Abstract
Improved intensive care therapies have increased the survival of children born preterm. Yet, many preterm children experience long-term neurodevelopmental sequelae. Indeed, preterm birth remains a leading cause of lifelong neurodevelopmental disability globally, posing significant challenges to the child, family, and society. Neurodevelopmental disability in children born preterm is traditionally linked to acquired brain injuries such as white matter injury and to impaired brain maturation resulting from neonatal illness such as chronic lung disease. Socioeconomic status (SES) has long been recognized to contribute to variation in outcome in children born preterm. Recent brain imaging data in normative term-born cohorts suggest that lower SES itself predicts alterations in brain development, including the growth of the cerebral cortex and subcortical structures. Recent evidence in children born preterm suggests that the response to early-life brain injuries is modified by the socioeconomic circumstances of children and families. Exciting new data points to the potential of more favorable SES circumstances to mitigate the impact of neonatal brain injury. This review addresses emerging evidence suggesting that SES modifies the relationship between early-life exposures, brain injury, and neurodevelopmental outcomes in children born preterm. Better understanding these relationships opens new avenues for research with the ultimate goal of promoting optimal outcomes for those children born preterm at highest risk of neurodevelopmental consequence.
Similar content being viewed by others
Introduction
Over the past two decades, improved neonatal intensive care unit (NICU) therapies have reduced the mortality and increased the survival of preterm newborns, resulting in an increase in preterm-born children in North America and around the world.1 Advances in NICU care have also reduced several short-term medical morbidities for preterm children.2 Despite these intensive care advances, preterm birth remains a leading, and rising, cause of childhood and lifelong disability in North America and globally. The concomitant rise in preterm birth makes promoting the neurodevelopmental outcome of these children a major imperative for families and child health care providers. The prevalent neurodevelopmental consequences of preterm birth impact multiple domains of development: cognitive, language, behavioral, sensory, and motor functions. The burdens and costs associated with these neurodevelopmental disabilities make understanding how these sequelae emerge, and how their consequences can be mitigated an urgent priority for the pediatric research community.3,4,5,6,7
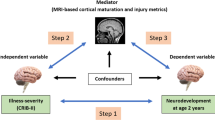
Socioeconomic status (SES) is a well-recognized predictor of neurodevelopmental outcome in preterm children, particularly cognition. Newer literature reveals complex relationship between SES and the developing brain. To synthesize emerging evidence relating SES of the preterm child as a modifier of brain injury and subsequent cognitive skills, we review the established relationship between SES and neurodevelopmental outcome in preterm children, early-life exposures and brain maturation in preterm neonates, and new evidence indicating SES is a predictor of brain structure and a modifier of the brain’s response to injury (Fig. 1).
To examine how SES might modify the response of the child to preterm birth and associated brain injuries, we performed a search strategy based on a population, intervention, or exposure (PECO)-framed focused research question:8 population-preterm infants; exposure-low SES; comparison: high SES; outcome: brain development or brain injury in context of cognitive outcome. To focus on the more recent literature, we searched PubMed without language restrictions from 1 January 2000 to 1 January 2019 with the terms (preterm AND socioeconomic status AND brain development AND cognitive outcome) and again with the terms (preterm AND socioeconomic status AND brain injury AND cognitive outcome). The papers are identified in Table 1 with findings from these studies discussed in the appropriate sections of this narrative review.
SES is strongly related to neurodevelopmental outcomes in preterm children
SES is a construct that refers to an individual’s access to material resources, as well as the social standing, or status that comes from these resources.9 SES is thus multidimensional, derived from many different aspects of life that can provide material resources and social status. Correspondingly, SES can also be measured in different ways, which represent sources of resources and status.10 Most commonly, metrics of education, income, and employment status are used to approximate one’s SES.11,12,13 While relying on one or two measures is not ideal and might prevent a broad understanding of the contributions of different components of SES to the observed associations, this is the most common approach in research given the practicalities of measurement and the moderate correlations among components of SES.14
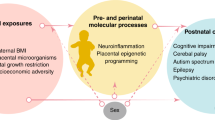
Research on SES has uncovered its remarkably strong relationship with health.10 We have come to understand that SES is a key determinant of an astounding array of exposures, and triggers a vast range of biological, behavioral, and other mechanisms associated with health. It has thus been termed a “fundamental cause” of health status. In the context of the current study, SES is a key determinant of the exposures, environments, and mechanisms through which brain maturation occurs. More specifically, higher SES is associated with secure, stable, resource-rich environments, while lower SES is associated with a breadth of biological risk exposures and psychosocial disadvantages that are directly relevant to the health of the fetus. These exposures include but are not limited to low maternal age, maternal malnutrition, maternal obesity, substance abuse, environmental exposures, parental mental health concerns and stress, and more limited access to perinatal health care services.
