Abstract
Objectives
To assess the prognostic significance of an early normal/mildly abnormal conventional EEG (cEEG) on seizure risk in neonates undergoing therapeutic hypothermia.
Methods
We reviewed the video-EEG recordings from a large cohort of neonates treated with therapeutic hypothermia for hypoxic–ischemic encephalopathy from 2008 to 2017 in a single tertiary center. Continuous video-EEG was started as soon as possible (median 8.2 h) and continued throughout hypothermia and rewarming. We studied those neonates with a normal/mildly abnormal EEG during the first 24 h of monitoring.
Results
A total of 331 neonates were treated with hypothermia and 323 had cEEG recordings available for review; 99 were excluded because of a moderately/severely abnormal cEEG background and/or seizure during the first 24 h of recording, and an additional eight because of early rewarming. The remaining 216 had a normal/mildly abnormal cEEG in the first 24 h. None of these patients subsequently developed seizures.
Conclusion
A normal/mildly abnormal cEEG during the first 24 h indicates a very low risk of subsequent seizures. This suggests that cEEG monitoring can be safely discontinued after 24 h if it has remained normal or excessively discontinuous and no seizures are detected, limiting the need for this resource-intensive and expensive tool.
Similar content being viewed by others
Introduction
Over the last decade, therapeutic hypothermia (TH) has become a standard of care for neonates with moderate to severe neonatal encephalopathy secondary to birth asphyxia.1 It is now a widely used treatment that has been shown to be both safe and effective in improving neuro-developmental outcome.2
Seizures are common in neonates with hypoxic–ischemic encephalopathy (HIE)3,4,5,6 and are associated with short- and long-term unfavorable developmental outcomes7 and an increased risk of moderate to severe injury on magnetic resonance imaging (MRI).8,9 Conventional electroencephalography (cEEG) monitoring is the gold standard for evaluating brain function and for recording seizures. However, it is resource intensive and requires a large commitment in terms of equipment, time, and personnel.10 Furthermore, cEEG is not uniformly available in neonatal units.11 Hence, amplitude-integrated EEG (aEEG) is routinely used in many units because it is easier to apply and interpret at the bedside by a neonatologist. However, it has its own specific limitations, including an interpreter-dependent sensitivity and specificity and limited ability to detect short, focal, or low amplitude seizure.12 In 2011, the American Clinical Neurophysiology Society recommended monitoring with cEEG for at least 24 h all neonates at risk of seizure, including those with HIE.13 Monitoring with either cEEG or aEEG during TH is also recommended by the American Academy of Pediatrics, although the duration of monitoring is not specified.1
Identifying neonates at higher risk of seizure is challenging, as no clinical variables—including mental status, clinically suspected seizures prior to monitoring, or biological signs (pH, base excess, or 10-min Apgar score)—have been strongly associated with the occurrence of seizure during TH. The only variable associated with the development of a seizure is an abnormal EEG background.6,14 It is also known that seizures tend to arise within the first 24 h following the acute insult.4,6 However, neonates with seizures during rewarming have been described,6,8,15,16,17 prompting many neonatal units to monitor neonates undergoing TH for the entire duration of cooling and rewarming.4,5,18 This appears to be the current clinical practice in centers using cEEG to monitor neonates undergoing TH.
The aim of this study was to assess the prevalence of seizures in neonates with an initially normal or mildly abnormal background on cEEG. We hypothesized that the number of neonates with seizure onset after 24 h of a normal background with no seizure activity would be low. This would refine the guidelines for cEEG monitoring in neonates and help directing resource utilization and clinical management.
