Abstract
Background
A strong correlation between the bilirubin/albumin (B/A) ratio and unbound bilirubin (UB) levels in newborns ≥35 weeks of gestation has been reported. However, in preterm infants, the usefulness of B/A ratios remains unclear.
Methods
We obtained serum from 381 newborns <35 weeks of gestation. UB levels were measured using the glucose oxidase-peroxidase method. Total serum bilirubin (TB) and albumin (Alb) concentrations were measured spectrophotometrically. Samples were then stratified into two groups based on the infant’s phototherapy use. B/A ratios were calculated and correlated with UB levels. Samples taken from infants prior to or never receiving phototherapy (No PTx) were then stratified by gestational age (GA) epochs: 22–27, 28–29, 30–31, and 32–34 weeks and B/A ratios correlated with UB levels.
Results
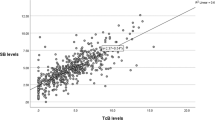
B/A ratios significantly correlated with UB levels in samples from the No PTx cohort (n = 1250; y = 1.83x − 0.15, r2 = 0.93) when compared with samples from infants post-phototherapy (Post-PTx, n = 2039; y = 1.05x + 0.09, r2 = 0.69). Even when stratified by GA, the correlation remained.
Conclusions
In preterm infants <35 weeks of gestation, B/A ratios correlated with UB levels better in infants prior to or never receiving phototherapy than in those infants receiving phototherapy.
Impact
-
The bilirubin/albumin (B/A) ratio significantly correlates with unbound bilirubin (UB) levels in preterm infants <35 weeks of gestation.
-
The B/A ratio can be used as an index of UB levels in preterm infants <35 weeks of gestation.
-
The B/A ratio is useful, especially when UB measurements are not available, for managing hyperbilirubinemia in preterm infants.
Similar content being viewed by others
Introduction
Bilirubin, the causative agent of neonatal jaundice, is neurotoxic. Therefore, significant hyperbilirubinemia can lead to bilirubin encephalopathy and/or long-term neurological sequelae. In circulation, bilirubin is primarily bound to albumin (Alb), and the remainder is present as “free” or unbound bilirubin (UB).1 UB is a known sensitive biomarker of bilirubin encephalopathy because of its high permeability to the central nervous system.2 In a multicenter study, Morioka et al. have shown the utility of using measurement of UB levels to assess an infant’s risk of developing bilirubin encephalopathy. They reported that 39% of very low birth weight (VLBW) infants, who ultimately developed bilirubin encephalopathy, had high serum levels of UB but not high levels of total serum bilirubin (TB).3 However, measurements of serum UB concentrations are only routinely performed currently in Japan.
Therefore, to identify infants at risk for developing bilirubin neurotoxicity, several other parameters have been evaluated, including the bilirubin-binding capacity (BBC), which is an index of the ability of Alb to bind bilirubin,4,5 and the bilirubin/Alb (B/A) ratio.6 Hematofluorometry, which is based on bilirubin fluorescence, has been used to measure BBC;7 however, it has only been used in the research setting and is not applicable for routine use in clinical practice.8 In contrast, the B/A ratio has been used and can be easily calculated.6,9,10,11
The 2004 American Academy of Pediatrics guideline has recommend the use of the B/A ratio to guide the initiation of exchange transfusions in infants ≥35 weeks of gestation.12 Previously, our group has demonstrated a strong correlation between B/A ratios and serum UB levels in infants ≥35 weeks of gestation.13 Conversely, Amin et al. reported only a weak correlation between the B/A ratio and peak UB levels in infants born between 24 and 33 weeks of gestation after controlling for confounding factors.14 Hence, the clinical utility of the B/A ratio in infants <35 weeks of gestation is still controversial.