SES is predictive of a broad range of important life outcomes being positively related to physical health and lifespan and negatively related to serious illnesses such as cardiovascular disease, stroke, cancer, and diabetes.14 Also, cognition and academic achievement both show positive gradients with SES: higher SES is associated with higher performance across a range of cognitive outcomes. von Stumm and Plomin et al.15 analyzed longitudinal data on intelligence from the Twins Early Development Study (TEDS) cohort from 2 to 16 years. In the TEDS cohort, children from more advantaged SES backgrounds had higher intelligence scores in infancy with a 6-point difference in intelligence quotient (IQ) at 2 years. Importantly, these children experience greater gains with time exhibiting a15-point difference in IQ by age 16 years.15 These data suggest a critical long-term compounding of SES influences on cognitive development.
Specifically, in the preterm child, SES is predictive of cognitive outcome, such that a lower level of parental education is predictive of cognitive impairment.16,17,18 The relationship between SES and cognition is remarkably unwavering. In contrast with the influence of child-specific characteristics, like sex or low birth weight, on general cognition that largely diminish in later childhood, the influence of parental education persists through middle childhood.19 While the impact on cognitive outcome implies different areas of development, Joseph et al.,19 found that the most pronounced and consistent associations of lower SES in the preterm infant, as measured by maternal education, were with verbal reasoning, language ability, executive functions, and academic skills. These neurodevelopmental domains are remarkably similar to those reported for low SES term-born community samples.20
The beneficial effects of early childhood intervention programs on later neurodevelopmental outcomes in normative full-term populations are promising.21,22 In the Carolina Abecedarian (ABC) Project, children from low-income families who were randomized to receive early educational intervention had greater developmental and education achievements.23 Highlighting the breadth of influence that SES exerts on overall health, those in the intervention group also exhibited a lower prevalence of cardiovascular and metabolic disease beyond 30 years of age.21 Importantly, the beneficial impact of these interventions are not consistent when applied in preterm neonates.24 One potential explanation for this discrepancy is the existence of brain injury in children born preterm.
Through the 1990s, several studies highlight the important intersection of preterm birth and SES in predicting cognitive outcomes in childhood.12,25 Several of these and other recent studies excluded children with major medical impairments.12,25,26,27,28 Thus, the importance of biological factors in mediating the relationship between preterm birth and lower IQ may be underestimated.25 The availability of accurate brain imaging measures of brain injury and development offer contemporary opportunities to unravel the potentially interacting relationships between preterm birth, early-life NICU exposures, and SES.
Early-life NICU exposures and brain maturation of preterm neonates
Traditionally, research on the root causes of preterm birth-related neurodevelopmental challenges has focused on the type and extent of brain injuries and other biological and physiological manifestations that occur at birth, such as intraventricular hemorrhage (IVH) and overt white matter injury (WMI). Often however, neurodevelopmental challenges occur in the absence of observed IVH and WMI.29,30,31 Moreover, even when focal brain injuries are detected by diagnostic imaging, the multi-domain neurodevelopmental impairments that often become apparent over time suggest a more widespread brain abnormality than is apparent on clinical brain imaging.6
Experimental studies and human imaging studies are now shifting the paradigm of a “one-hit brain injury” being the key driver of neurodevelopmental outcomes to the importance of the trajectory of brain maturation.6 In the preterm brain, WMI is prevalent, which is seen in ~1/3 of very preterm neonates as a specific pattern of injury on clinical MRI.3,31 WMI is so prevalent because early lineage oligodendroglia are vulnerable to insults that do not affect mature myelin-forming oligodendrocytes.32,33,34 WMI is tightly linked with brain dysmaturation: the primary mechanism of myelination failure in preterm neonates is impaired maturation, which is a disrupted cellular response whereby pre-oligodendrocytes fail to differentiate (i.e., dysmaturation).35 More recently, neuronal dysmaturation is recognized.36,37,38,39 Brain dysmaturation is increasingly emerging as the most important predictor of the high burden of neurodevelopmental impairments in preterm neonates.38,40,41
Brain imaging studies of the preterm neonate have established the cumulative importance of everyday practices for brain maturation during the neonatal period, including pain and nutrition. For example, preterm neonates often spend months in the NICU, where they are treated with many painful procedures that are essential to life-saving care. Pain is now recognized as an important mediator of brain maturation in preterm neonates as they develop from early life to term age.4,38,42,43,44,45,46,47,48,49,50 Nutrition in the first 2 weeks of life is another “everyday” exposure and key predictor of early brain maturation, reflected in magnetic resonance imaging (MRI) volumetric and diffusion MRI measures.51 Recognizing the potential of SES to modify these clinical exposures may help to identify new opportunities for supportive intervention as the brain maturation abnormalities identified through these studies are robust predictors of neurodevelopmental outcomes in children born preterm.38
An understanding of how SES might impact these early-life exposures is critically needed given recent observations as to how SES influences the quality of NICU care in the United States.52 This is especially relevant as recent studies highlight the importance of considering SES, which is itself an everyday predictor of brain maturation and outcomes.20,53,54 More recently, we recognize the significant potential for social factors to mitigate neurodevelopmental consequence of early-life brain injury.53,55,56 Thus, an understanding of how SES might also directly impact the “everyday” experience of the preterm neonate in the NICU, including the burden of pain and the quality of nutrition, is urgently needed.