Patients and methods
Consecutive newborns with encephalopathy presumed secondary to HIE who underwent TH with whole-body cooling at University of California, San Francisco, over a 9-year period between April 2008 and April 2017 were considered for inclusion in this cohort study. According to our center guidelines, TH was provided to infants >36 weeks gestational age, with evidence of perinatal acidosis by one of the following: pH < 7.0 or base deficit >12 on cord or first patient blood gas, 10-min Apgar score <5, or a history of prolonged resuscitation at birth, and moderate to severe encephalopathy at any time within 6 hours of birth. To note, in our center as well as in most centers in the United States, EEG findings are not part of the inclusion criteria for TH. Exclusion criteria for TH included coagulopathy with active bleeding and prenatally diagnosed syndromes or metabolic disorders not compatible with life. Whole-body cooling was initiated as soon as possible using a servo-regulated cooling blanket device (Cincinnati Sub-Zero Blanketrol III). Target temperature of 33.5 °C was maintained for 72 h, from the time core rectal temperature was achieved, followed by gradual rewarming over 6–12 h. Infants born in outside hospitals were passively cooled, once screened as candidates for TH. The majority of transports occurred under a passive cooling protocol with continuous rectal temperature monitoring, but more recently by active cooling at target temperature using a portable servo-regulated cooling blanket device (Tecotherm-Neo, Inspiration Medical LTD).19 All patients received morphine infusions (10–25 mcg/kg/h and 5–10 mcg/kg boluses as needed) throughout TH to reduce discomfort attributable to encephalopathy and to counteract the stress response induced by hypothermia, which might reduce the effectiveness of hypothermia.20,21
As part of our clinical protocol, continuous video-EEG was initiated as soon as possible after admission to the intensive care nursery using a Nicolet One video-EEG system (April 2008–Feb 2015) or Natus Networks video-EEG system (February 2015–April 2017) and continued until rewarming was completed and at least for 24 h after the last seizure. A trained technician applied surface electrodes according to the international 10–20 system, modified for neonates. EEG recordings were interpreted by pediatric neurophysiologists for clinical use in the acute setting. Using stored files, video-EEG recordings of the first 24 h were evaluated by a neonatal neurophysiologist (M.R.C.) blinded to all clinical factors except patient age and setting of TH, for the purpose of this study. EEG recordings were classified based on the worst EEG grade that persisted for at least 1 h, as previously reported.3 A normal cEEG was defined as a normal pattern for gestational age; a mildly abnormal cEEG was defined as an excessively discontinuous pattern with persistence of discontinuous activity occupying more than 50% of the recording and consisting of bursts of normal activity separated by interburst intervals >6 and ≤15 s in duration, and amplitude between 5 and 25 μV. Neonates with an EEG background characterized by continuous low voltage, burst suppression pattern, or low voltage undifferentiated were grouped together in a moderate to severe EEG background group (Fig. 1). A seizure was defined as a repetitive, evolving, and stereotyped EEG pattern, with a definite beginning and end, a minimum duration of 10 s, and a minimal amplitude of 2 μV. Clinical seizures without cEEG correlate were not considered. Seizures were treated with antiepileptic drugs (AED) including lorazepam, phenobarbital, fosphenytoin, and levetiracetam according to institutional guidelines. Exclusion criteria for this study were: cEEG unavailable for review or started after 24 h of life, failure to complete 72 h of TH, a moderate to severe cEEG background, and/or the presence of seizure in the first 24 h of recording. cEEG of the included neonates were reviewed until the end of recording to assess for background and the presence of seizure. Demographic data and MRI results were extracted from medical records. The infants were imaged shortly after rewarming using a specialized neonatal head coil. Imaging sequences included T1- and T2-weighted MRI and diffusion-weighted imaging. Brain injury was categorized as “none-mild” or “moderate-severe” as previously published.3,9 The Committee on Human Research at UCSF approved this study.
Distinction between excessively discontinuous and burst suppression pattern on EEG. a Excessively discontinuous: burst of normal activity and amplitude, intermixed with interburst interval (IBI) lasting more than 6 s. b Burst suppression: short high-amplitude burst with no normal activity, in between prolonged period of very low amplitude. Note the electrocardiogram artifact, most evident in the Fp2-T4 leads. No discernible brain activity is recorded during the interburst interval.