In a prospective cohort study by Ritter et al., the B/A ratio was found to be highly elevated in VLBW infants who later develop bilirubin encephalopathy.9 Similarly, Govaert et al. retrospectively found that 5 preterm infants with kernicterus associated with the changes in the globus pallidus had increased B/A ratios (0.58–0.76) but TB levels below exchange transfusion thresholds.10
In contrast, Amin et al. prospectively examined the usefulness of the B/A ratio and UB levels as compared with TB in predicting bilirubin encephalopathy by assessing auditory brainstem responses (ABRs) in infants of 28–32 weeks of gestation. They reported that peak UB levels, but not B/A ratios, were significantly higher in infants with abnormal ABR maturation than in those with normal maturation.11 Recently, Hulzebos et al. reported that neurodevelopmental outcomes in infants <32 weeks of gestation were not improved after including the B/A ratio into the management criteria in their randomized controlled trial.15 The discrepancy between these studies might be due to the lack of universally established hyperbilirubinemia management criteria regarding the use of B/A ratios in combination with TB levels.
Since 1992, we have routinely measured UB levels in our infants to manage neonatal hyperbilirubinemia in Japan.16 Therefore, in this study, we determined the usefulness of B/A ratios in the management of hyperbilirubinemia in preterm infants <35 weeks of gestation by retrospectively examining the correlation between B/A ratios and UB levels.
Methods
Patients and samples
In this retrospective observational study, medical records from all newborn infants ≤35 weeks of gestation born at the Kobe University Hospital, a tertiary perinatal center located in Kobe city, were reviewed from January 2014 to December 2018. The treatment thresholds for TB and UB concentrations were based on the treatment criteria established by Morioka et al. (Supplementary Fig. S1).17 This study was approved by the Institutional Review Board of Kobe University Graduate School of Medicine (#B190144, 29/Aug/2019). All parents gave written informed consent for the use of personal medical data in research.
After collection, whole blood samples were promptly centrifuged and serum was obtained. UB, TB, direct bilirubin (DB), and Alb levels were then measured. Following our treatment criteria, infants received phototherapy based on TB or UB threshold levels stratified by post-conceptional age.16,17 We recorded the following clinical characteristics: gestational age (GA), birth weight (BW), Apgar scores, sex, TB, DB, Alb, and UB up to 14 days of age. We only included test results of blood collected at the earliest time point if the test was performed multiple times on the same day to prevent any possibility of selection bias. In addition, we excluded samples when the DB ≥2.0 mg/dL, since it is known that the UB values determined by the UB analyzer (Arrows, Osaka, Japan) are inaccurate when DB value exceeds this value.18
Because when exposed to light, bilirubin is converted into structural isomers that can affect its binding to Alb,19,20 we categorized the samples into two groups: (1) those collected from infants prior to (n = 338) or never receiving (n = 43) phototherapy (No PTx); and (2) those collected from infants after receiving phototherapy (Post-PTx, n = 338, Fig. 1). We decided that it was feasible to combine samples from infants prior to receiving and who never received phototherapy because TB, Alb, UB, B/A ratios (Supplementary Fig. S2) and correlations were similar in samples from infants who never received phototherapy (y = 1.55x − 0.10, r2 = 0.85, p < 0.0001, Supplementary Fig. S3a) and in samples taken from infants prior to receiving phototherapy (y = 1.86x − 0.15, r2 = 0.89, p < 0.0001, Supplementary Fig. S3b).
Of the 381 patients enrolled, 3395 samples were collected from 0 to 14 days of age (Fig. 1). Our No PTx cohort consisted of 196 samples taken from infants who had never received phototherapy (n = 43) and 1088 samples taken from infants prior to receiving phototherapy (n = 338) for a total of 1284. Of these, based on our criteria, 4 and 30 samples were excluded, respectively, due to incomplete data (missing at least one TB, UB, DB, or Alb value), resulting in a final total of 1250. Our Post-PTx cohort consisted of 2111 samples that were collected from infants after receiving phototherapy (n = 338). Of these, 72 samples were excluded due to incomplete data (n = 33) or having DB ≥2.0 mg/dL (n = 39) for a total of 2039.
Measurement of UB, TB, Alb, and DB levels
Serum UB levels were measured using an automated UB Analyzer (Arrows) via the glucose oxidase-peroxidase (GOD-POD) method as previously described.21,22 TB, Alb, and DB levels were measured using spectrophotometry, the modified bromocresol purple method, and the bilirubin oxidase method, respectively.