SES and the maturation of brain structures underlying cognition and language
The brain undergoes dramatic development over the first years of life and this early brain growth is a critical determinant for later-life cognition, language skills, and behavior. The brain structures most strongly associated with SES in term-born children share remarkable similarity with those neuronal structures most vulnerable to alterations related to preterm birth, including the cerebral cortex, thalamus, and hippocampus.54 Concurrent with new evidence suggesting that SES modifies the NICU experience of preterm neonates, evidence linking SES and brain structure in normative populations is emerging.
Recent brain imaging studies have revealed a critical link between SES and the development of brain structures related to cognition and language outcomes.20,57,58,59 SES has been related to different volumes and hemispheric specialization of the inferior frontal gyrus, the brain region containing Broca’s area critical for expressive language function.59 Hippocampal volume, a brain region involved in learning and memory, has also been related to SES,58,60,61 as well as amygdala volumes.60,61 Moreover, Noble et al.20 have demonstrated in the largest study to date (1099 children) the association of cognitive outcome with SES and cortical surface area in addition to subcortical structures. Whereas stress-sensitive brain regions have been the focus of initial research, there is increasing evidence of a maturation lag in brain development related to exposure to low SES environments. This maturation lag has been associated with stress-sensitive brain regions, including the hippocampus and amygdala, as well as more widespread relationships to cortical gyrification, as well as cortical gray matter and white matter volumes in children aged 6–12 years.62,63 Remarkably, up to 20% of the gap in cognitive and academic achievement in a longitudinal cohort study of 389 typically developing children and adolescents could be explained by maturational lags in the frontal and temporal lobes.64 Together, these findings reinforce the hypothesis that structural brain development may mediate the relationship between SES and impaired academic performance.64
Moreover, the influence of SES seems to become stronger with increasing exposure to less favorable SES factors with evidence of profound impacts on development and outcomes across the life course.11,20,59,64 The role of environment and inputs to the brain can be considered critical in the basis of network formation during early life.65 These studies suggest a “sensitive” period where the brain is particularly responsive to experiences in the form of patterned activity.65 The differences in brain structure reported by Betancourt et al.57 in the first month of life suggest that SES starts to play an important role early in life. These structural and functional changes reinforce the concept of experience-dependent plasticity of the infant brain demonstrated through extensive scientific literature both in animals and humans.66
Importantly, however, the range of exposures mediated by SES do not only predict adversity in brain health. Robust neuroscience studies in animal models demonstrate that animals raised in enriched environments, including social stimulation, exercise, and novelty, have improved brain structure and functional outcomes, including learning, memory, and plasticity.67,68,69,70,71 Moreover, enriched environments are also shown to improve brain development, reduce progressive cognitive decline, and mitigate the impact of early-life brain injuries, attenuating or reversing sequelae of brain insults, such as seizures, ischemia, and cortical lesions among other important brain conditions.66,72,73,74,75
Complementary studies in humans have also shown a remarkable capacity for brain recovery and repair that could counteract the brain vulnerability and susceptibility to low SES-related exposures. Examples of this are the studies that demonstrate how placing a child before 2 years of life in high-quality foster care when previously institutionalized from birth leads to a dramatic increase in IQ that would otherwise be in the low 70s.65,76 We can also see how strategies focused on early childhood intervention programs have demonstrated long-lasting benefits.21,22 As noted above, in the ABC Project, children from low-income families who were randomized to receive early educational intervention had greater developmental and education achievements, and improved health in adulthood.23
Does SES modify the response of the child to preterm birth and associated brain injury?
The importance of SES as a modifier of the preterm child’s response to early-life brain injury is highlighted in a recent prospective cohort study in which higher maternal education mitigated the neurodevelopmental impact of brain injury identified on neonatal MRI studies.53
Most studies examining the neurodevelopmental outcomes of brain injury in preterm-born children have not sensitively accounted for the contribution of SES to neurodevelopmental outcomes.6,31,77,78,79 Other studies have considered SES in detail without contemporary measures of brain injury.26,28,77,80,81,82
The differential risk of adverse outcome in the presence of brain injury in preterm infants exposed to a range of SES is not yet fully understood. Voss et al.,83 in a German cohort of 200 preterm infants born between 1993 and 1998, found that maternal educational background was the strongest predictor of intelligence in children at 10–13 years of age, followed by IVH or periventricular leukomalacia (PVL). SES had a significant positive effect on neurodevelopmental trajectories between 6 and 10–13 years after birth, especially in those preterm infants with IVH. Another study from the same time period in Australia by Doyle et al.84 reported a strong persistent influence of IVH on cognitive outcome from 2 to 18 years, while SES was associated with cognitive outcome only from 8 years on. In this cohort, maternal education of less than a high school completion level ultimately accounted for a decrement of half standard deviation in general intellectual ability. While Voss et al.83 included severe IVH (grades III–IV; n = 16(6%)) and SES in the same model as independent variables, Doyle et al.84 analyzed the association of all grades of IVH (n = 49(33%)) on cognitive outcome with other biological variables (sex, postnatal steroids, bronchopulmonary dysplasia, long-term ventilation, PVL) and separately studied the impact of social factors (education, social class, multilingual family, and not having both biological parents at home from birth) on the outcome in a different model.
