Abstract
Intensive care unit (ICU) staff continue to face recurrent work-related traumatic events throughout the COVID-19 pandemic. Intrusive memories (IMs) of such traumatic events comprise sensory image-based memories. Harnessing research on preventing IMs with a novel behavioural intervention on the day of trauma, here we take critical next steps in developing this approach as a treatment for ICU staff who are already experiencing IMs days, weeks, or months post-trauma. To address the urgent need to develop novel mental health interventions, we used Bayesian statistical approaches to optimise a brief imagery-competing task intervention to reduce the number of IMs. We evaluated a digitised version of the intervention for remote, scalable delivery. We conducted a two-arm, parallel-group, randomised, adaptive Bayesian optimisation trial. Eligible participants worked clinically in a UK NHS ICU during the pandemic, experienced at least one work-related traumatic event, and at least three IMs in the week prior to recruitment. Participants were randomised to receive immediate or delayed (after 4 weeks) access to the intervention. Primary outcome was the number of IMs of trauma during week 4, controlling for baseline week. Analyses were conducted on an intention-to-treat basis as a between-group comparison. Prior to final analysis, sequential Bayesian analyses were conducted (n = 20, 23, 29, 37, 41, 45) to inform early stopping of the trial prior to the planned maximum recruitment (n = 150). Final analysis (n = 75) showed strong evidence for a positive treatment effect (Bayes factor, BF = 1.25 × 106): the immediate arm reported fewer IMs (median = 1, IQR = 0–3) than the delayed arm (median = 10, IQR = 6–16.5). With further digital enhancements, the intervention (n = 28) also showed a positive treatment effect (BF = 7.31). Sequential Bayesian analyses provided evidence for reducing IMs of work-related trauma for healthcare workers. This methodology also allowed us to rule out negative effects early, reduced the planned maximum sample size, and allowed evaluation of enhancements. Trial Registration NCT04992390 (www.clinicaltrials.gov).
Similar content being viewed by others
Introduction
Throughout the COVID-19 pandemic, frontline healthcare workers have been repeatedly exposed to potentially psychologically traumatic events, such as untimely and excess deaths of patients. After trauma, it is common to experience intrusive memories (IMs) of the event. These are emotional, sensory, and primarily visual memories (mental imagery) of the traumatic event that intrude repeatedly into mind, comprising a core clinical feature of post-traumatic stress disorder (PTSD) [1]. Anecdotal examples of an IM include a vivid mental image of the eyes of a young patient dying while resuscitation fails; or an image of an ambulance stretcher bearing a former colleague. IMs can be distressing and disruptive. Even before COVID-19, emergency-room nurses reported high levels of IMs of work-related trauma [2], and meta-analysis estimates show that healthcare workers are twice as likely to develop PTSD compared to the general public [3]. Due to the COVID-19 pandemic, around 40% of healthcare workers in UK hospitals reported a level of symptoms consistent with a diagnosis of PTSD as of June/July 2020 [4]—five times higher than in 2015 [5]. Reports of mental health disorders in healthcare workers increased further to 64% in winter 2020 of the pandemic [6].
The urgent need for scalable approaches to support the mental health of frontline healthcare workers, such as Intensive Care Unit (ICU) staff, was highlighted early in the pandemic [7]. To address this, novel approaches are needed for this population. Given their high workload demands, a brief and flexible intervention approach would be beneficial. Moreover, the nature of the trauma exposure facing healthcare workers is not single-event trauma but is repeated and ongoing through the pandemic. Harnessing research on preventing IMs with a novel behavioural intervention on the day of trauma [8], here we take critical next steps to develop this approach as a treatment for ICU staff who are already experiencing IMs days, weeks or months post-trauma. The new intervention approach to reduce IMs here aims to be readily repeatable for different traumas as well as brief, flexible and low stigma.
An imagery-competing task intervention approach (which includes computer gameplay) to prevent IMs was developed from insights from cognitive neuroscience and experimental research [9, 10] by taking a mechanistically informed single-symptom approach [11]. The aim was to offer a new low-intensity intervention post-trauma. The intervention included a memory reminder cue plus playing a computer game with high visuospatial demands (Tetris®) using mental rotation, theorised to disrupt the consolidation of sensory elements of the trauma memory. More specifically, the intervention exploits principles of working memory theory (i.e., limited capacity to process similar cognitive information simultaneously), and the malleability of memory to updating, by using a competing cognitive task to interfere with mental imagery-based (mainly visuospatial) trauma memory. The disruption of visuospatial processing while memory is being stored or updated should render the memory less likely to be triggered, i.e. from becoming intrusive [8,9,10, 12, 13]. Laboratory studies indicating specificity of the intervention to target intrusive trauma memory whilst leaving voluntary non-traumatic memory are consistent with this hypothesised mechanism [14, 15].
A first proof-of-concept translation study from lab to clinic included patients in the emergency department (ED) within 6 h of a motor vehicle accident [8]. It was predicted that if the intervention was administered in the first hours after a traumatic event, the number of IMs would be reduced. The randomised controlled trial (RCT) compared the imagery-competing task intervention with an attention-placebo control. Results indicated the efficacy of the intervention in that there were fewer IMs reported in the week post-intervention. Participants found the single session intervention easy, helpful, and minimally distressing. Similar findings were found in a subsequent study in the ED [16] including more trauma types than motor vehicle accidents. Further, addressing a previous limitation, results showed the reduction in IMs at 1 week persisted to 1 month. A similar intervention approach with mothers after traumatic childbirth showed an effect of reducing IMs [17].
While showing promise for the “prevention” of IMs soon after trauma [8, 12, 16, 17], critically we also need interventions for the treatment of “established” IMs i.e. rather than on the day of trauma, treatment delivery when a longer time has elapsed (days, weeks or months later) for individuals already experiencing IMs. Accordingly, to adapt the imagery-competing task intervention we drew on insights from memory reconsolidation studies on older established memories [10, 12, 18, 19]. In laboratory [20] and case studies [21, 22] we adapted parameters of the intervention (e.g. timings) to promote a reduction of established IMs (Anemone™). We digitised intervention delivery procedures [23] so that they could be administered remotely due to contagion risk during the COVID-19 pandemic. In collaboration with healthcare workers with lived experiences of IMs, the tailored intervention was piloted in the pandemic [24].
In the current trial, we sought to optimise and evaluate a brief digital imagery-competing task intervention [8, 20] to reduce the number of IMs of work-related traumatic events for ICU staff. The imagery-competing task intervention consisted of a brief reminder cue to a specific intrusive memory, followed by playing the computer game Tetris® for 20 min with mental rotation. The first session was guided by a researcher. Thereafter the intervention could be used self-guided, i.e. was repeatable. Participants in the present trial were already experiencing IMs, with many facing ongoing trauma exposure. This is the first trial to evaluate the brief imagery-competing task intervention for traumatic events that could have taken place days, weeks or months ago, for healthcare workers, and using a remotely delivered digitised version of the intervention on i-spero®. The primary outcome diary was 4 weeks after the guided intervention session.