Calculation of the B/A ratio and BBC
Based on our previous reports,13 TB in mg/dL, UB in µg/dL, and Alb in g/dL were converted to µmol/L by dividing each by 0.0546, 54.6, and 0.0066741, respectively. The B/A ratio and Alb-bound bilirubin concentrations (binding constants) were calculated as follows:13
Correlation of B/A ratios and UB levels
The correlation of B/A ratios and serum UB levels was calculated for all enrolled newborns and then stratified by phototherapy use and by GA: (A) infants <28 weeks, (B) infants 28–29 weeks, (C) infants 30–31 weeks, and (D) infants 32–34 weeks. Binding constants were calculated and compared to evaluate the differences between the groups.
Cut-off value of B/A ratio for predicting UB = 0.6 µg/dL
Because we have reported that a UB value of 0.6 µg/dL is predictive of developing bilirubin encephalopathy, we determined the cut-off value of the B/A ratio.13
Statistical analyses
Data were described as median (range) or n (%). Regression analysis was performed to linearly compare between B/A ratios and UB levels, and regression equation and correlation coefficients (r2) were calculated. Statistical significance was determined using Mann–Whitney U test for comparisons of BBC between the groups. To determine the cut-off value of B/A ratio at UB ≥0.6 µg/dL, receiver operating characteristic (ROC) curves by logistic regression analyses were generated, and the point where area under the curve is maximal was taken as the cut-off point. Correlations and differences were deemed statistically significant when p < 0.05.
Results
The clinical characteristics of the 381 enrolled infants are shown in Table 1. The medians (ranges) of GA and BW were 32 (22–34) weeks and 1604 g (284–2962), respectively. Two hundred and five infants (54%) were male and median (range) Apgar scores were 4 (0–9) at 1 min and 7 (2–9) at 5 min. One hundred and eighty-five infants (49%) received antibiotic treatment with ampicillin (ABPC), 72 infants (19%) received antibiotic treatment with ABPC with amikacin, and 124 infants (33%) received no antibiotics. In addition, 63 infants (17%) received indomethacin for the treatment of patent ductus arteriosus, and 338 infants (89%) received phototherapy (Table 1).
Correlations of B/A ratios with UB levels for both No PTx and Post-PTx groups are shown in Fig. 2. B/A ratios significantly correlated with UB levels only in the No PTx group (y = 1.83x − 0.15, r2 = 0.93, p < 0.0001, Fig. 2a). In the Post-PTx group, the correlation was, however, weaker (y = 1.05x + 0.09, r2 = 0.69, p < 0.0001, Fig. 2b).
When we stratified the No PTx samples based on GA [(A) <28 weeks (n = 65, Supplementary Fig. S4a), (B) 28–29 weeks (n = 51, Supplementary Fig. S4b), (C) 30–31 weeks (n = 69, Supplementary Fig. S4c), and (D) 32–34 weeks (n = 196, Supplementary Fig. S4d), respectively], B/A ratios significantly correlated with UB levels at all GAs: (A) y = 1.83x − 0.15, r2 = 0.88; (B) y = 2.02x − 0.18, r2 = 0 .93; (C); y = 1.91x − 0.16, r2 = 0.94; and (D) y = 1.76x − 0.14, r2 = 0.94, p < 0.0001 for all the groups.
The distribution of the B/A ratio as a function of binding constants for the No PTx group is shown in Fig. 3. When the relationship between the B/A ratio and the binding constant was examined, we found that the binding constant was similar in samples with a B/A ratio ≥0.2 (n = 797) but was significantly lower in samples with a B/A ratio <0.2 (n = 453, p < 0.0001, Table 2 and Supplementary Fig. S5).
A serum UB ≥0.6 µg/dL is widely used as a criterion for the initiation of phototherapy in Japan. Thus we calculated the cut-off value of the B/A ratio to predict serum UB levels ≥0.6 µg/dL by ROC curves. We found that a B/A ratio cut-off value was 0.36 in all patients (sensitivity: 93%, specificity: 88%), 0.29 in A group (sensitivity: 100%, specificity: 93%), 0.33 in B group (sensitivity: 92%, specificity: 87%), 0.36 in C group (sensitivity: 92%, specificity: 91%), and 0.39 in D group (sensitivity: 90%, specificity: 87%) (Table 3).