In the absence of overt injury, SES has been found to be both a mitigating and compounding predictive factor for outcome. Among infants without severe neurologic injury, Kilbride et al.28 found similar IQs among high SES extremely low birth weight (ELBW) children and low SES term-born children, and, concomitantly, a gap of 2 standard deviations in IQ among low SES ELBW children compared to high SES term-born children.
Studies of children born preterm also highlight opportunities to better characterize the full extent of brain changes related to SES. Tich et al.85 reported lower cognitive scores in 236 preterm infants at 2 years being related to neonatal metrics if brain size, and being male, and having higher social risk, lower birth weight, postnatal corticosteroids, and moderate or severe WMI. Yet, a recent longitudinal study by Gui et al.,27 evaluating brain volumes and outcomes, showed a positive association between SES and cognitive outcomes to 5 years of age, which is independent of neonatal brain volumes. Importantly, this cohort excluded children with significant IVH and WMI, so that these brain injuries were not examined as modifier of the link between SES and cognitive outcome. These new data suggest pathways between SES and cognition in early childhood that are not reflected in changes to brain volume from early in life to term-equivalent age in preterm neonates. Studies with other quantitative brain imaging tools such as diffusion tensor imaging are now needed to delineate this critical pathway so that opportunities for intervention can be identified.
A recent study of a Canadian cohort of 234 very preterm children, recruited from 2006 to 2013, found that brain injury (punctate WMI and severe IVH) and SES had the same effect size for predicting cognitive outcomes. Among pre-, peri-, and postnatal clinical exposures, brain injury, maternal level of education, and bronchopulmonary dysplasia (BPD) were the most important factors in predicting 4.5-year cognitive outcome. Surprisingly, the postnatal development of brain injury and of BPD were not found to be associated with lower full-scale IQ in preterm children born to mothers with postgraduate education.53 These new data suggest the potential of higher SES to mitigate the consequence of neonatal brain injury in children born preterm. In the absence of brain injury, the higher SES group achieves a predicted IQ 7.4 points higher than the lower SES group. In the presence of brain injury, the effect size of SES increases, with the higher SES group having a mean increase of 13.7 points relative to the lower SES group, suggesting an attenuation on the impact of brain injury on cognitive outcomes.53 Together, these studies support a paradigm shift from brain injury meaning inevitable disability to one in which early diagnosis enables new opportunities to promote better neurodevelopmental outcomes.
SES and the preterm neonate: knowledge gaps and opportunities for intervention
Even with significant advances in neonatal intensive care, cognitive outcomes among preterm children have not improved significantly since the 1990s.12,25,80,86,87 Preterm-born children as a group still perform almost 1 SD below full-term children on intelligence tests87,88 with a steeper difference of almost 2 SDs in those born before 26 weeks of gestation.16 The difference in intellectual ability and academic performance in preterm children relative to those born at term is remarkable stable over time to at least late adolescence.84,89 Graded associations of social disadvantage and worse neurocognitive and academic outcomes are already evident at school age in children born extremely preterm.19 These relationships do not only impact school performance and educational achievement as individuals born preterm in lower SES, but it also manifest an increased risk of living in poverty and increased social security dependency.25,80,90 Thus, better understanding the bi-directionality of the relationships between SES and cognitive outcomes are needed. With increased awareness of the negative impact of SES disadvantage on outcomes in preterm children, the co-occurrence of both conditions, preterm birth and SES disadvantage, is now recognized to be a situation where biological and social risks have synergistic associations with long-term outcome.19,91
To date, the heterogeneity in study design, cohort definition, statistical approach, age at assessment, and SES indicators used hampers the estimation of a pooled statistical effect size for SES on preterm outcome.92 Despite the heterogeneity in measures of SES, maternal level of education as the most widely used indicator is repeatedly and strongly associated with cognitive outcomes in the preterm child through the school years.16,92,93,94,95 While many studies focus on maternal level of education as the primary indicator of SES, the family environment in which cognitive development takes place must also be considered.96
While specific interventions to improve neurodevelopmental outcomes are beneficial in normative populations, they are not necessarily effective in preterm children.24 The failure to generalize benefit of interventions in normative term-born cohorts to preterm children may reflect the existence of brain injury in the preterm population. Some observational studies in preterm infants do suggest the potential benefits on early cognition of interventions that promote sensitive parenting,97 and increase access to resources such as high-quality day care.98 Moreover, a recent review and meta-analysis by Spittle et al.99 has shown that, despite heterogeneity between studies given the variety of early developmental intervention programs, there is enough evidence to suggest that early developmental interventions improve cognitive outcomes up to at least preschool age.
