Abstract
Dedifferentiated chondrosarcoma (DDCS) is an aggressive bone sarcoma characterized by low-intermediate grade cartilage component with abrupt transition to a high-grade non-chondrosarcomatous component. Generally, the dedifferentiated (DD) component is large. However, rare cases have minimal (<1 cm) or small (1–2 cm) areas of DD. We describe the clinicopathologic features of such tumors and evaluate the prognostic significance of this finding compared to cases with large DD (>2 cm). Available slides were re-reviewed for assessment of histologic features. The medical record was reviewed for imaging studies and clinical characteristics. Thirty-five cases were included. Six patients had minimal DD, four had small DD and 25 had large DD. None of the minimal DD showed definitive imaging evidence of DD. Two minimal DD (33%) locally recurred and 2 (33%) developed distant metastases. None of the small DD cases showed definitive imaging evidence of DD. None of the small DD locally recurred and at least 1 (25%) developed distant metastases. There was no significant difference in age, gender, pelvic site, tumor size >8 cm, tumor necrosis or undifferentiated pleomorphic sarcoma-like morphology between minimal or small DD compared to large DD, though osteosarcomatous differentiation was significantly more common in large DD. There was no significant difference in overall survival between minimal or small DD compared to large DD (p = 0.81 and p = 0.17, respectively), or in progression-free survival (p = 0.47 and 0.29, respectively), or metastasis-free survival (p = 0.06 and 0.62, respectively). DDCS with minimal or small DD show similar demographic distribution, anatomic localization and histologic features to large DD. DD in these cases is unlikely to be detected on imaging. Furthermore, at least a subset of these tumors is extremely aggressive despite the limited extent of DD. This highlights the need for thorough gross and histologic examination and sampling.
Similar content being viewed by others
Introduction
Dedifferentiated chondrosarcoma (DDCS) is an uncommon bone sarcoma characterized by a low to intermediate grade conventional cartilage component with abrupt transition to a high-grade non-cartilaginous sarcomatous component usually presenting in older adults1,2. DDCS comprise ~10% of chondrosarcomas and arise as an intramedullary mass, though rare cases develop on the surface from an osteochondroma (dedifferentiated peripheral chondrosarcoma)3,4,5. The dedifferentiated (DD) component frequently shows morphologic features of osteosarcoma, spindle cell sarcoma, or undifferentiated pleomorphic sarcoma (UPS), but can also display rhabdomyosarcomatous, angiosarcomatous or leiomyosarcomatous differentiation6,7,8. In addition, very rare cases are documented with glandular, squamous or adamantinoma-like basaloid features9,10,11,12. IDH1/2 mutations are found in 50–87% of DDCS and both components appear to arise from a common origin1,13,14,15,16.
A number of studies have evaluated prognostic factors in DDCS. Larger tumor size, presence of extraosseous extension, presence of pathologic fracture, lymph node involvement, metastasis at diagnosis, positive margin status, pelvic location, UPS component and high percentage of DD component have been associated with adverse outcome6,17,18,19,20,21,22,23. However, even without negative prognostic factors the overall prognosis remains dismal. While in the great majority of cases the DD component is large, there are rare cases with only minimal or small areas of DD. The prognostic significance of this finding is unknown. Here, we describe the clinical, imaging and pathologic features of DDCS with minimal (<1 cm) or small (1–2 cm) DD components and compare these cases to DDCS with large (>2 cm) foci of DD.
Methods
This study was approved by the institutional review boards of both institutions. The departmental pathology archives were retrospectively searched for primary resections of DDCS. At Washington University in St. Louis School of Medicine, the pathology departmental archive and database of one of the authors (D.J.M.) were queried for all primary resections of DDCS, and at the University of Texas MD Anderson Cancer Center the archive was searched for all primary resections of “minimal” or “early” DDCS. Available slides were reviewed by two pathologists with experience in bone pathology for diagnosis confirmation and assessment of histologic features.