Given the rapid need for novel approaches during a pandemic, it was advantageous to evaluate the intervention, both swiftly and robustly. However, RCTs are notoriously lengthy, spanning several years before an intervention is optimised and evaluated. The COVID-19 pandemic has led to difficulties with participant recruitment and testing, underscoring the need to minimise sample sizes where possible [25]. Our solution to the challenge of being able to assess evidence rapidly while upholding, and arguably even improving, the standards of RCTs was to apply advances in Bayesian statistical methodology. Bayesian methods provide powerful tools for inference (see “Statistical analysis”), and have been applied widely in medical research, e.g. for SARS-CoV-2 virus [26]. However, reporting of frequentist null hypothesis significance testing is still the prevailing norm in clinical trials [27].
The sequential Bayesian design used here has previously been recommended for vaccine development [28] and used in COVID-19 trials to lower required sample sizes without loss of scientific integrity [25]. For example, in early-phase COVID-19 vaccine trials, aspects of treatment such as dosage were modified based on early evidence, allowing later confirmatory trials to test optimised versions of a treatment [25, 29]. Compared to traditional designs, a sequential Bayesian approach typically requires 50% to 70% smaller samples to conclude the presence of an effect and has the same or lower rate of false inference [30]. Using a sequential Bayesian approach, with the ability to quantify and track evidence over time, allowed for continuous learning from the data to guide decision making (such as early stopping and optimising the intervention). It provided the possibility of taking early action if we saw evidence of a negative effect (greater number of IMs), as well as to act on evidence of a positive treatment effect (fewer IMs) (see “Results”).
In sum, we aimed to optimise a brief digital imagery-competing task intervention to help reduce the number of IMs of work-related trauma being experienced by ICU staff. To this end, we used Bayesian statistical methodology to optimise trial design and guide decisions. Taken all together, our aim was that this intervention and the Bayesian methodology would help address the need for accelerated mental health treatment development for healthcare staff working during the COVID-19 pandemic.
Material and methods
Study design and participants
We conducted a two-arm, parallel-group, randomised, adaptive Bayesian optimisation trial of a remotely delivered digital intervention. Ethical approval for the trial was granted by the Wales Research Ethics Committee (Wales REC 6, 21/WA/0173). The trial was registered prospectively at Clinical.Trials.gov (CTR: NCT04992390). The study protocol was added to a public depository (osf.io/2xn5m), and the trial had a data monitoring committee (DMC).
The study was advertised via email and Twitter directly from the Intensive Care Society to its membership network, mailing list, and existing social media followers, supplemented by advertisements through Facebook. Advertisements contained a link to the study website: https://www.p1vital-gains.com/, which included a summary of study information, a video explaining IMs, and a participant information sheet.
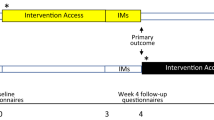
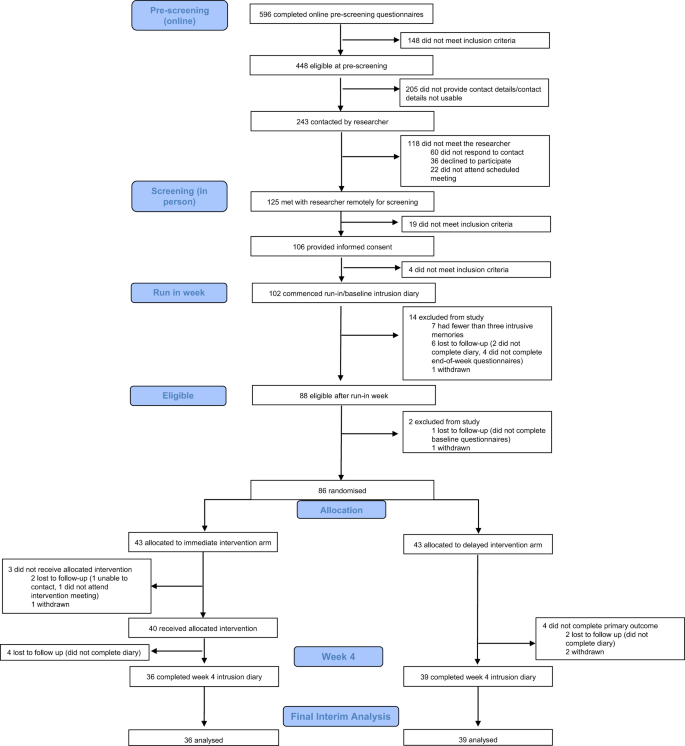
Eligible participants were adults aged 18 years or older, who worked in a clinical role in an NHS ICU or equivalent during the COVID-19 pandemic (e.g. as a member of ICU staff or deployed to work in the ICU during the pandemic), who had experienced at least one traumatic event related to their work (meeting criterion A of the DSM-5 criteria for PTSD: “exposure to actual or threatened death, serious injury, or sexual violence” by “directly experiencing the traumatic event(s)” or “witnessing, in person, the event(s) as it occurred to others”), had IMs of the traumatic event(s), and had experienced at least three IMs in the week prior to screening. Further, participants had internet access; were willing and able to be contacted by the research team during the study period; were able to read, write, and speak English; and were willing and able to provide informed consent and complete study procedures. Exclusion criteria were having fewer than three IMs during the baseline week after informed consent (i.e. the run-in week on Fig. 1). We did not exclude those undergoing other treatments for PTSD or its symptoms, so that the study was as inclusive as possible to meet the challenges that ICU staff were facing during the COVID-19 pandemic. Written informed consent was obtained before participation (using an electronic signature via email).
Randomisation and masking
Participants were randomly assigned (1:1) using a remote, secure web-based clinical research system (P1vital® ePRO) to either immediate intervention arm (immediate access to the brief digital imagery-competing task intervention plus symptom monitoring for 4 weeks) or delayed intervention arm (usual care for 4 weeks followed by access to the intervention plus symptom monitoring for 4 weeks).
Randomisation occurred following the baseline week, and after baseline questionnaires (see protocol; osf.io/2xn5m). The allocation sequence was computer-generated consecutively using minimisation. Minimisation allocated the first participant to either arm, thereafter allocation was preferential to the arm with the fewest participants to minimise the difference in group sizes. The randomisation allocation percentage was originally set to 66% and altered to 85% after 61 participants were randomised (to ensure balance of groups after early stopping decision, see “Results”). Overall, 86 participants were randomly assigned to the delayed (n = 43) or immediate (n = 43) arm.