In addressing future research directions, it is worth considering opportunities to address methodological challenges arising when relating SES and development of preterm infants.13 Some opportunities for child health researchers include defining the most predictive marker of socioeconomic disadvantage, understanding why some children are vulnerable to disadvantage while others are resilient, and addressing the distinct contributions of prenatal versus postnatal SES exposures. Addressing these issues will be facilitated by advances in statistical models that assess mediation and moderation, and that allow for individual and sub-group-level differences. Our review highlights the important potential of new biomarkers to unravel the complex relationships between SES and neurodevelopmental outcome in children born preterm. While we focus our review on brain imaging, other emerging biomarkers, such as epigenetic marks reflected in DNA methylation,100,101 also offer promise for perinatal researchers to better understand the important link between SES and brain development.
The next frontier in the care of preterm neonates is to optimize neurodevelopmental outcomes and reduce childhood and lifelong disabilities. The available evidence points to the potential of early experience to promote optimal neurodevelopmental outcomes following preterm birth. By recognizing how SES modifies the relationship between preterm birth and the brain, timely interventions to improve neurodevelopmental outcomes can be identified, evaluated, and ultimately implemented.
References
Statistics Canada. Births. Statistics Canada Catalogue. 2007: 84-F02-10X.
Stoll, B. J. et al., Human Development Neonatal Research Network. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 314, 1039–1051 (2015).
Miller, S. P. et al. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J. Pediatr. 147, 609–616 (2005).
Chau, V. et al. Effect of chorioamnionitis on brain development and injury in premature newborns. Ann. Neurol. 66, 155–164 (2009).
Grunau, R. E., Whitfield, M. F. & Fay, T. B. Psychosocial and academic characteristics of extremely low birth weight (< or =800 g) adolescents who are free of major impairment compared with term-born control subjects. Pediatrics 114, e725–e732 (2004).
Back, S. A. & Miller, S. P. Brain injury in premature neonates: a primary cerebral dysmaturation disorder? Ann. Neurol. 75, 469–486 (2014).
Synnes, A. R. et al. School entry age outcomes for infants with birth weight ≤800 grams. J. Pediatr. 157, 989–994.e981 (2010).
Morgan, R. L., Whaley, P., Thayer, K. A. & Schunemann, H. J. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ. Int. 121, 1027–1031 (2018).
Krieger, N. A glossary for social epidemiology. J. Epidemiol. Community Health 55, 693–700 (2001).
Braveman, P. A. et al. Socioeconomic status in health research: one size does not fit all. JAMA 294, 2879–2888 (2005).
Leijser, L. M., Siddiqi, A. & Miller, S. P. Imaging evidence of the effect of socio-economic status on brain structure and development. Semin. Pediatr. Neurol. 27, 26–34 (2018).
Bhutta, A. T., Cleves, M. A., Casey, P. H., Cradock, M. M. & Anand, K. J. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA 288, 728–737 (2002).
Cirino, P. T. et al. Measuring socioeconomic status: reliability and preliminary validity for different approaches. Assessment 9, 145–155 (2002).
Farah, M. J. The neuroscience of socioeconomic status: correlates, causes, and consequences. Neuron 96, 56–71 (2017).
von Stumm, S. & Plomin, R. Socioeconomic status and the growth of intelligence from infancy through adolescence. Intelligence 48, 30–36 (2015).
Linsell, L., Malouf, R., Morris, J., Kurinczuk, J. J. & Marlow, N. Prognostic factors for poor cognitive development in children born very preterm or with very low birth weight: a systematic review. JAMA Pediatr. 169, 1162–1172 (2015).
Ko, G., Shah, P., Lee, S. K. & Asztalos, E. Impact of maternal education on cognitive and language scores at 18 to 24 months among extremely preterm neonates. Am. J. Perinatol. 30, 723–730 (2013).
Vohr, B. R. et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics 105, 1216–1226 (2000).
Joseph, R. M., O’Shea, T. M., Allred, E. N., Heeren, T. & Kuban, K. K. Maternal educational status at birth, maternal educational advancement, and neurocognitive outcomes at age 10 years among children born extremely preterm. Pediatr. Res 83, 767–777 (2018).
Noble, K. G. et al. Family income, parental education and brain structure in children and adolescents. Nat. Neurosci. 18, 773–778 (2015).
Campbell, F. et al. Early childhood investments substantially boost adult health. Science 343, 1478–1485 (2014).
Heckman, J., Pinto, R. & Savelyev, P. Understanding the mechanisms through which an influential early childhood program boosted adult outcomes. Am. Econ. Rev. 103, 2052–2086 (2013).
Campbell, F. A. & Ramey, C. T. Effects of early intervention on intellectual and academic achievement: a follow-up study of children from low-income families. Child Dev. 65, 684–698 (1994).
McCormick, M. C. et al. Early intervention in low birth weight premature infants: results at 18 years of age for the Infant Health and Development Program. Pediatrics 117, 771–780 (2006).