Demographic information, imaging findings, tumor site, tumor size, resection margin status, extraosseous extension, size of DD component, DD component focality, mitotic rate, tumor necrosis, histomorphology of DD component, local recurrence, distant metastases, neoadjuvant and adjuvant treatment, overall survival (OS) and follow-up duration were noted. Histologic, gross and imaging findings (if available) were correlated and discussed with subspecialty trained musculoskeletal radiologists with at least 10 years of experience with oncological imaging (B.A., J.W.J.) and integrated to determine the size and focality of the DD component. In cases with multiple foci of DD or with focality unknown, the largest measurable focus of DD was recorded. OS, progression-free survival (PFS) and metastasis-free survival (MFS) were assessed using Kaplan–Meier and Cox regression methods in graphpad.
Results
Overall, 35 cases of DDCS were included. Twenty-nine patients were male (83%) and six patients female (17%). The median age was 62 years (range: 37–89). Tumors involved the femur (16), innominate bone/sacrum (8), tibia (3), fibula (1), humerus (2), sternum (3), rib (1) and mobile spine (1) (Fig. 1). Median tumor size was 13.5 cm (range 3.6–37). The size of the DD component ranged from 0.2 to 37 cm and was unifocal (25), multifocal (5) and focality unknown (5). In six patients, the DD component was minimal (<1 cm), in four patients the DD component was small (1–2 cm), and in the remainder (25) the DD component was large (>2 cm).
The histomorphology of the DD component in the whole cohort was mixed pleomorphic and spindle cell in 20 cases (57%), predominantly spindled without clear line of differentiation in 11 cases (31%) and predominantly pleomorphic in four cases (12%). Osteosarcomatous differentiation was present in 12 cases (34%) and rhabdoid foci were seen in a minority (3 cases, 9%). Soft tissue extension was seen in 28 cases (80%), cortical breakthrough without evident invasion through the periosteum in 2 cases (6%), cortical invasion without breakthrough in 2 cases (6%), 2 cases (6%) without soft tissue extension not further specified and unknown in 1 case (3%). The median follow-up time was 20 months (range 1–208). Twenty-two patients (63%) had adjuvant treatment. Eight tumors (23%) were resected with positive margins, 26 tumors (74%) with negative margins and unknown margin status in one case (3%). Eleven tumors (31%) locally recurred and 15 (43%) developed distant metastases.
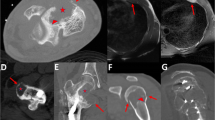
Regarding the six cases with minimal DD component (<1 cm), three patients were male (50%) and three were female (50%) with a median age of 57 years (range: 37–71). Tumors were located in the femur (2), innominate bone/sacrum (2), mobile spine (1) and sternum (1) (Fig. 1). Tumors were imaged with a variable combination of conventional radiography (5), CT (4), bone scan (1), FDG PET/CT (2) and MRI (5). None showed definitive imaging bimorphic features8 characteristic of DDCS and none showed pathologic fracture (Fig. 2). Median tumor size was 12.5 cm (range 6–15.5) and the size of the DD component ranged from 0.2 to 0.8 cm; four of which were unifocal and two were multifocal. Five minimal DD cases showed <5% DD and one case showed 5% DD.
A Plain film shows expansile lytic lesion of the right proximal femur with endosteal scalloping and cortical thinning. B MRI shows T2 bright intramedullary tumor without evidence of bimorphic pattern. C Gross photograph of a cartilaginous tumor without obvious fleshy solid dedifferentiated component.