Discussion
We have found that B/A ratios significantly correlated with UB levels in preterm infants <35 weeks of gestation. In infants not undergoing phototherapy, a UB level of 0.6 µg/dL correlated with a B/A ratio of 0.36 in all infants, and 0.29 at 22–27, 0.33 at 28–29, 0.36 at 30–31, and 0.39 at 32–34 weeks of gestation.
Similar to our previous study with infants ≥35 weeks of gestation,13 we found a strong correlation between B/A ratios and UB levels even in preterm infants <35 weeks of gestation. To date, there have been a few studies reporting that there is no clinical utility in using B/A ratios to manage hyperbilirubinemia in preterm infants. Amin et al. characterized the early postnatal changes of jaundice markers such as the B/A ratio and binding affinity in 166 preterm infants <33 weeks of gestation and concluded that the B/A ratio was not a useful index of UB values in this population.14 They did not, however, categorize infants by phototherapy use in their study. Based on our results, the correlation of B/A ratios and UB values is dependent upon light exposure. There have been several reports showing that phototherapy has little or no effect on BBC.23,24 During phototherapy, bilirubin is converted into water-soluble optical isomers, which results in increased intestinal and urinary excretion of bilirubin.18 Bilirubin isomers however have been reported to have a reduced binding to Alb.19,20 Hegyi et al. have shown in a retrospective study of 117 preterm infants that a correlation between TB and UB was stronger in samples taken from infants who never received phototherapy (r2 = 0.26) but weaker in those taken from infants who underwent phototherapy (r2 = 0.08)25 confirming our findings that B/A ratios can be used as an index of UB values in infants in our No PTx cohort.
In a randomized controlled trial of 615 preterm infants who were born <32 weeks of gestation, Hulzebos et al. showed that B/A ratios used in combination with TB levels to manage infants with hyperbilirubinemia did not improve neurodevelopmental outcomes compared with using TB alone.15 They defined their treatment threshold of TB and B/A ratio based on BW and risk factors from previously published criteria.26,27
In this study, we found that B/A ratios are highly correlated with UB levels even in very preterm infants. Preterm infants are known to be prone to bilirubin-induced neurological dysfunction due to susceptibility of their developing and immature central nervous system to bilirubin toxicity.28,29,30 As mentioned above, Morioka et al. examined the TB and UB levels in extremely low birth weight (ELBW) infants who were subsequently diagnosed with kernicterus and found that 89% had high UB levels; whereas only 56% had high TB levels. They concluded that high UB levels predicted kernicterus in ELBW infants with a sensitivity of 90%.3 Since the number of samples evaluated in this study was sufficiently large compared with previous reports, we were able to adequately examine the correlation between B/A ratios and UB levels stratified by GA epochs.
In addition, we also showed that the binding constants were constant when the B/A ratio is >0.2. Based on our results, all the samples with a B/A ratio ≤0.2 have a TB value of <6.0 (median 2.6 mg/dL [0.9–5.7]), thus we believe that B/A ratios can be used as an index of UB levels when TB is ≥6.0 mg/dL. This indicates that the B/A ratio is a good predictor of UB in most preterm infants who require treatment for extreme hyperbilirubinemia but not in infants with very low TB levels (even when assuming constant Alb levels), such as immediately after birth.
Our study had some limitations. First, the binding state of bilirubin and Alb was not measured directly. UB obtained by the GOD-POD method and binding affinity was obtained by calculation. There is concern that the peroxidase method might be affected by substances that compete with the peroxidation reaction such as hemoglobin and vitamins.31 Although there are reports that state that these substances do not affect the binding affinity, a more accurate relationship between UB and B/A ratio can be obtained by measuring BBC.4 Second, our total sample numbers included multiple time points for some of the infants, which may have led to a bias in our analyses. Moreover, our No PTx cohort included samples from infants never receiving phototherapy and from infants prior to phototherapy, and a larger multicenter study comparing B/A ratios and UB levels in samples taken from the same infant pre- and post-phototherapy is warranted and now underway. In addition, we did not stratify the subjects by clinical factors such as hypoxia, acidosis, sepsis, or use of a bilirubin displacer, which might also affect bilirubin–Alb binding affinity. Our criteria for the management of neonatal hyperbilirubinemia does not require stratification by clinical factors16,17 and is different from the criteria used in US, where risk is stratified based on the clinical risk.12,26,27 Thus we have tried to reduce this bias by stratifying based on GA. However, in order to extrapolate our findings to the clinical setting, it is necessary to prospectively examine the correlation between B/A ratios and UB levels stratified by clinical risk factors.