Results
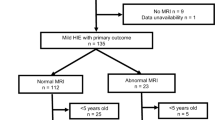
During the study period, 331 newborns were treated with hypothermia and 323 had continuous video-EEG available for review. Of these, 99 neonates (31%) were excluded because of an abnormal EEG and/or seizure in the first 24 h. In particular, 13 (4%) had seizures in the first 12 h of recording in the context of a normal/mildly abnormal EEG background, and 86 (27%) had a moderately to severely abnormal EEG background. Two-hundred and twenty-four neonates (69%) had a normal/mildly abnormal EEG background and no seizures in the first 24 h of life. Of them, eight were rewarmed early (seven had severe persistent pulmonary hypertension of the newborn, and one was rewarmed per parental request).
Hence, 216 neonates were included in the study. Their demographics are presented in Table 1. cEEG was started at a median age of 8.2 h (interquartile range 5.7–10.6 h of life) and continued for a median of 87 h (interquartile range 79–94 h). No patients with an initially normal/excessively discontinuous EEG evolved into a more severe pattern afterward and no seizures were detected in this large cohort. Hence, the probability of seizure occurrence, after 24 h of a normal/mildly abnormal cEEG, calculated from our cohort is extremely low (<1.38%; 95% confidence interval).
Interestingly, 21 (10%) received AED prior to cEEG recording for paroxysmal events that were presumed to be and treated as seizure based on clinical observation. Those patients were treated with phenobarbital (n = 13), lorazepam (n = 5), or both (n = 3). None of these 21 neonates had subsequently EEG-confirmed seizures during the monitoring.
About 214/216 patients had an MRI performed after TH (median: 4 days of life). Of those, 208 (97%) had no to mild brain injury.
Discussion
In this large single-center study of neonates continuously monitored with video-EEG during TH and rewarming, the patients with a normal or mildly abnormal cEEG background who did not experience seizures in the first 24 h, did not develop seizure during the rest of monitoring. Furthermore, neonates with normal/mildly abnormal EEG background from the beginning had overall a low risk for seizures (13/229; 5.7%), and in all cases, seizures occurred within the first 12 h of recording, corresponding to the first 24 h of life. The low incidence of seizures in neonates with a normal or mildly abnormal EEG background is in keeping with previous studies. In 1998, Laroia et al. demonstrated that in neonates at risk of seizures, early EEG background predicts seizure occurrence (only one of 24 neonates with a normal or mildly abnormal EEG background had seizure compared to 22/25 with an abnormal background).14 In the cohort reported by Del Balzo et al., of 20 neonates undergoing TH, none of the 10 neonates with a normal EEG background had seizures.22 In contrast, in a recent multi-center study, a third of neonates (18/54) with a normal/excessively discontinuous background had seizures6; however, only one of them had seizure onset during rewarming. Seizure onset after 24 h of EEG monitoring is reported in the literature.5,6,8,17,23,24 Information on EEG background in those cases are scarce, although most reported neonates had severe encephalopathy. Some studies have included in the definition of excessively discontinuous pattern also more severe patterns such as burst-suppression and longer interburst interval.25,26 This could explain the higher percentage of seizures and the poor outcome reported for those patients.