Morphologically, the DD component in the minimally DD cases was spindled in three cases (50%) and pleomorphic in three cases (50%) (Fig. 3). One case (17%) showed osteosarcomatous differentiation and three cases (50%) showed UPS-like morphology (mixed spindle cell and pleomorphic, or pleomorphic). The DD component formed a distinct nodule or expansion of the fibrous tissue between cartilaginous lobules. The median mitotic rate of the DD component was 3/10 hpf (range: 0–8/10). Of note, these foci where quite limited in extent, which may at least partially account for some of the very low mitotic rates. Median percent tumor necrosis was 0% (range: 0–10). All six cases had a chondrosarcomatous component with soft tissue extension. The median follow-up time was 39.5 months (range 20–65). Four patients (67%) received adjuvant treatment. One case (17%) had positive margins, four cases (67%) had negative margins and margin status was unknown in one case (17%). None of the tumors underwent curettage prior to resection. Two cases (33%) recurred locally and two (33%) developed distant metastases. Both locally recurrent tumors showed DD. The metastatic lesions were identified on imaging studies but not biopsied so the histology could not be evaluated.
The four cases with small DD component (1–2 cm) presented in three females (75%) and one male (25%) with a median age of 64 years (range: 56–89) and the tumors arose in the femur (3) and innominate bone/sacrum (1) (Fig. 1). Tumors were imaged with a variable combination of conventional radiography (4), CT (3), bone scan (3) and MRI (4). None showed definitive imaging bimorphic features8 characteristic of DDCS and none showed pathologic fracture. The median tumor size was 11.6 cm (range: 4.5–19.6) with the DD component ranging from 1.2 to 1.6 cm; all four of were unifocal. Three small DD cases showed <5% DD and 1 case (4.5 cm tumor) showed 10% DD.
Two of the cases with small areas of DD (50%) showed spindled morphology, 1 (25%) had pleomorphic morphology and 1 (25%) showed mixed pleomorphic and spindle cell morphology. None showed osteosarcomatous differentiation, and 2 showed UPS-like morphology. The DD component formed a distinct nodule or expansion of the fibrous tissue between cartilaginous lobules. The median mitotic rate of the DD component was 5/10 hpf (range: 1–18/10) and tumor necrosis was not identified. Two of the four cases (50%) had chondrosarcomatous components with soft tissue extension and all were resected with negative margins with 1 (25%) receiving adjuvant treatment. None of the tumors underwent curettage prior to resection. The median follow-up for this group was 20 months (range: 8–163), none locally recurred and at least 1 case (25%) developed distant metastases. Histologic examination of the metastatic lesion showed DD. A second patient with DDCS with small DD component had a lung lesion. Biopsy showed necrotic tissue favored to be necrotic tumor; however, a definitive diagnosis of metastatic DDCS could not be made, though was suspected clinically.
Results for the 25 cases with large DD component (>2 cm) showed a median age of 62 years (range: 37–79). Seventeen cases (68%) occurred in males and 8 cases (32%) in females. Tumors were located in the femur (11), innominate bone/sacrum (5), humerus (2), tibia (3), fibula (1), rib (1) and sternum (2) (Fig. 1). Tumor size ranged from 5.5 to 37 cm with a median tumor size of 14.1 cm. The median size of the DD component was 10.5 cm (range: 2.1–37) and 17 cases (68%) presented as a single focus of DD, 3 cases (12%) were multifocal and for 5 cases (20%) focality could not be definitively determined.
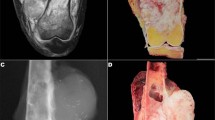
Of the tumors with large areas of DD, 20 cases (80%) showed mixed pleomorphic and spindle cell morphology and 5 cases (20%) showed spindled cell morphology (Fig. 4). Eleven cases (44%) showed areas of osteosarcomatous differentiation and 20 cases (80%) had UPS-like morphology. The median mitotic rate of the DD component was 23/10 hpf (range: 3–99/10) and median percent tumor necrosis was 10% (range: 0–80). Soft tissue extension was noted in 20 cases (80%), cortical breakthrough without evident invasion through the periosteum in 2 cases (8%), cortical invasion without breakthrough in 2 cases (8%), and unknown in 1 case (4%). Six cases (24%) were resected with positive margins and 19 cases (76%) were resected with negative margins. Eighteen cases (72%) had adjuvant treatment and the median follow-up was 13 months (1–129); 9 cases locally recurred (37.5%) and 12 cases (50%) developed with distant metastases.