Participants were blinded to group allocation. The statistician who conducted the interim analyses (VR), and researchers who contacted participants and facilitated the conduct of the task intervention were not blinded to group allocation. A researcher supported the technical use of the intervention in the first guided session in i-spero® and was aware that the participant completed a cognitive task. It is noted that all outcome data (including the primary outcome 4 weeks later) was self-reported by participants independently and directly into the online platform (P1vital® ePRO) removing the need for researcher input and reducing the risk of bias. That is, outcome assessments were masked to group allocation since they were self-reported by participants in the digital platform. Scoring of data was automatic in P1vital® ePRO, and thus independent from the researchers guiding the intervention session.
The intervention
Straight after randomisation, participants in the immediate intervention arm gained access to the digital imagery-competing task intervention with symptom monitoring of IMs for 4 weeks. The intervention was delivered on a secure web platform (i-spero®) via smartphone, tablet, or computer. Participants had an initial researcher-guided session (~1 h, via Microsoft Teams) and thereafter used i-spero® in a self-directed manner (~25 min; with the option for support). The researcher-guided session consisted of step-by-step instructions, animated videos and multiple-choice questions. Participants were instructed to list their different IMs by typing a brief description. They selected one IM from their list, and very briefly brought the image to mind. After instructions on playing the computer game Tetris® using mental rotation, they played for 20 min. Finally, they were instructed on monitoring IMs in i-spero® and encouraged to use the intervention to target each memory on their list.
The brief digital intervention on i-spero® and P1vital® ePRO are owned and manufactured by P1vital Products Ltd. Tetris® has been licensed for use within i-spero® from The Tetris Company. P1vital® ePRO, i-spero® and the brief digital intervention have been developed following a formal computerised system validation methodology which complies with Good Clinical Practice, FDA 21CFR Part 11 and ISO13485 Quality Management System.
Assessments
Baseline
After informed consent, participants completed a daily IM diary online for 7 days (baseline week, day 0–6 i.e. run-in week on Fig.1) to record the number of IMs of traumatic events. This diary was adapted from previous studies [8, 20] for digital delivery using P1vital® ePRO. Participants were asked “Have you had any intrusive memories today?” and if answered “yes” selected how many, prompted by email/SMS once daily. Those who reported three or more IMs during baseline week and completed baseline questionnaires (sent after baseline week to those meeting the study entry criteria, see Fig. 1 and Supplementary Table 1) were randomised. The total number of IMs of traumatic event(s) recorded during the baseline week is used as a baseline covariate when modelling.
Primary outcome
During week 4, participants in both arms were asked to again complete the daily IM diary for 7 days (i.e. from day 22 to 28, where day 1 is the guided session in immediate arm/equivalent timeframe in delayed arm) to record the number of IMs of traumatic event(s); The primary outcome measure was the total number of IMs recorded by participants in this daily IM diary in week 4.
The outcome measure was derived from diaries used in clinical practice [31], laboratory [20] and patient studies [8]. Positive relationships between diary IMs and the Impact of Events Intrusion subscale indicates convergent validity with PTSD symptoms [32]. Count data provides greater sensitivity than questionnaires with finite categories, with no upper bound. Daily completion can reduce retrospective recall biases of completing measures later. Service users report the diary is straightforward with typically good adherence and limited missing data [32]. Remote and digital completion of the diary here meant it was assessor blinded.
The clinical meaning of a change in score may depend on the trauma population, as single-event trauma incurs fewer IMs than repeated trauma. For a PTSD diagnosis, the Clinician-Administered PTSD Scale for DSM–5 (CAPS-5) [33] requires at least two IMs over the past month. The CAPS-5 maximum score is “daily”, and reducing this to “once-or-twice a week”/“never” (CAPS-5 mild-minimum) represents a clinically meaningful outcome target [34]. The CAPS-5 was not administered in the current study due to considerations around participant burden of adding a clinician interview. However, as it is a gold standard measurement in the field, we here note its scoring scale range to shed light regarding the number of IMs typically assessed clinically.
Safety
Adverse events were monitored through a standardised question (“have you experienced any untoward medical occurrences or other problems?”) at week 4 and week 8, as well as through any spontaneous reports from participants at any time point during the study.
Other outcomes
The present article focuses solely on the sequential Bayesian analyses on the primary outcome measure. A standard analysis (using frequentist statistics) of the final study population including secondary outcome measures will be reported elsewhere [35] (CTR: NCT04992390).
Statistical analysis
Informed by power estimates based on an effect size of d = 0.63 (based on pooled information from three previous related RCTs [8, 16, 17]) for the primary outcome, we planned to recruit up to 150 participants, with the potential to end recruitment earlier based on interim analyses. Therefore, we employed a sequential Bayesian design with maximal sample size [30, 36]. This allowed for interim analyses to guide decision-making, such as when to adjust aspects of the intervention to optimise its effect, and when sufficient evidence has been collected to end the optimisation trial, and proceed to a follow-up pragmatic RCT to test the clinical effectiveness of the optimised intervention.
The fundamental idea to a Bayesian approach is simple [37]: the parameters that we are trying to estimate are treated as random variables with distributions that represent our initial beliefs and uncertainty. After observing data, initial beliefs can be updated with the new information to get improved beliefs. This contrasts with the prevailing “frequentist” statistical frameworks where these parameters are fixed, and probabilities are seen as long-run frequencies generated by some unknown process. The principal outcome of fitting a Bayesian model is the posterior distribution: a probability distribution that indicates how probable particular parameter values are, given the prior distribution (representing initial beliefs) and the observed data. In a Bayesian model, the 95% credibility interval states that there is 95% chance that the true population value falls within this interval.
All analyses were completed in R (version 4.1.2) on an intention-to-treat basis. We fitted a Bayesian model, where the primary outcome (intrusive memory count) was modelled using a Poisson linear mixed model. The baseline number of IMs, and treatment assignment were fitted as fixed effects with a random intercept effect for participant. As daily IM diary data used to calculate the primary outcome was collected sequentially over time (baseline or week 4), we used time series methods and an expectation-maximisation algorithm [38] to impute missing values. We present the median and interquartile range (IQR) to account for outliers and skewed primary outcome data (Fig. 2 and Supplementary Fig. 1); other summary statistics such as the mean and standard deviation (SD) can be found in Supplementary Table 2. The posterior mean of the treatment assignment parameter and the associated 95% credible interval will also be presented. The Supplementary Information provides full details regarding software, model assumptions, priors, missing data, model fit (Supplementary Figs. 2 and 3 and Supplementary Table 3), and sensitivity analyses (Supplementary Figs. 4–7).