In our cohort, 69% of neonates had a normal or mildly abnormal EEG and no seizures in the first 24 h of recording and only 30% had a moderately to severely abnormal EEG (either presence of seizure in the context of a normal/mild background or a moderate/severe background). This proportion of neonates with normal or mildly abnormal EEG is higher than previously reported in neonates treated with TH.5,27 Possible explanations include a drift toward cooling neonates with milder encephalopathy as described in other cohorts,28,29 earlier recognition of encephalopathy, and faster implementation of cooling leading to the possibility that, by the time EEG is started, the brain activity has already improved in some neonates. Furthermore, our definition of excessively discontinuous background encompasses a wide range of interburst intervals (up to 15 s). In the setting of TH, an excessively discontinuous cEEG pattern is not infrequently seen possibly due to the effects of concomitant medications such as morphine30 and/or the decreased temperature itself. This could explain why, even in the presence of an excessively discontinuous background on cEEG, no neonates had seizure onset after 24 h. Furthermore, consistent with other studies,3,31 we did not observe evolution from normal/excessively discontinuous background into more severe patterns. In the context of TH for presumed HIE, EEG background appears to have the greatest prognostic value at 24 h of monitoring for later seizure occurrence.3,27,32
In our large cohort, the vast majority of neonates with a normal/excessively discontinuous EEG background (97%) had no or mild MRI brain injury. The present data are in line with previous studies showing that 100% of neonates with an early normal background and 73–75% of those with an excessively discontinuous pattern after rewarming had no or only mild MRI injury.3,27 This association between a normal/mildly abnormal initial EEG and none or mild MRI brain injury supports the observation that an excessively discontinuous background in the setting of TH is usually not associated with a poor outcome.
Lately, many centers have started offering TH to neonates with mild encephalopathy,28,29 despite lack of data in support of this practice. This changing attitude has been explained by many arguments, including reports of adverse neurodevelopmental outcome in neonates with mild HIE,33,34 difficulty in grading neonatal encephalopathy soon after birth, and concern about missing the 6-h window for initiating cooling.28 Since the clinical evolution of the encephalopathy can be rapid, it is possible that in our cohort some neonates who were cooled had only a mild encephalopathy.
Because none of the neonates with no seizure activity and a normal/mildly abnormal EEG during the first 24 h subsequently developed seizures, it may be reasonable to discontinue cEEG monitoring at that time and in that context. Our finding could help refine the guidelines for cEEG monitoring in patients with HIE. Although cEEG monitoring is the gold standard for detecting seizures, it is labor intensive as trained technologists and physicians are required to review traces, often at all hours of the day and night.10 Discontinuing cEEG after 24 h may lead to better allocation of resources. This recommendation should be balanced with the risk that, without EEG monitoring, some neonates may be diagnosed with seizures based on suspicious movements, exposing them to unnecessary AED treatment.18 The role of aEEG for seizure screening and monitoring of background evolution (including return to sleep wake cycling) merits additional study.
To our knowledge, this is the largest reported cohort of neonates with HIE treated with hypothermia and continuously monitored with video-EEG. Nevertheless, this study has some limitations. First, background assessment was performed by visual interpretation of a single rater. Although a single EEG rater provides consistency, this approach may limit the generalizability of the findings. A recent study showed that the interrater agreement for the classification of cEEG background comparing three raters was 72% full agreement.35 More accurate ways of classifying cEEG background in neonates, including automated quantitative analysis of EEG activity, may help in overcoming those challenges and provide objective basis for studies across all centers worldwide.36,37 Additionally, 10% of neonates in our cohort had received AED prior to cEEG recording for paroxysmal events that were presumed to be seizures on a clinical basis. The significance of these events remains unknown, but AED might have protected them from developing further seizures in the next hours or days, biasing our results toward a lower seizure incidence. However, in previous studies, AED administration prior to monitoring was not associated with a lower or higher EEG-proven seizure risk .6
Conclusion
Our data demonstrate that the risk of developing seizures is extremely low in infants treated with TH with a normal/mildly abnormal cEEG and no seizure activity during the first 24 h. Furthermore, none of these infants evolved into a more severe pattern and very few have evidence of moderate to severe brain injury on MRI.
We suggest that cEEG may be safely discontinued after 24 h of normal recording in neonates who are treated with TH. This practice could lead to better resource allocation focused on higher risk and more fragile infants. Discontinuation of cEEG after 24 h in HIE patients with a very low risk of seizures would optimize the utilization of resources and workload without compromising seizure detection.
References
Papile, L.-A. Hypothermia and neonatal encephalopathy. Pediatrics 133, 1146–1150 (2014).
Jacobs, S. E. et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 1, 1465–1858 (2013).