A Gross photograph of dedifferentiated chondrosarcoma with extensive dedifferentiated component (red oval indicates grossly evident conventional cartilaginous component). B Well-differentiated areas and dedifferentiated areas. Higher magnification of dedifferentiated area (C) which representing the majority of the lesion (D).
Age and gender were not significantly different between cases with minimal DD component compared to cases with a large DD component (p = 0.38 and 0.28, respectively) nor between cases with small DD component compared to cases with large DD component (p = 0.24 and 0.31, respectively). Further, pelvic site was not significantly different between cases with minimal or small DD components compared to tumors with large DD areas (p = 0.38 and 0.35, respectively). Overall tumor size was not significantly different between tumors with minimal DD component and large DD component (p = 0.49) or between small DD component and large DD component (p = 0.56) and similar results held when evaluating the study groups for tumors >8 cm (p = 0.32 and 0.63, respectively). Osteosarcomatous differentiation was significantly more common in cases with large DD component compared to cases with minimal (p = 0.03) and compared to cases with small foci of DD (p = 0.03). The presence of UPS-like morphology was not significantly different between cases with large DD component and cases with minimal (p = 0.43) or small foci of DD (p = 0.40). The mitotic rate was significantly higher in cases with large DD component compared to cases with minimal DD (p = 0.02) but not compared to cases with small foci of DD (p = 0.10). This may be result of more area available for evaluation in cases with large DD. Tumor necrosis in cases with a large DD component was not significantly different compared to cases with minimal DD (p = 0.06), or compared to cases with small foci of DD (p = 0.10). Margin positive resection was not significantly different between cases with minimal or small DD components compared to tumors with large DD areas (p = 0.32 and 0.55, respectively)
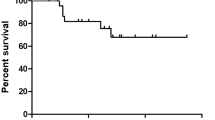
There was no significant difference in OS between minimally DD tumors and tumors with large DD components (p = 0.81), or between tumors with small DD components and tumors with large DD component (p = 0.17). Likewise, no significant difference in PFS was observed between tumors with minimal or small areas of DD and tumors with large DD components (p = 0.47 and 0.29, respectively). Further, minimally DD tumors and tumors with small DD components compared with tumors with large DD component did not show significantly different MFS (p = 0.06 and 0.62, respectively).
Discussion
DDCS typically presents with large DD areas however rare tumors present with only minimal or small DD components, which we defined as areas of DD measuring <1 cm and 1–2 cm, respectively. The clinical, imaging and pathologic features of such cases have not been the focus of previous studies. Further, while Staals and colleagues showed that DDCS with >50% DD was a poor prognostic factor for OS survival6, our study sought to investigate if minimal or small foci of DD, rather than a percentage of the tumor, was associated with outcome. To that end we described six DDCS with minimal DD foci and four DDCS with small DD foci, and compared such cases to 25 more typical DDCS with DD components >2 cm.
In terms of demographics, our overall study cohort was fairly typical of DDCS with a male predominance and a median age of 62 years (range: 37–89). No significant difference in age and gender was observed between minimally DD cases or cases with small foci of DD and tumors with large DD component. Likewise, tumors with minimal and small DD components arose in a similar distribution to tumors with large DD component, with tumors most frequently occurring in the pelvis and femur. Of note, the case that arose in the mobile spine, an unusual site for DDCS20,24, was minimally DD. However, larger studies are needed to conclusively determine if there is any association between mobile spinal localization and minimally DDCS.