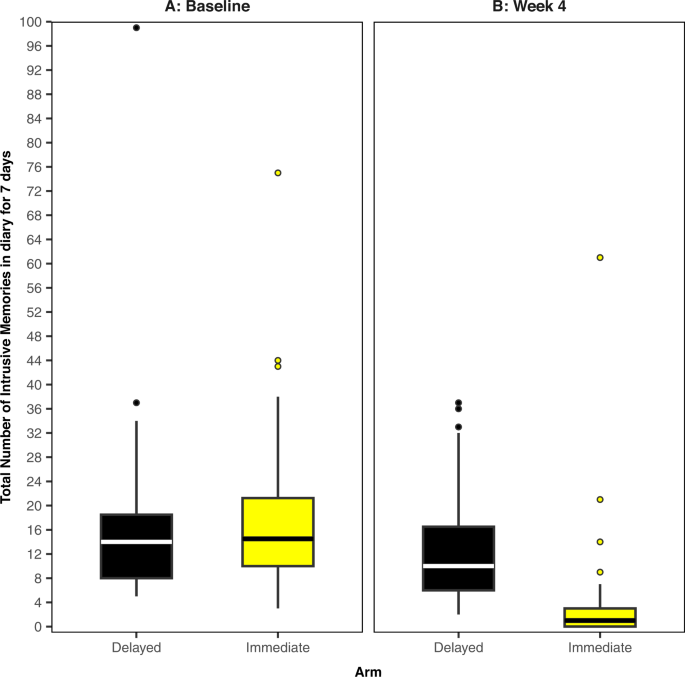
The midline of the boxplot is the median value, with the upper and lower limits of the box being the third and first quartile (75th and 25th percentile), and the whiskers covering 1.5 times the IQR. The dots depict outliers (each dot represents one participant that departed more than 1.5 times the IQR above the third quartile and below the first quartile). All outliers are included in this figure. A Baseline measure for each arm. Total number of IMs of traumatic events recorded by participants in a brief daily online intrusive memory diary for 7 days during the baseline week (i.e. run-in week) for both arms (black = delayed arm (control); n = 39: usual care for 4 weeks; yellow = immediate arm; n = 36: immediate access to the intervention following the baseline week: the intervention consisted of a cognitive task involving a trauma reminder-cue plus Tetris® computer gameplay using mental rotation plus symptom monitoring), showing that the two arms did not differ at baseline (i.e., before the intervention was provided to the immediate arm). B The primary outcome measure for each arm. Total number of IMs of traumatic events recorded by participants in a brief daily online intrusive memory diary for 7 days during week 4 for each arm (black = delayed arm (control); n = 39: usual care for 4 weeks; yellow = immediate arm; n = 36: immediate access to the intervention following the baseline week: the intervention consisted of a cognitive task involving a trauma reminder-cue plus Tetris® computer gameplay using mental rotation plus symptom monitoring), showing that the immediate arm had fewer IMs at week 4 compared to the delayed arm, and that the number of IMs for the immediate arm decreased between the baseline week and week 4.
In Bayesian hypothesis testing, a metric known as a Bayes Factor (BF) [37] provides a continuous measure quantifying how well a hypothesis predicts the data relative to a competing hypothesis. Conventional significance tests using p values do not provide any information about the alternative hypothesis. Theory shows that if the Bayes Factor (BF10) equals 5, this indicates that the data are five times more likely under Hypothesis 1 (H1) than under Hypothesis 0 (H0). This means that H1 provides better probabilistic prediction for the observed data than does H0 [36]. The Bayesian framework requires the explicit specification of (at least) two models to compare, whereas the frequentist framework relies on only one [39]. Clinical researchers are often interested in several questions when developing a treatment: does the treatment work better, worse, or no differently than an existing placebo or active control? This approach to model comparison through a Bayesian framework allows for a more direct way of answering such questions.
In contrast to p values, BFs retain their meaning in situations where data are provided over time, regardless of any sampling decisions, therefore data can be analysed repeatedly as it becomes available, without needing special corrections (see Schönbrodt et al. [30]). Therefore, the Bayesian approach allows interim analyses to guide decision-making during RCTs, making them attractive when using adaptive trial designs [40, 41].
For this study, BFs were computed repeatedly during interim analyses, starting with 20 participants and approximately every 4–10 participants thereafter, up to a maximum of 150. Early stopping of the trial for either sufficient evidence of negative or positive effect was considered if the respective BFs exceeded a pre-defined threshold of 20 which would suggest strong evidence [37]. This sequential BF stopping rule is a suggestion, not a prescription [30]; in Bayesian analyses we are able to sample until hypotheses have been convincingly proven/disproven, or until resources run out [30, 42].
We first calculated a BF for a negative effect of the intervention (i.e. whether participants in the immediate arm had a greater number of IMs at week 4 than the delayed arm). If this BF exceeded 20, we concluded that there was strong evidence for a negative effect of the intervention and the trial may need to be altered or stopped. We then calculated a BF for positive treatment effect (i.e. whether those in the immediate arm had fewer IMs at week 4 than the delayed arm, as opposed to having no difference). If this BF exceeded 20, we concluded that there was strong evidence for the effectiveness of the intervention and consideration could be given to stopping the trial early (Supplementary Information).
Originally, we planned to explore potential “mechanistic” optimisations to improve the effectiveness of the intervention (e.g., time playing Tetris®). Given the rapid accumulation of evidence in favour of a positive treatment effect (see “Results”), we focused on practical “usability enhancements” to aid smooth digital delivery and user experience (Supplementary Information): this included repeating the intrusive memory visualisation step, adding a summary instruction video, and adding a graphical representation of daily IMs for the 4 weeks. An optimisation enhancement round was conducted on Feb 7, 2022, after 55 participants had been randomised. When testing for the effect of these enhancements, sample size analyses were first conducted to estimate the number of participants needed to test for a positive treatment effect, and to compare pre-and post-optimisation groups (Supplementary Figs. 8 and 9).
Results
Between Aug 16, 2021 and Apr 19, 2022, 125 participants were screened by the study team following pre-screening questionnaires (Fig. 1). In total, 102 eligible participants provided informed consent and commenced their baseline week diary. Seven participants were then excluded for having fewer than three IMs during baseline week, seven were lost to follow-up, and two participants withdrew prior to randomisation. Eighty-six participants were randomly assigned to the delayed (n = 43) or immediate (n = 43) arm. Four weeks after randomisation, primary outcome data were available for 75 (87.2%) randomised participants (Fig. 1), who were taken as our intention-to-treat population [43].
Baseline characteristics
Trial participants had a mean age of 38.7 years (SD 9.9), were predominantly women (n = 69; 80.2%) and working full time (n = 66; 76.7%). The number of IMs experienced in the baseline week were similar between trial arms (Fig. 2A) (combined median = 14, IQR = 9–20). Baseline characteristics (Supplementary Table 1) appear balanced between arms.