Conclusions
In this retrospective study, we found that the B/A ratio can be used as an index of serum UB levels in preterm infants <35 weeks of gestation. This might be useful, especially when UB measurements are not available, for managing hyperbilirubinemia in preterm infants.
References
Cashore, W. J., Gartner, L. M., Oh, W. & Stern, L. Clinical application of neonatal bilirubin-binding determinations: current status. J. Pediatr. 93, 827–833 (1978).
Ostrow, J. D., Pascolo, L. & Tiribelli, C. Reassessment of the unbound concentrations of unconjugated bilirubin in relation to neurotoxicity in vitro. Pediatr. Res. 54, 926 (2003).
Morioka, I. et al. Serum unbound bilirubin as a predictor for clinical kernicterus in extremely low birth weight infants at a late age in the neonatal intensive care unit. Brain Dev. 37, 753–757 (2015).
Amin, S. B. Bilirubin binding capacity in the preterm neonate. Clin. Perinatol. 43, 241–257 (2016).
Morioka, I., Iwatani, S., Koda, T., Iijima, K. & Nakamura, H. Disorders of bilirubin binding to albumin and bilirubin-induced neurologic dysfunction. Semin. Fetal Neonatal Med. 20, 31–36 (2015).
Hulzebos, C. V. & Dijk, P. H. Bilirubin-albumin binding, bilirubin/albumin ratios, and free bilirubin levels: where do we stand? Semin. Perinatol. 38, 412–421 (2014).
Brown, A. K. et al. A rapid fluorometric method for determining bilirubin levels and binding in the blood of neonates: comparisons with a diazo method and with 2-(4’-hydroxybenzene)azobenzoic acid dye binding. Pediatrics 65, 767–776 (1980).
Morioka, I. et al. Monitoring bilirubin binding parameters in a cohort of Japanese neonates. J. Clin. Lab. Med. https://doi.org/10.16966/12572-19578.16119 (2018).
Ritter, D. A., Kenny, J. D., Norton, H. J. & Rudolph, A. J. A prospective study of free bilirubin and other risk factors in the development of kernicterus in premature infants. Pediatrics 69, 260–266 (1982).
Govaert, P. et al. Changes in globus pallidus with (pre)term kernicterus. Pediatrics 112, 1256–1263 (2003).
Amin, S. B. et al. Bilirubin and serial auditory brainstem responses in premature infants. Pediatrics 107, 664–670 (2001).
American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 114, 297–316 (2004).
Sato, Y. et al. Is bilirubin/albumin ratio correlated with unbound bilirubin concentration? Pediatr. Int. 54, 81–85 (2012).
Amin, S. B. & Wang, H. Bilirubin albumin binding and unbound unconjugated hyperbilirubinemia in premature infants. J. Pediatr. 192, 47–52 (2018).
Hulzebos, C. V. et al. The bilirubin albumin ratio in the management of hyperbilirubinemia in preterm infants to improve neurodevelopmental outcome: a randomized controlled trial–BARTrial. PLoS ONE 9, e99466 (2014).
Nakamura, H., Yonetani, M., Uetani, Y., Funato, M. & Lee, Y. Determination of serum unbound bilirubin for prediction of kernicterus in low birthweight infants. Acta Paediatr. Jpn. 34, 642–647 (1992).
Morioka, I. Hyperbilirubinemia in preterm infants in Japan: new treatment criteria. Pediatr. Int. 60, 684–690 (2018).
Lee, Y. K., Daito, Y., Katayama, Y., Minami, H. & Negishi, H. The significance of measurement of serum unbound bilirubin concentrations in high-risk infants. Pediatr. Int. 51, 795–799 (2009).
Lamola, A. A., Flores, J. & Blumberg, W. E. Binding of photobilirubin to human serum albumin. Estimate of the affinity constant. Eur. J. Biochem. 132, 165–169 (1983).