Overall tumor sizes were not significantly different between the three groups. Histologically, all cases showed a conventional cartilaginous component (with features ranging from enchondroma-like to grade 2 chondrosarcoma) with a sharp transition to a high-grade non-cartilaginous sarcoma diagnostic of DDCS. Tumors with minimal or small foci of DD showed similar morphologic patterns compared to tumors with large areas of DD including spindle cell, pleomorphic, and mixed pleomorphic and spindle cell patterns, though osteosarcomatous differentiation was significantly more common in tumors with large foci of DD. The mitotic rate was significantly higher in cases with large foci of DD compared to minimally DD cases, however the difference was not significant compared to tumors with small foci of DD. Tumor necrosis was not significantly different in cases with large DD compared to minimal foci of DD, or in cases with small DD.
We hypothesized that even minimal or small areas of DD would portend a poor outcome as DDCS is an extraordinarily aggressive neoplasm. However, it was possible that the presence of only minimal or small areas of DD could reflect more limited growth potential of the DD component, which one could reasonably speculate would lead to less aggressive behavior. Alternatively, and more likely, minimal or small areas of DD could simply reflect resection at a relatively early time point in the growth of the DD component. From that perspective, the behavior could be hypothesized to be less aggressive due to less time to spread distantly, less time to accumulate additional mutations with selection of more aggressive clones, and less likely to be present in unresected tumor in cases with positive margins.
In our study, two patients with foci of DD measuring 1.6 and 1.2 cm died at 8 and 23 months, respectively. Even foci measuring 0.5 and 0.5 cm led to death from metastatic sarcoma at 31 and 48 months, respectively. Further, there was no significant difference in OS, MFS or PFS when comparing cases with minimal or small foci of DD to tumors with large foci of DD. This is in line with the fact that the most important negative prognostic factor in conventional cartilaginous neoplasia is the presence of DD. The median survival of patients with DDCS is 13 months, and 2- and 5-year survival rates are reported at only 34% and 24%, respectively6. This is in contrast to patients with atypical cartilaginous tumor/grade 1 chondrosarcoma and grade 2 chondrosarcoma who have 5- and 10-year survival of 93% and 88%, and 74% and 62%, respectively25. This underscores the importance of thorough sampling and careful gross and microscopic examination as minimal and small areas of DD can portend an aggressive clinical course and are unlikely to be detected on imaging studies.
It is our practice to thoroughly section chondrosarcomas (usually in a plane to give the largest tumor face unless other considerations supersede). Gross sections are carefully examined for “fleshy” areas and other relevant findings such as soft tissue extension and tumor-margin relationships. Tumors are frequently mapped, though this is not required or standard across institutions. Regardless, at least one section of tumor per cm of the largest tumor dimension is submitted for microscopic examination. Sections are selected to represent any heterogeneity grossly apparent in the tumor and in particular to include sampling of “fleshy” areas suspicious for dedifferentiation.
At least a subset of minimal and small DDCS can exhibit highly aggressive behavior, however it is noteworthy that while a statistically significant difference was not observed, 4/6 (66.7%) patients with minimally DD tumors and 2/4 (50%) of patients with tumors with small DD component were alive with no evidence of disease compared to 10/25 (40%) of patients with large DD components. This suggests that while the behavior of at least a subset of DDCS with minimal or small foci of DD is clearly highly aggressive, the course may be more variable and perhaps more favorable compared to tumors with large DD component and our sample size was not large enough to detect the difference.
This interpretation is in line with the findings of Staals et al. who showed that the smaller the estimated percentage of DD the better the prognosis6 as the minimal and small DDCS in our series were overall quite large tumors. However, we choose direct measurement of the largest dimension of the largest focus of DD instead of estimated percentage of DD as our methodology as a practical consideration. In routine practice a minimal or small focus of DD can be easily measured on the slide. This approach is analogous to the convention of using somewhat arbitrary but clearly defined cut-offs for the largest dimension of limited foci of DD in dedifferentiated liposarcoma to divide tumors into a descriptive category for atypical lipomatous tumor/well-differentiated liposarcoma with DD foci measuring ≤1 cm and “minimal dedifferentiation” for tumors with DD foci measuring 1–2 cm26. However, we have also included the percentages of DD for the DDCS cases with minimal or small foci of DD (see results above) to make our results more comparable with other studies.