Treatment effects
Bayesian analyses of the primary outcome involved seven sequential analyses (after 20, 23, 29, 37, 41, 45, and 75 participants completed the primary outcome). From the first analysis (at n = 20) there was strong evidence against a negative treatment effect (BF = 59.8) (Supplementary Table 4). Thereafter, strong evidence in favour of the hypothesis that there was a positive treatment effect rapidly accumulated (Fig. 3). Supportive evidence for the positive treatment effect was reached well before the originally proposed sample size had been randomised (Supplementary Table 4). Following DMC recommendation to the trial steering committee, the trial was concluded early as there was sufficient evidence for the effectiveness of the intervention (final BF = 1.25 × 106) .
Sequential Bayesian analyses showed that the evidence (BFlog, i.e., the logarithmically scaled Bayes factor) in favour of the hypothesis that the intervention caused a positive treatment effect rapidly increased from the point of the first Bayesian analysis (n = 20, raw Bayes factor = 1.27) to the point where on consultation with the DMC the study was concluded (n = 45, raw Bayes factor = 14,700) pending a round of testing of digital optimisations. The study completed with 86 randomised participants, 75 of which were analysed (black line), thus saving 64 participants compared to the original estimated sample size (n = 150).
For the final sample (n = 75), median number of IMs of traumatic events over the 7-day period at week 4 was 1 (IQR = 0–3) in the immediate arm, compared with a median of 10 (IQR = 6–16.5) in the delayed arm (Fig. 2B), with an estimated Cohen’s d effect size of 0.85 (95% CI 0.36–1.33). In the fitted Bayesian model, the categorical treatment assignment parameter, which provides a comparison of the immediate arm to the delayed arm (taken as reference level), has posterior mean −1.9 (95% credible interval −2.49, −1.37). This result indicates that when controlling for baseline number of IMs, we would expect the logged number of IMs to be 1.9 lower for those in the immediate arm than the delayed arm. Equivalently, on the unlogged scale, those in the immediate arm tend to have 0.15 times as many IMs at week 4 as those in the delayed arm.
For the round of “usability enhancement” optimisations (see “Method”) conducted on Feb 7, 2022, there was evidence for a positive treatment effect of the optimised intervention (BF = 7.31) based on analyses of 28 participants who entered the trial under the optimised intervention.
Sensitivity analyses were completed for variation in Bayesian priors or model used, and to assess impact of outliers and missing data imputation (Supplementary Figs. 4–7). There was no considerable variation in the posterior distributions of our model parameters when varying the Bayesian prior or model used. Outliers were identified as observations with large residuals and large Cook’s distance and leverage; analyses excluding these outliers led to the same pattern of results. In the final analyses, only two participants (one on immediate arm, and one on delayed arm) had one value in their week 4 daily IM diary imputed; analyses excluding these participants also led to similar results (Supplementary Information and Supplementary Fig. 7).
Safety
By using sequential analyses, we could early (at n = 20) assure that there was strong evidence against a negative treatment effect (BF = 59.8). All adverse and serious adverse events were unrelated to the study (see Supplementary Tables 5 and 6): there were 19 adverse events (in 14 participants) in the delayed arm, and 13 adverse events (in 11 participants) and a single serious adverse event (admitted to hospital for chest infection with reduced fetal movement) in the immediate arm.
Discussion
Results showed strong evidence that ICU staff experiencing IMs after work-related traumatic events in the COVID-19 pandemic, had fewer IMs (median = 1 per week, IQR = 0–3) when they were given access to the brief digital imagery-competing task intervention, as opposed to usual care for 4 weeks (median = 10 per week, IQR = 6–16.5) (Fig. 2). Sequential Bayesian analyses allowed us to rule out any negative effects early in the trial (by n = 20). Subsequently we were able to conclude the study early—cutting our maximum proposed sample size (n = 150) substantially. Further, we implemented and assessed intervention enhancements in the same trial, providing evidence that a positive treatment effect was still present after changes. To our knowledge, this is the first RCT to optimise an intervention for the treatment of IMs after traumatic events, and one of the first in mental health to use an adaptive Bayesian approach [44,45,46,47].
At trial entry, participants reported a very high number of work-related traumatic events during the pandemic (on average more than 35 traumas, Supplementary Table 1), most of which had taken place over 3 months ago. Many participants had ongoing trauma exposure during the trial. Prior to the intervention, participants experienced a high number of IMs in daily life—median of 14 per week (baseline, Fig. 2 and Supplementary Table 2), reflecting the symptom burden faced by healthcare workers. In the immediate arm, IMs reduced to a median of one per week, with an average 78% reduction in the number of IMs compared to baseline (Supplementary Table 2), and 36% experiencing zero IMs at week 4 (Supplementary Fig. 1).
There remains an urgent need for scalable approaches to support the mental health of frontline healthcare workers. Given their high workload demands, we developed a brief and flexible digital imagery-competing task intervention approach to reduce IMs. After one initial session with research guidance, the intervention could thereafter be used independently and was repeatable to treat different IMs (e.g. intrusive image of a dying patients face; intrusive image of colleague on ambulance stretcher; etc) and new trauma. Compared to studies on the day of trauma [8, 16], current results offer the possibility to deliver treatment when a longer time has elapsed (i.e., weeks or months post-trauma), and thus be useful for individuals already experiencing IMs such as ICU staff.
Adopting new statistical approaches for RCTs is an essential step in speeding up the development of new interventions, and associated moral and ethical decisions in the use of RCTs. By utilising advances in Bayesian trial methodology in the present study to optimise the brief digital imagery-competing task intervention, we substantially reduced the sample size and therefore the time and resources needed to run the trial (Fig. 3). This allowed a more efficient trial without sacrificing statistical and/or scientific rigour. By the explicit model comparison aspect of the Bayesian approach and ability to quantify evidence for the hypotheses of interest using BFs, we could determine, more rapidly, whether the intervention provided a negative or positive effect on the frequency of IMs. The fact that BFs retain their meaning in situations where data is collected over time, allowed us to monitor evidence in an almost continuous manner using sequential analyses, affording the opportunity to exploit the advantages of adaptive trial designs. Based on recent studies on the relationship between p values and BFs, our results are arguably even more robust than if we had merely determined a frequentist p value to some level of significance [48].
In general, the practical consequences of using more efficient adaptive Bayesian trial designs to develop therapeutic approaches are clear—they can provide information rapidly to support a go/no-go clinical development decision, thus helping treatment innovation by reducing the time required to progress to subsequently assess efficacy. Here, it helped us examine the effects of a new intervention approach. Methods advances are needed given the relatively slow progress of behavioural interventions since the 1960s [49, 50]. This Bayesian study focuses on the primary outcome—a fuller set of analyses using frequentist statistical approaches will be reported in a companion article [35].