Kerr-Wilson, C. O., Mackay, D. F., Smith, G. C. & Pell, J. P. Meta-analysis of the association between preterm delivery and intelligence. J. Public Health (Oxf.) 34, 209–216 (2012).
Bruckert, L. et al. White matter plasticity in reading-related pathways differs in children born preterm and at term: a longitudinal analysis. Front. Hum. Neurosci. 13, 139 (2019).
Gui, L. et al. Longitudinal study of neonatal brain tissue volumes in preterm infants and their ability to predict neurodevelopmental outcome. Neuroimage 185, 728–741 (2019).
Kilbride, H. W., Thorstad, K. & Daily, D. K. Preschool outcome of less than 801-gram preterm infants compared with full-term siblings. Pediatrics 113, 742–747 (2004).
Schmidt, B. et al. Prediction of late death or disability at age 5 years using a count of 3 neonatal morbidities in very low birth weight infants. J. Pediatr. 167, 982–986.e982 (2015).
Synnes, A. et al. Determinants of developmental outcomes in a very preterm Canadian cohort. Arch. Dis. Child Fetal Neonatal Ed. 102, F235–F234 (2017).
Guo, T. et al. Quantitative assessment of white matter injury in preterm neonates: association with outcomes. Neurology 88, 614–622 (2017).
Back, S. A. & Rivkees, S. A. Emerging concepts in periventricular white matter injury. Semin. Perinatol. 28, 405–414 (2004).
Back, S. A. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment. Retard Dev. Disabil. Res. Rev. 12, 129–140 (2006).
Back, S. A. & Rosenberg, P. A. Pathophysiology of glia in perinatal white matter injury. Glia 62, 1790–1815 (2014).
Buser, J. R. et al. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann. Neurol. 71, 93–109 (2012).
McClendon, E. et al. Prenatal cerebral ischemia triggers dysmaturation of caudate projection neurons. Ann. Neurol. 75, 508–524 (2014).
Duerden, E. G. et al. Midazolam dose correlates with abnormal hippocampal growth and neurodevelopmental outcome in preterm infants. Ann. Neurol. 79, 548–559 (2016).
Duerden, E. G. et al. Early procedural pain is associated with regionally-specific alterations in thalamic development in preterm neonates. J. Neurosci. 38, 878–886 (2018).
Ball, G. et al. Development of cortical microstructure in the preterm human brain. Proc. Natl. Acad. Sci. USA 110, 9541–9546 (2013).
Chau, V. et al. Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology 81, 2082–2089 (2013).
Schneider, J. et al. Evolution of T1 relaxation, ADC, and fractional anisotropy during early brain maturation: a serial imaging study on preterm infants. Am. J. Neuroradiol. 37, 155–162 (2016).
Vinall, J. et al. Slower postnatal growth is associated with delayed cerebral cortical maturation in preterm newborns. Sci. Transl. Med 5, 168ra168 (2013).
Tam, E. W. et al. Preterm cerebellar growth impairment after postnatal exposure to glucocorticoids. Sci. Transl. Med. 3, 105ra105 (2011).
Tam, E. W. et al. Cerebellar development in the preterm neonate: effect of supratentorial brain injury. Pediatr. Res. 66, 102–106 (2009).
Tam, E. W. et al. Differential effects of intraventricular hemorrhage and white matter injury on preterm cerebellar growth. J. Pediatr. 158, 366–371 (2011).
Adams, E. et al. Tractography-based quantitation of corticospinal tract development in premature newborns. J. Pediatr. 156, 882–888, 888.e881 (2010).
Brummelte, S. et al. Procedural pain and brain development in premature newborns. Ann. Neurol. 71, 385–396 (2012).
Chau, V. et al. Postnatal infection is associated with widespread abnormalities of brain development in premature newborns. Pediatr. Res. 71, 274–279 (2012).
Bonifacio, S. L. et al. Extreme premature birth is not associated with impaired development of brain microstructure. J. Pediatr. 157, 726–732.e721 (2010).
Card, D. et al. Brain metabolite concentrations are associated with illness severity scores and white matter abnormalities in very preterm infants. Pediatr. Res. 74, 75–81 (2013).
Schneider, J. et al. Nutrient intake in the first two weeks of life and brain growth in preterm neonates. Pediatrics 141, e20172169 (2018).
Horbar, J. D. et al. Racial segregation and inequality in the neonatal intensive care unit for very low-birth-weight and very preterm infants. JAMA Pediatr. 173, 455–461 (2019).
Benavente-Fernández, I. et al. Association of socioeconomic status and brain injury with neurodevelopmental outcomes of very preterm children. JAMA Netw. Open 2, e192914–e192914 (2019).
McDermott, C. L. et al. Longitudinally mapping childhood socioeconomic status associations with cortical and subcortical morphology. J. Neurosci. 39, 1365–1373 (2019).