Prior studies have identified multiple other poor prognostic factors. A worse outcome has been observed if the DD component was UPS-like6,20. In our study there was no significant difference in UPS-like morphology between cases with minimal or small areas of DD compared to cases with large DD components. Further, increasing age, larger tumor size, tumor size >8 cm, margin positive resection and pelvic location have been previously associated with adverse outcome6,17,18,19,20,21,22. In our study, these variables were not significantly different between tumors with minimal or small DD components and tumors with large areas of DD.
Limitations of this study include difference in treatment and management of patients over time and between institutions and referral bias. Furthermore, the study is limited by relatively small sample size, which is due to the rarity of DDCS and even greater scarcity of DDCS with minimal or small DD components. Larger studies at other institutions could be performed to confirm or refute our findings though initial presented results from an independent group appear similar27.
In conclusion, DDCS with minimal or small foci of DD show similar demographic distribution, anatomic localization and histologic features to more typical DDCS with large areas of DD. DD in these cases is unlikely to be detected on imaging studies. Furthermore, while larger studies are needed to fully characterize the behavior of these neoplasms, at least a subset of these tumors can still behave in a highly aggressive manner despite their small size of the DD component. This highlights the need for careful gross and histologic examination and sampling.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Inwards, C. Y. & Hogendoorn, P. C. W. Dedifferentiated chondrosarcoma. In: WHO Classification of Tumours Soft Tissue and Bone Tumours (ed WHO Classification of Tumours Editorial Board) (IARC Press, 2020).
Dahlin, D. C. & Beabout, J. W. Dedifferentiation of low-grade chondrosarcomas. Cancer 28, 461–466 (1971).
Frassica, F. J., Unni, K. K., Beabout, J. W. & Sim, F. H. Dedifferentiated chondrosarcoma. A report of the clinicopathological features and treatment of seventy-eight cases. J. Bone Jt. Surg. Am. 68, 1197–1205 (1986).
Rozeman, L. B. et al. Dedifferentiated peripheral chondrosarcomas: regulation of EXT-downstream molecules and differentiation-related genes. Mod. Pathol. 22, 1489–1498 (2009).
Staals, E. L., Bacchini, P., Mercuri, M. & Bertoni, F. Dedifferentiated chondrosarcomas arising in preexisting osteochondromas. J. Bone Jt. Surg. Am. 89, 987–993 (2007).
Staals, E. L., Bacchini, P. & Bertoni, F. Dedifferentiated central chondrosarcoma. Cancer 106, 2682–2691 (2006).
Reith, J. D., Bauer, T. W., Fischler, D. F., Joyce, M. J. & Marks, K. E. Dedifferentiated chondrosarcoma with rhabdomyosarcomatous differentiation. Am. J. Surg. Pathol. 20, 293–298 (1996).
Littrell, L. A. et al. Radiographic, CT, and MR imaging features of dedifferentiated chondrosarcomas: a retrospective review of 174 de novo cases. Radiographics 24, 1397–1409 (2004).
Jour, G., Liu, Y., Ricciotti, R., Pritchard, C. & Hoch, B. L. Glandular differentiation in dedifferentiated chondrosarcoma: molecular evidence of a rare phenomenon. Hum. Pathol. 46, 1398–1404 (2015).
Zhang, Y., Paz Mejia, A., Temple, H. T., Trent, J. & Rosenberg, A. E. Squamous cell carcinoma arising in dedifferentiated chondrosarcoma proved by isocitrate dehydrogenase mutation analysis. Hum. Pathol. 45, 1541–1545 (2014).
Gambarotti, M. et al. Dedifferentiated chondrosarcoma with “adamantinoma-like” features: a case report and review of literature. Pathol. Res. Pr. 213, 698–701 (2017).
Ling, L. L. & Steiner, G. C. Primary multipotential malignant neoplasm of bone: chondrosarcoma associated with squamous cell carcinoma. Hum. Pathol. 17, 317–320 (1986).