In retrospect, we could have conducted an even more efficient trial. As the evidence progression shows, there was sufficient evidence (BF > 20) when 29 participants had completed the trial to conclude that there were positive effects of the intervention. However, at that point to ensure the robustness of the results we conducted a more thorough sensitivity analyses. Simultaneously, we strived for equivalent allocation to the two arms (immediate and delayed), a balancing that ultimately delayed the conclusion of the study. Such sensitivity and balancing issues can be mitigated beforehand [51]. Further limitations of this trial include the use of a wait list control (i.e. at the time of the primary outcome, prior to the crossover). The design was chosen due to the early stage of the intervention development, the lack of a comparator treatment for IMs, and ethical considerations around the participant population. The statistician running Bayesian analyses (VR) was not blinded to group allocation and was a part of the wider study team. There is a reduction of IMs in both arms over time, and placebo effects cannot be ruled out. The next study should use a comparison arm rather than wait list.
The current study raises numerous questions for future research. For example, further analysis is important to identify whether the improvement is linked to intervention load. Interestingly, we think it is unlikely that there is a simple correlation between more sessions and improved outcomes—rather, when the intervention works optimally it is hypothesised that the participant would only need one session with the intervention per different intrusive memory. Thus, paradoxically we may expect that fewer sessions would be related to better outcomes, and a high number of sessions to be related to those who either have difficulties with using the intervention or who have a high number of different IMs on their list. Future work could also analyse individual differences in baseline number of IMs to determine how this impacts treatment response, as well as pre-existing mental health difficulties. Again, it may be the case that treatment response is not simply determined by the overall number of baseline IMs, but rather the number of different IMs on their list (i.e. distinct images/scenes from a traumatic event). It would be of interest to add a re-administration of the intrusive memory list post-intervention at the time of the primary outcome. This would allow a test of the hypothesis that use of the intervention is associated with a change in the sensory elements in the intrusive memory descriptions (hotspots) rather than say affective or cognitive elements, indicated by recent studies in both lab [52] and clinic [53].
As stated in the Introduction, the disruption of visuospatial processing while memory is being stored or updated should render the memory less likely to be triggered, i.e. from becoming intrusive [10, 13]. Theory related to memory updating which informed the development of the intervention suggests we should be able to observe a reduction in the number of intrusive memories while leaving the underlying episodic memory component intact [54], see also [55]. While this dissociation has been shown in laboratory settings [14, 15], it remains to be examined in clinical samples. Further, it is of great interest to understand how this works mechanistically for consolidated memories, as we have begun to address elsewhere [56]. To render an intrusive memory from an intrusive memory state to a non-intrusive memory state indeed involves new learning [18], and we hypothesise that the Tetris® game play task provides a form of prediction error compared to the usual cognitive activity occurring after recall of the trauma. Further work is clearly needed at various mechanistic levels from mathematics [56], though biology [57], pharmacology [58] and behavioural work such as that presented here.
There is an urgent and unmet need to develop novel approaches to support the mental health of healthcare workers to continue to manage the emotionally traumatic nature of their clinical work [7]. Current clinical guidelines show we lack treatment approaches for people facing ongoing trauma exposure, such as those working in the ICU [59]. Addressing the mental health challenges of healthcare workers is important for them as individuals but is also important for the sustainability of the provision of healthcare services, particularly during a pandemic and in the recovery of health services post-pandemic. In this study, we addressed the need for accelerating treatment development by the use of Bayesian methodology, essentially cutting sample size in half while allowing for testing more hypotheses than in a traditional frequentist trial. Results showed strong evidence in favour of a positive treatment effect of the brief (one guided-session), remotely delivered digital intervention in reducing the number of IMs after trauma. Next steps include a trial with a control comparator and longer term follow up period. Overall, for ICU staff with unwanted intrusive images of traumatic events from work, this optimisation trial during the pandemic suggests that a brief digital imagery-competing task intervention may help reduce the frequency at which trauma memories intrude. This novel intervention shows promise for further development.
Data availability
Anonymised databases with the individual participant data and the metadata for Bayesian analyses, along with a data dictionary are available on the Open Science Framework (OSF) (osf.io/m5cvj) for anyone who wishes to access the data for any purpose. The Study Protocol and Bayesian Statistical Analysis Plan are available on the OSF platform (osf.io/2xn5m). All data and supporting information mentioned above will be shared indefinitely and with no end date on the OSF platform.
Code availability
Analytical code files (R scripts) are available on the Open Science Framework (OSF) (osf.io/m5cvj) for anyone who wishes to access the code for any purpose. This will be shared indefinitely with no end date on the OSF platform.
References
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed., vol. 21. Washington DC: American Psychiatric Publishing, 2013.
Kleim B, Bingisser MB, Westphal M, Bingisser R. Frozen moments: flashback memories of critical incidents in emergency personnel. Brain Behav. 2015;5:e00325.
Carmassi C, Foghi C, Dell’Oste V, Cordone A, Bertelloni CA, Bui E, et al. PTSD symptoms in healthcare workers facing the three coronavirus outbreaks: what can we expect after the COVID-19 pandemic. Psychiatry Res. 2020;292:113312.
Greenberg N, Weston D, Hall C, Caulfield T, Williamson V, Fong K. Mental health of staff working in intensive care during Covid-19. Occup Med. 2021;71:62–67.
Colville G, Hammond J, Perkins-Porras L. Post-traumatic stress symptoms in intensive care staff working in adult and paediatric settings. Crit Care. 2015;19:P531.
Hall CE, Milward J, Spoiala C, Bhogal JK, Weston D, Potts HWW, et al. The mental health of staff working on intensive care units over the COVID-19 winter surge of 2020 in England: a cross sectional survey. Br J Anaesth. 2022;128:971–9.
Holmes EA, O’Connor RC, Perry VH, Tracey I, Wessely S, Arseneault L, et al. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020;7:547–60.
Iyadurai L, Blackwell SE, Meiser-Stedman R, Watson PC, Bonsall MB, Geddes JR, et al. Preventing intrusive memories after trauma via a brief intervention involving Tetris computer game play in the emergency department: a proof-of-concept randomized controlled trial. Mol Psychiatry. 2018;23:674–82.
Holmes EA, James EL, Kilford EJ, Deeprose C. Key steps in developing a cognitive vaccine against traumatic flashbacks: visuospatial Tetris versus verbal Pub Quiz. PLoS ONE. 2010;5:e13706.
Singh L, Espinosa L, Ji JL, Moulds ML, Holmes EA. Developing thinking around mental health science: the example of intrusive, emotional mental imagery after psychological trauma. Cogn Neuropsychiatry. 2020;25:348–63.
Holmes EA, Ghaderi A, Harmer CJ, Ramchandani PG, Cuijpers P, Morrison AP, et al. Psychological treatments research in tomorrow’s science: seeing further. Lancet Psychiatry. 2018;5:237–86.
Astill Wright L, Horstmann L, Holmes EA, Bisson JI. Consolidation/reconsolidation therapies for the prevention and treatment of PTSD and re-experiencing: a systematic review and meta-analysis. Transl Psychiatry. 2021;11:453.
