/10.1038/s41372-017-0034-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-017-0034-2