Amary, M. F. et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J. Pathol. 224, 334–343 (2011).
Meijer, D. et al. Genetic characterization of mesenchymal, clear cell, and dedifferentiated chondrosarcoma. Genes Chromosomes Cancer 51, 899–909 (2012).
Chen, S. et al. Diagnostic utility of IDH1/2 mutations to distinguish dedifferentiated chondrosarcoma from undifferentiated pleomorphic sarcoma of bone. Hum. Pathol. 65, 239–246 (2017).
Bovée, J. V. et al. Molecular genetic characterization of both components of a dedifferentiated chondrosarcoma, with implications for its histogenesis. J. Pathol. 189, 454–462 (1999).
Grimer, R. J. et al. Dedifferentiated chondrosarcoma: prognostic factors and outcome from a European group. Eur. J. Cancer 43, 2060–2065 (2007).
Lex, J. R. et al. Dedifferentiated chondrosarcoma of the pelvis: clinical outcomes and current treatment. Clin. Sarcoma Res. 8, 23 (2018).
Liu, C. et al. Dedifferentiated chondrosarcoma: radiological features, prognostic factors and survival statistics in 23 patients. PLoS ONE 12, e0173665 (2017).
Miao, R. et al. Prognostic factors in dedifferentiated chondrosarcoma: a retrospective analysis of a large series treated at a single institution. Sarcoma 2019, 9069272 (2019).
Mitchell, A. D. et al. Experience in the treatment of dedifferentiated chondrosarcoma. J. Bone Jt. Surg. Br. 82, 55–61 (2000).
Strotman, P. K., Reif, T. J., Kliethermes, S. A., Sandhu, J. K. & Nystrom, L. M. Dedifferentiated chondrosarcoma: a survival analysis of 159 cases from the SEER database (2001-2011). J. Surg. Oncol. 116, 252–257 (2017).
Yokota, K. et al. Clinical outcome for patients with dedifferentiated chondrosarcoma: a report of 9 cases at a single institute. J. Orthop. Surg. Res. 7, 38 (2012).
Unni, K. K. & Inwards, C. Y. Dahlin’s Bone Tumors 6th edn, 76–83 (Lippincott Williams & Wilkins, 2010).
van Praag Veroniek, V. M. et al. Incidence, outcomes and prognostic factors during 25 years of treatment of chondrosarcomas. Surg. Oncol. 27, 402–408 (2018).
Goldblum, J. R., Folpe, A. L. & Weiss, S. W. Enzinger and Weiss’s Soft Tissue Tumors. 7th edn (Elsevier, 2020).
Hakim, M. et al. Abstracts from USCAP 2021: bone and soft tissue pathology (33-65): dedifferentiated chondrosarcoma: how much really counts? Mod. Pathol. 34, 48–49 (2021).
Author information
Authors and Affiliations
Contributions
C.A.D., W.-L.W., D.J.M., and J.S.A.C. performed study concept and design; C.A.D., N.M. W.-L.W., D.J.M. and J.S.A.C. performed development of methodology and writing; C.A.D., N.M. B.A., J.W.J., W.-L.W., D.J.M., and J.S.A.C. performed review and revision of the paper; C.A.D., B.A., N.M., J.W.J., W.-L.W., D.J.M., and J.S.A.C. provided acquisition, analysis and interpretation of data, and statistical analysis. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the Institutional Review Boards.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dehner, C.A., Maloney, N., Amini, B. et al. Dedifferentiated chondrosarcoma with minimal or small dedifferentiated component. Mod Pathol 35, 922–928 (2022). https://doi.org/10.1038/s41379-022-01008-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-022-01008-8
This article is cited by
-
Construction of novel predictive tools for post-surgical cancer-specific survival probability in patients with primary chondrosarcoma and external validation in Chinese cohorts: a large population-based retrospective study
Journal of Cancer Research and Clinical Oncology (2023)