Bisby JA, Burgess N. Differential effects of negative emotion on memory for items and associations, and their relationship to intrusive imagery. Curr Opin Behav Sci. 2017;17:124–32.
Lau-Zhu A, Henson RN, Holmes EA. Intrusive memories and voluntary memory of a trauma film: differential effects of a cognitive interference task after encoding. J Exp Psychol Gen. 2019;148:2154–80.
Lau-Zhu A, Henson RN, Holmes EA. Selectively interfering with intrusive but not voluntary memories of a trauma film: accounting for the role of associative memory. Clin Psychol Sci. 2021;9:1128–43.
Kanstrup M, Singh L, Göransson KE, Widoff J, Taylor RS, Gamble B, et al. Reducing intrusive memories after trauma via a brief cognitive task intervention in the hospital emergency department: an exploratory pilot randomised controlled trial. Transl Psychiatry. 2021;11:30.
Horsch A, Vial Y, Favrod C, Harari MM, Blackwell SE, Watson P, et al. Reducing intrusive traumatic memories after emergency caesarean section: a proof-of-principle randomized controlled study. Behav Res Ther. 2017;94:36–47.
Monfils MH, Holmes EA. Memory boundaries: opening a window inspired by reconsolidation to treat anxiety, trauma-related, and addiction disorders. Lancet Psychiatry. 2018;5:1032–42.
Milton AL, Holmes EA. Of mice and mental health: facilitating dialogue and seeing further. Philos Trans R Soc B: Biol Sci. 2018;373:20170022.
James EL, Bonsall MB, Hoppitt L, Tunbridge EM, Geddes JR, Milton AL, et al. Computer game play reduces intrusive memories of experimental trauma via reconsolidation-update mechanisms. Psychol Sci. 2015;26:1201–15.
Kessler H, Holmes EA, Blackwell SE, Schmidt AC, Schweer JM, Bücker A, et al. Reducing intrusive memories of trauma using a visuospatial interference intervention with inpatients with posttraumatic stress disorder (PTSD). J Consult Clin Psychol. 2018;86:1076–90.
Thorarinsdottir K, Holmes EA, Hardarson J, Stephenssen ES, Jonasdottir MH, Kanstrup M, et al. Using a brief mental imagery competing task to reduce the number of intrusive memories: exploratory case series with trauma-exposed women. JMIR Form Res. 2022;6:e37382.
Gamble B, Depa K, Holmes EA, Kanstrup M. Digitalizing a brief intervention to reduce intrusive memories of psychological trauma: qualitative interview study. JMIR Ment Health. 2021;8:e23712.
Singh L, Kanstrup M, Depa K, Falk AC, Lindström V, Dahl O, et al. Digitalizing a brief intervention to reduce intrusive memories of psychological trauma for health care staff working during COVID-19: exploratory pilot study with nurses. JMIR Form Res. 2021;5:e27473.
Stallard N, Hampson L, Benda N, Brannath W, Burnett T, Friede T, et al. Efficient adaptive designs for clinical trials of interventions for COVID-19. Stat Biopharm Res. 2020;12:483–97.
Volz E, Baguelin M, Bhatia S, Boonyasiri A, Cori A, Cucunubá Z, et al. Report 5: phylogenetic analysis of SARS-CoV-2. Imperial College London: MRC Centre for Global Infectious Disease Analysis; 2020. https://doi.org/10.25561/77169.
Yarnell CJ, Abrams D, Baldwin MR, Brodie D, Fan E, Ferguson ND, et al. Clinical trials in critical care: can a Bayesian approach enhance clinical and scientific decision making? Lancet Respir Med. 2021;9:207–16.
Liu M, Li Q, Lin J, Lin Y, Hoffman E. Innovative trial designs and analyses for vaccine clinical development. Contemp Clin Trials. 2021;100:106225.
Griffiths G, Fitzgerald R, Jaki T, Corkhill A, Marwood E, Reynolds H, et al. AGILE-ACCORD: a randomized, multicentre, seamless, adaptive phase I/II platform study to determine the optimal dose, safety and efficacy of multiple candidate agents for the treatment of COVID-19: a structured summary of a study protocol for a randomised platform trial. Trials. 2020;21:544.
Schönbrodt FD, Wagenmakers EJ, Zehetleitner M, Perugini M. Sequential hypothesis testing with Bayes factors: efficiently testing mean differences. Psychol Methods. 2017;22:322–39.
Holmes EA, Grey N, Young KAD. Intrusive images and ‘hotspots’ of trauma memories in Posttraumatic Stress Disorder: an exploratory investigation of emotions and cognitive themes. J Behav Ther Exp Psychiatry. 2005;36:3–17.
Singh L, Pihlgren SA, Holmes EA, Moulds ML. Using a daily diary for monitoring intrusive memories of trauma: a translational data synthesis study exploring convergent validity. Int J Methods Psychiatr Res. 2022;32:e1936.
Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, et al. The clinician-administered ptsd scale for DSM-5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30:383–95.
Holmes EA, Ghaderi A, Eriksson E, Lauri KO, Kukacka OM, Mamish M, et al. ‘I Can’t Concentrate’: a feasibility study with young refugees in Sweden on developing science-driven interventions for intrusive memories related to trauma. Behav Cogn Psychother. 2017;45:97–109.
Iyadurai L, Highfield J, Kanstrup M, Markham A, Ramineni V, Guo B, et al. Reducing intrusive memories after trauma via an imagery-competing task intervention in COVID-19 intensive care staff: a randomised controlled trial (under review). 2023.
Schönbrodt FD, Wagenmakers EJ. Bayes factor design analysis: planning for compelling evidence. Psychon Bull Rev. 2018;25:128–42.
Kass RE, Raftery AE. Bayes factors. J Am Stat Assoc. 1995;90:773–95.
Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm. J R Stat Soc: Ser B Methodol) 1977;39:1–22.
Dienes Z. How Bayes factors change scientific practice. J Math Psychol. 2016;72:78–89.
Ewings S, Saunders G, Jaki T, Mozgunov P. Practical recommendations for implementing a Bayesian adaptive phase I design during a pandemic. BMC Med Res Methodol. 2022;22:25.
Bothwell LE, Avorn J, Khan NF, Kesselheim AS. Adaptive design clinical trials: a review of the literature and ClinicalTrials.gov. BMJ Open. 2018;8:e018320.
Edwards W, Lindman H, Savage LJ. Bayesian statistical inference for psychological research. Psychol Rev. 1963;70:193–242.
ICH E9 Expert Working Group. Statistical principles for clinical trials (ICH harmonised tripartite guideline E9). Stat Med. 1999;18:1905–42.
Lijffijt M, Murphy N, Iqbal S, Green CE, Iqbal T, Chang LC, et al. Identification of an optimal dose of intravenous ketamine for late-life treatment-resistant depression: a Bayesian adaptive randomization trial. Neuropsychopharmacology. 2022;47:1088–95.