Vinall, J., Miller, S. P., Synnes, A. R. & Grunau, R. E. Parent behaviors moderate the relationship between neonatal pain and internalizing behaviors at 18 months corrected age in children born very prematurely. Pain 154, 1831–1839 (2013).
O’Brien, K. et al. Effectiveness of family integrated care in neonatal intensive care units on infant and parent outcomes: a multicentre, multinational, cluster-randomised controlled trial. Lancet Child Adolesc. Health 2, 245–254 (2018).
Betancourt, L. M. et al. Effect of socioeconomic status (SES) disparity on neural development in female African-American infants at age 1 month. Dev. Sci. 19, 947–956 (2016).
Hanson, J. L., Chandra, A., Wolfe, B. L. & Pollak, S. D. Association between income and the hippocampus. PLoS ONE 6, e18712 (2011).
Raizada, R. D., Richards, T. L., Meltzoff, A. & Kuhl, P. K. Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. Neuroimage 40, 1392–1401 (2008).
Noble, K. G., Houston, S. M., Kan, E. & Sowell, E. R. Neural correlates of socioeconomic status in the developing human brain. Dev. Sci. 15, 516–527 (2012).
Noble, K. G., Korgaonkar, M. S., Grieve, S. M. & Brickman, A. M. Higher education is an age-independent predictor of white matter integrity and cognitive control in late adolescence. Dev. Sci. 16, 653–664 (2013).
Jednoróg, K. et al. The influence of socioeconomic status on children’s brain structure. PLoS ONE 7, e42486 (2012).
Luby, J. et al. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr. 167, 1135–1142 (2013).
Hair, N. L., Hanson, J. L., Wolfe, B. L. & Pollak, S. D. Association of child poverty, brain development, and academic achievement. JAMA Pediatr. 169, 822–829 (2015).
Fox, S. E., Levitt, P. & Nelson, C. A. 3rd How the timing and quality of early experiences influence the development of brain architecture. Child Dev. 81, 28–40 (2010).
Cioni, G., Inguaggiato, E. & Sgandurra, G. Early intervention in neurodevelopmental disorders: underlying neural mechanisms. Dev. Med. Child Neurol. 58(Suppl. 4), 61–66 (2016).
Diamond, M. C., Krech, D. & Rosenzweig, M. R. The effects of an enriched environment on the histology of the rat cerebral cortex. J. Comp. Neurol. 123, 111–120. (1964).
Kempermann, G., Kuhn, H. G. & Gage, F. H. More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495 (1997).
Bennett, E. L., Rosenzweig, M. R. & Diamond, M. C. Rat brain: effects of environmental enrichment on wet and dry weights. Science 163, 825–826 (1969).
Leger, M. et al. Environmental enrichment duration differentially affects behavior and neuroplasticity in adult mice. Cereb. Cortex 25, 4048–4061 (2015).
Brenes, J. C. et al. Differential effects of social and physical environmental enrichment on brain plasticity, cognition, and ultrasonic communication in rats. J. Comp. Neurol. 524, 1586–1607 (2016).
Faverjon, S. et al. Beneficial effects of enriched environment following status epilepticus in immature rats. Neurology 59, 1356–1364 (2002).
Sale, A., Berardi, N. & Maffei, L. Environment and brain plasticity: towards an endogenous pharmacotherapy. Physiol. Rev. 94, 189–234 (2014).
Nithianantharajah, J. & Hannan, A. J. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 7, 697–709 (2006).
van Praag, H., Kempermann, G. & Gage, F. H. Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 1, 191–198 (2000).
Nelson, C. A. 3rd et al. Cognitive recovery in socially deprived young children: the Bucharest Early Intervention Project. Science 318, 1937–1940 (2007).
Kidokoro, H. et al. Brain injury and altered brain growth in preterm infants: predictors and prognosis. Pediatrics 134, e444–e453 (2014).
Woodward, L. J., Clark, C. A., Bora, S. & Inder, T. E. Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PLoS ONE 7, e51879 (2012).
Woodward, L. J., Anderson, P. J., Austin, N. C., Howard, K. & Inder, T. E. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N. Engl. J. Med. 355, 685–694 (2006).
Joseph, R. M. et al. Neurocognitive and academic outcomes at age 10 years of extremely preterm newborns. Pediatrics 137 (2016).
Nguyen, T. N. et al. Developmental trajectory of language from 2 to 13 years in children born very preterm. Pediatrics 141 (2018).
Scherjon, S., Briet, J., Oosting, H. & Kok, J. The discrepancy between maturation of visual-evoked potentials and cognitive outcome at five years in very preterm infants with and without hemodynamic signs of fetal brain-sparing. Pediatrics 105, 385–391 (2000).
Voss, W., Jungmann, T., Wachtendorf, M. & Neubauer, A. P. Long-term cognitive outcomes of extremely low-birth-weight infants: the influence of the maternal educational background. Acta Paediatr. 101, 569–573 (2012).
Doyle, L. W. et al. Biological and social influences on outcomes of extreme-preterm/low-birth weight adolescents. Pediatrics 136, e1513–e1520 (2015).