Nash, K. B. et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neurology 76, 556–562 (2011).
Lynch, N. E. et al. The temporal characteristics of seizures in neonatal hypoxic ischemic encephalopathy treated with hypothermia. Seizure 33, 60–65 (2015).
Mahfooz, N. et al. Optimal Duration of Continuous Video-Electroencephalography in Term Infants With Hypoxic-Ischemic Encephalopathy and Therapeutic Hypothermia. J. Child Neurol. 32, 522–527 (2017).
Glass, H. C. et al. Risk factors for EEG seizures in neonates treated with hypothermia: A multicenter cohort study. Neurology 82, 1239–1244 (2014).
Uria-Avellanal, C., Marlow, N. & Rennie, J. M. Outcome following neonatal seizures. Semin Fetal Neonatal Med. 18, 224–232 (2013).
Shah, D. K. et al. Electrographic seizures are associated with brain injury in newborns undergoing therapeutic hypothermia. Arch. Dis. Child - Fetal Neonatal Ed. 99, F219–F224 (2014).
Glass, H. C. et al. Seizures and magnetic resonance imaging-detected brain injury in newborns cooled for hypoxic-ischemic encephalopathy. J. Pediatr. 159, 731–735.e1 (2011).
Ney, J. P., van der Goes, D. N., Nuwer, M. R., Nelson, L. & Eccher, M. A. Continuous and routine EEG in intensive care: utilization and outcomes, United States 2005-2009. Neurology 81, 2002–2008 (2013).
Boylan, G., Burgoyne, L., Moore, C., O’Flaherty, B. & Rennie, J. An international survey of EEG use in the neonatal intensive care unit. Acta Paediatr. 99, 1150–1155 (2010).
Rakshasbhuvankar, A., Paul, S., Nagarajan, L., Ghosh, S. & Rao, S. Amplitude-integrated EEG for detection of neonatal seizures: A systematic review. Seizure 33, 90–98 (2015).
Shellhaas, R. A. et al. The American Clinical Neurophysiology Society’s Guideline on Continuous Electroencephalography Monitoring in Neonates. J. Clin. Neurophysiol. 28, 611–617 (2011).
Laroia, N., Guillet, R., Burchfiel, J. & McBride, M. C. EEG backgrounds as predictor of electrographic seizures in high-risk neonates. Epilepsia 39, 545–551 (1998).
Battin, M., Bennet, L. & Gunn, A. J. Rebound seizures during rewarming. Pediatrics 114, 1369 (2004).
Kendall, G. S., Mathieson, S., Meek, J. & Rennie, J. M. Recooling for rebound seizures after rewarming in neonatal encephalopathy. Pediatrics 130, e451–e455 (2012).
Lynch, N. E. et al. The temporal evolution of electrographic seizure burden in neonatal hypoxic ischemic encephalopathy. Epilepsia 53, 549–557 (2012).
Jan, S., Northington, F. J., Parkinson, C. M. & Stafstrom, C. E. EEG Monitoring Technique Influences the Management of Hypoxic-Ischemic Seizures in Neonates Undergoing Therapeutic Hypothermia. Dev. Neurosci. 21205, 1–7 (2017).
Akula, V. P. et al. A randomized clinical trial of therapeutic hypothermia mode during transport for neonatal encephalopathy. J. Pediatr. 166, 856–61-2 (2015).
Thoresen, M. et al. Twenty-Four Hours of Mild Hypothermia in Unsedated Newborn Pigs Starting after a Severe Global Hypoxic-Ischemic Insult Is Not Neuroprotective. Pediatr. Res. 50, 405–411 (2001).
Simbruner, G., Mittal, R. A., Rohlmann, F. & Muche, R., neo.nEURO.network Trial Participants. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics 126, e771–e778 (2010).
Del Balzo, F. et al. Electroencephalogram and magnetic resonance imaging comparison as a predicting factor for neurodevelopmental outcome in hypoxic ischemic encephalopathy infant treated with hypothermia. Pediatr. Rep. 6, 5532 (2014).