Schmitz JM, Stotts AL, Vujanovic AA, Weaver MF, Yoon JH, Vincent J, et al. A sequential multiple assignment randomized trial for cocaine cessation and relapse prevention: tailoring treatment to the individual. Contemp Clin Trials. 2018;65:109–15.
Suchting R, Green CE, de Dios C, Vincent J, Moeller FG, Lane SD, et al. Citalopram for treatment of cocaine use disorder: a Bayesian drop-the-loser randomized clinical trial. Drug Alcohol Depend. 2021;228:109054.
Freeman TP, Hindocha C, Baio G, Shaban NDC, Thomas EM, Astbury D, et al. Cannabidiol for the treatment of cannabis use disorder: a phase 2a, double-blind, placebo-controlled, randomised, adaptive Bayesian trial. Lancet Psychiatry. 2020;7:865–74.
Wagenmakers EJ. Approximate objective Bayes factors from p-values and sample size: the 3p√n rule. PsyArXiv. 2022.
Goodwin GM, Holmes EA, Andersson E, Browning M, Jones A, Lass-Hennemann J, et al. From neuroscience to evidence based psychological treatments—the promise and the challenge, ECNP March 2016, Nice, France. Eur Neuropsychopharmacol. 2018;28:317–33.
Holmes EA, Craske MG, Graybiel AM. Psychological treatments: a call for mental-health science. Nature. 2014;511:287–9.
Yuan W, Chen M-H, Zhong J. Bayesian design of superiority trials: methods and applications. Stat Biopharm Res. 2022;14;433–43.
Singh L, Garate B, Hoppe JM, Holmes EA. Qualitative analysis of hotspots and intrusive memories after viewing an aversive film highlights their sensory and spatial features. Sci Rep. 2022;12:7049.
Hoppe JM, Walldén YSE, Kanstrup M, Singh L, Agren T, Holmes EA, et al. Hotspots in the immediate aftermath of trauma—mental imagery of worst moments highlighting time, space and motion. Conscious Cogn. 2022;99:103286.
Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12:256–8.
Visser RM, Lau-Zhu A, Henson RN, Holmes EA. Multiple memory systems, multiple time points: How science can inform treatment to control the expression of unwanted emotional memories. Philos Trans R Soc B: Biol Sci. 2018;373:20170209.
Holmes EA, Espinosa L, Visser RM, Bonsall MB, Singh L. Psychological interventions as they relate to intrusive thinking: intrusive, emotional mental imagery after traumatic and negative events. Strüngmann Forum Reports, MIT Press; 2020.
Popik B, Amorim FE, Amaral OB, de Oliveira Alvares L. Shifting from fear to safety through deconditioning-update. Elife. 2020;9:e51207.
Sevenster D, Beckers T, Kindt M. Prediction error governs pharmacologically induced amnesia for learned fear. Science. 2013;339:830–3.
National Guideline Alliance (UK). Evidence reviews for psychological, psychosocial and other non-pharmacological interventions for the treatment of PTSD in adults. National Institute for Health and Care Excellence (NICE); NICE Guidelines; NG116; 2018.
Acknowledgements
The study was funded by the Wellcome Trust (223016/Z/21/Z). TJ is supported by a grant from UK Medical Research Council (MC_UU_00002/14). For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. Tetris® has been licenced for use within i-spero® from The Tetris Company. The authors would like to thank the Intensive Care Society, in particular Dr Sandy Mather and Alex Day; Our Data Monitoring Committee members comprising Prof Andreas Reif (chair, Frankfurt am Main—Goethe University), Prof Steve Hollon (Vanderbilt University), Prof Ian Penton-Voak (Bristol University), and adjunct member Prof Michael B. Bonsall (University of Oxford); Trial Steering Committee including Prof Guy Goodwin, Pooyan Behbahani, and Rebecca Dias; Expert Advisors including Dr Nick Grey, Prof Sir Simon Wessely, and Prof Jonathon Bisson. Members of study team including Veronika Kubičková, Alfred Markham, Zunaid Islam and Marie Kanstrup for support; Our Data Management Team including Mark Dziedzic, Sameer Iqbal, and Nikita Shukan.
Funding
The study was funded by the Wellcome Trust (223016/Z/21/Z). The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. P1vital Products Ltd was the trial sponsor. Open access funding was provided by Uppsala University.
Author information
Authors and Affiliations
Contributions
EAH and JK conceived the trial, gained funding, and contributed to the study design. CS and MBB provided expert input into the conception of the trial, and MBB and TJ into the study design. LI delivered the intervention, acquired the data, and with JK, JH and CS contributed to interpretation of the work. VR and MBB accessed and verified the underlying data. VR did the statistical analyses, and with MBB, EAH and PM contributed to the analysis of the work and interpretation of data. VR, PM, EAH and MBB wrote the first draft of the article. All authors contributed intellectual content, critically revised the article, and approved the submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
The study was funded by the Wellcome Trust (223016/Z/21/Z). JK is shareholder and director of P1vital Products Ltd which is the study sponsor and manufacturer of i-spero®. VR and LI are employed by P1vital Products Ltd. MBB is an adjunct member of the DMC. CS salary is partly funded by National Institute for Health Research (NIHR133788) and Medical Research Council (MR/S035753/1 and MR/X005070/1). TJ is supported by a grant from UK Medical Research Council (MC_UU_00002/14). EAH salary is part funded by Wellcome Trust (223016/Z/21/Z) via consultancy to P1vital Products Ltd. EAH is on the Board of Trustees of the MQ Foundation. EAH also receives funding from the Swedish Research Council, Rannís The Icelandic Research Fund, OAK foundation, The Lupina Foundation and AFA Försäkring. EAH developed the intervention approach and training in using it (Anenome™). EAH receives book royalties from Guildford Press and Oxford University Press, and receives occasional honoraria for conference keynotes and clinical workshops. All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41380_2023_2062_MOESM9_ESM.pdf
Supplementary Figure 8: Bayes Factor vs Sample Size plot to Test for a Positive Treatment Effect under the Optimised Intervention.
41380_2023_2062_MOESM10_ESM.pdf
Supplementary Figure 9: Bayes Factor vs Sample Size plot to Compare the Optimised Intervention to the Former Un-optimised Intervention.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramineni, V., Millroth, P., Iyadurai, L. et al. Treating intrusive memories after trauma in healthcare workers: a Bayesian adaptive randomised trial developing an imagery-competing task intervention. Mol Psychiatry 28, 2985–2994 (2023). https://doi.org/10.1038/s41380-023-02062-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02062-7
This article is cited by
-
Memory persistence: from fundamental mechanisms to translational opportunities
Translational Psychiatry (2024)
-
Reducing intrusive memories after trauma via an imagery-competing task intervention in COVID-19 intensive care staff: a randomised controlled trial
Translational Psychiatry (2023)