Tich, S. N. et al. Neurodevelopmental and perinatal correlates of simple brain metrics in very preterm infants. Arch. Pediatr. Adolesc. Med. 165, 216–222. (2011).
Groenendaal, F., Termote, J. U., van der Heide-Jalving, M., van Haastert, I. C. & de Vries, L. S. Complications affecting preterm neonates from 1991 to 2006: what have we gained? Acta Paediatr. 99, 354–358 (2010).
Twilhaar, E. S. et al. Cognitive outcomes of children born extremely or very preterm since the 1990s and associated risk factors: a meta-analysis and meta-regression. JAMA Pediatr. 172, 361–367 (2018).
Wolke, D. & Meyer, R. Cognitive status, language attainment, and prereading skills of 6-year-old very preterm children and their peers: the Bavarian Longitudinal Study. Dev. Med. Child Neurol. 41, 94–109 (1999).
Burnett, A. C., Cheong, J. L. Y. & Doyle, L. W. Biological and social influences on the neurodevelopmental outcomes of preterm infants. Clin. Perinatol. 45, 485–500 (2018).
Basten, M., Jaekel, J., Johnson, S., Gilmore, C. & Wolke, D. Preterm birth and adult wealth: mathematics skills count. Psychol. Sci. 26, 1608–1619 (2015).
McGowan, E. C. & Vohr, B. R. Neurodevelopmental follow-up of preterm infants: What is new? Pediatr. Clin. N. Am. 66, 509–523 (2019).
Wong, H. S. & Edwards, P. Nature or nurture: a systematic review of the effect of socio-economic status on the developmental and cognitive outcomes of children born preterm. Matern. Child Health J. 17, 1689–1700 (2013).
Asztalos, E. V. et al., Canadian Neonatal Follow-up Network I. Association between primary caregiver education and cognitive and language development of preterm neonates. Am. J. Perinatol. 34, 364–371 (2017).
Beaino, G. et al. Predictors of cerebral palsy in very preterm infants: the EPIPAGE prospective population-based cohort study. Dev. Med. Child Neurol. 52, e119–e125 (2010).
Potharst, E. S. et al. High incidence of multi-domain disabilities in very preterm children at five years of age. J. Pediatr. 159, 79–85 (2011).
Mangin, K. S., Horwood, L. J. & Woodward, L. J. Cognitive development trajectories of very preterm and typically developing children. Child Dev. 88, 282–298 (2017).
Wolke, D., Jaekel, J., Hall, J. & Baumann, N. Effects of sensitive parenting on the academic resilience of very preterm and very low birth weight adolescents. J. Adolesc. Health 53, 642–647 (2013).
Vandell, D. L., Belsky, J., Burchinal, M., Steinberg, L. & Vandergrift, N., Network NECCR. Do effects of early child care extend to age 15 years? Results from the NICHD study of early child care and youth development. Child Dev. 81, 737–756 (2010).
Spittle, A., Orton, J., Anderson, P. J., Boyd, R. & Doyle, L. W. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst. Rev. CD005495 (2015).
Provenzi, L., Guida, E. & Montirosso, R. Preterm behavioral epigenetics: A systematic review. Neurosci. Biobehav. Rev. 84, 262–271 (2018).
Santos, H. P. Jr. et al. Epigenome-wide DNA methylation in placentas from preterm infants: association with maternal socioeconomic status. Epigenetics 14, 751–765 (2019).
Acknowledgements
We received financial support from the Bloorview Children’s Hospital Chair in Pediatric Neuroscience, Canadian Institutes of Health Research, Kids Brain Health Network, Ontario Brain Institute and Cerebral Palsy Alliance (S.P.M.), Canada Research Chair in Population Health Equity (A.S.), and the European Regional Development Fund-Junta de Andalucía Health Board 2014-2020/PI-0052-2017 (I.B.-F.).
Author information
Authors and Affiliations
Contributions
I.B.-F., A.S. and S.P.M. made substantial contributions to conception and design of this review, drafting the manuscript and revising it critically for important intellectual content; all three authors agree with the final version of this review.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Benavente-Fernández, I., Siddiqi, A. & Miller, S.P. Socioeconomic status and brain injury in children born preterm: modifying neurodevelopmental outcome. Pediatr Res 87, 391–398 (2020). https://doi.org/10.1038/s41390-019-0646-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0646-7
This article is cited by
-
Advancing brain MRI as a prognostic indicator in hypoxic-ischemic encephalopathy
Pediatric Research (2024)
-
Neuroprotective therapies in the NICU in preterm infants: present and future (Neonatal Neurocritical Care Series)
Pediatric Research (2024)
-
Growth and neuro-developmental outcomes of probiotic supplemented preterm infants—a systematic review and meta-analysis
European Journal of Clinical Nutrition (2023)
-
Ultrasonographic evaluation of the early brain growth pattern in very low birth weight infants
Pediatric Research (2023)
-
Big data, machine learning, and population health: predicting cognitive outcomes in childhood
Pediatric Research (2023)