Meisel, P., Biebler, K. E., Gens, A. & Jaehrig, K. Albumin binding of photobilirubin II. Biochem. J. 213, 25–29 (1983).
Nakamura, H. & Lee, Y. Microdetermination of unbound bilirubin in icteric newborn sera: an enzymatic method employing peroxidase and glucose oxidase. Clin. Chim. Acta 79, 411–417 (1977).
Shimabuku, R. & Nakamura, H. Total and unbound bilirubin determination using an automated peroxidase micromethod. Kobe J. Med. Sci. 28, 91–104 (1982).
Cashore, W. J., Karotkin, E. H., Stern, L. & Oh, W. The lack of effect of phototherapy on serum bilirubin-binding capacity in newborn infants. J. Pediatr. 87, 977–980 (1975).
Ebbesen, F. & Jacobsen, J. Bilirubin-albumin binding affinity and serum albumin concentration during intensive phototherapy (blue double light) in jaundiced newborn infants. Eur. J. Pediatr. 134, 261–263 (1980).
Hegyi, T. et al. Unbound bilirubin levels in phototherapy-treated preterm infants receiving soy-based lipid emulsion. Pediatr. Int. 62, 1357–1363 (2020).
Ahlfors, C. E. Criteria for exchange transfusion in jaundiced newborns. Pediatrics 93, 488–494 (1994).
Maisels, M. J. & Watchko, J. F. Treatment of jaundice in low birthweight infants. Arch. Dis. Child. Fetal Neonatal Ed. 88, F459–F463 (2003).
Brites, D. & Fernandes, A. Bilirubin-induced neural impairment: a special focus on myelination, age-related windows of susceptibility and associated co-morbidities. Semin. Fetal Neonatal Med. 20, 14–19 (2015).
Gartner, L. M., Snyder, R. N., Chabon, R. S. & Bernstein, J. Kernicterus: high incidence in premature infants with low serum bilirubin concentrations. Pediatrics 45, 906–917 (1970).
Bhutani, V. K. & Johnson-Hamerman, L. The clinical syndrome of bilirubin-induced neurologic dysfunction. Semin. Fetal Neonatal Med. 20, 6–13 (2015).
Ahlfors, C. E. Effect of serum dilution on apparent unbound bilirubin concentration as measured by the peroxidase method. Clin. Chem. 27, 692–696 (1981).
Acknowledgements
We thank Dr. Takumi Kido, Dr. Yuki Kyono, Dr. Asumi Yoshida, Dr. Sachiyo Fukushima (Department of Pediatrics, Kobe University Graduate School of Medicine), and Dr. Ichiro Morioka (Department of Pediatrics and Child Health, Nihon University School of Medicine, Tokyo, Japan) for their support in the treatment of preterm infants. We deeply appreciate Professor Hajime Nakamura (Department of Pediatrics, Kobe University Graduate School of Medicine) for his insightful comment and constructive discussion on this manuscript. This work was supported by JSPS KAKENHI Grant Number 18K15710, and 20K08229 (Japan).
Author information
Authors and Affiliations
Contributions
S.A. and K.F. contributed to the conception and design of the study; S.A., K.F., R.N., S.S., M.A., and K.N. contributed to the acquisition of clinical data; S.A., K.F., and R.J.W. performed statistical analyses, interpretation of data, and drafting the manuscript; R.J.W. and K.I. critically reviewed the manuscript. All authors gave final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Informed consent for this study was obtained in the form of opt-out on the website. This study was approved by the Institutional Review Board of Kobe University Graduate School of Medicine (#B190144, 29/Aug/2019). All parents gave written informed consent for the use of personal medical data in research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abe, S., Fujioka, K., Nakasone, R. et al. Bilirubin/albumin (B/A) ratios correlate with unbound bilirubin levels in preterm infants. Pediatr Res 89, 1427–1431 (2021). https://doi.org/10.1038/s41390-020-01351-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01351-z
This article is cited by
-
Sustained lower bilirubin-binding affinity of albumin in extremely preterm infants
Pediatric Research (2023)
-
Early career investigator highlight biocommentary
Pediatric Research (2021)