Guidotti, I. et al. Hypothermia reduces seizure burden and improves neurological outcome in severe hypoxic–ischemic encephalopathy: an observational study. Dev. Med Child Neurol. 58, 1235–1241 (2016).
Low, E. et al. Cooling and seizure burden in term neonates: an observational study. Arch. Dis. Child - Fetal Neonatal Ed. 97, F267–F272 (2012).
Menache, C. C., Bourgeois, B. F. D. & Volpe, J. J. Prognostic value of neonatal discontinuous EEG. Pediatr. Neurol. 27, 93–101 (2002).
Walsh, B. H., Murray, D. M. & Boylan, G. B. The use of conventional EEG for the assessment of hypoxic ischaemic encephalopathy in the newborn: A review. Clin. Neurophysiol. 122, 1284–1294 (2011).
Elshorbagy, H. H. et al. Value of electroencephalographic monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. J. Pediatr. Neurosci. 11, 309–315 (2016).
Oliveira, V. et al. Therapeutic hypothermia in mild neonatal encephalopathy: a national survey of practice in the UK. Arch. Dis. Child. Fetal Neonatal Ed. (2017) [Epub ahead of print].
Kracer, B., Hintz, S. R., Van Meurs, K. P. & Lee, H. C. Hypothermia therapy for neonatal hypoxic ischemic encephalopathy in the state of California. J. Pediatr. 165, 267–273 (2014).
Young, G. B. & da Silva, O. P. Effects of morphine on the electroencephalograms of neonates: a prospective, observational study. Clin. Neurophysiol. 111, 1955–1960 (2000).
Murray, D. M., Boylan, G. B., Ryan, C. A. & Connolly, S. Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics 124, e459–e467 (2009).
Biagioni, E., Bartalena, L., Boldrini, A., Pieri, R. & Cioni, G. Constantly discontinuous EEG patterns in full-term neonates with hypoxic-ischaemic encephalopathy. Clin. Neurophysiol. 110, 1510–1515 (1999).
Walsh, B. H. et al. The frequency and severity of magnetic resonance imaging abnormalities in infants with mild neonatal encephalopathy. J. Pediatr. 187, 26–33.e1 (2017).
Murray, D. M. et al. Early EEG grade and outcome at 5 years after mild neonatal hypoxic ischemic encephalopathy. Pediatrics 138, e20160659–e20160659 (2016).
Wusthoff, C. J. et al. Interrater agreement in the interpretation of neonatal electroencephalography in hypoxic-ischemic encephalopathy. Epilepsia 58, 429–435 (2017).
Dereymaeker, A. et al. Interrater agreement in visual scoring of neonatal seizures based on majority voting on a web-based system: the Neoguard EEG database. Clin. Neurophysiol. 128, 1737–1745 (2017).
Dunne, J. M. et al. Automated electroencephalographic discontinuity in cooled newborns predicts cerebral MRI and neurodevelopmental outcome. Arch. Dis. Child. Fetal Neonatal Ed. 102, F58–F64 (2017).
Acknowledgements
Marie-Coralie Cornet received a grant from the Belgian American Educational Foundation to conduct this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Cornet, MC., Pasupuleti, A., Fang, A. et al. Predictive value of early EEG for seizures in neonates with hypoxic–ischemic encephalopathy undergoing therapeutic hypothermia. Pediatr Res 84, 399–402 (2018). https://doi.org/10.1038/s41390-018-0040-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0040-x
This article is cited by
-
Seizures after initiation of rewarming in cooled infants with hypoxic ischaemic encephalopathy
Pediatric Research (2024)
-
Review of Noninvasive Neuromonitoring Modalities in Children II: EEG, qEEG
Neurocritical Care (2023)
-
Neuromonitoring in neonatal critical care part I: neonatal encephalopathy and neonates with possible seizures
Pediatric Research (2023)