Abstract
Background
Phototherapy with blue light matching plasma absorption spectrum of the bilirubin–albumin complex with peak at 460 nm is standard treatment of neonatal hyperbilirubinemia.
Aim
To demonstrate clinically the action (efficacy) spectrum of phototherapy in hyperbilirubinemic neonates, through determination of the fraction of total serum bilirubin (TSB) decreased by phototherapy with peak emission wavelengths ≥478 nm (blue-green) compared with that of light of 459/452 nm (blue).
Methods
TSB values were compiled from three earlier trials, in which hyperbilirubinemic neonates were randomized to receive 24 h of either blue-green light (478/490/497 nm) (intervention groups) or blue light (459/452/459 nm) (control groups) with equal irradiance and exposed body surface areas. Ratios (efficacy) between the decrease in TSB between intervention and control groups were calculated and graphed versus peak wavelengths, demonstrating the course of the action spectrum.
Results
Calculated efficacy ratios were 1.31, 1.18, and 1.04 for light with peak wavelengths of 478, 490, and 497 nm, respectively. The action spectrum increases from 452/459 to maximum at 478 nm, from where it decreases to 1.18 and finally to 1.04.
Conclusion
For optimal phototherapeutic treatment, neonates need to be exposed to light with peak wavelength some 20 nm longer than is presently used.
Impact
-
The action (efficacy) spectrum of phototherapy for hyperbilirubinemic neonates has its peak wavelength at 478 nm.
-
The peak wavelength of this action spectrum is 20 nm longer than the wavelength presently believed to be most efficient. The peak is also different from the peak found in vitro.
-
For optimal phototherapeutic effect, neonates need to be treated with light of wavelengths some 20 nm longer than are presently used.
Similar content being viewed by others
Introduction
All neonates develop some degree of hyperbilirubinemia during the first days of life. It becomes visible as jaundice in about 60% of late preterm and term neonates and in nearly all neonates with lower gestational age. The native Z,Z-bilirubin molecule is toxic. Being lipophilic, the free fraction of bilirubin can pass the blood–brain barrier and may cause acute bilirubin encephalopathy. Extremely high plasma bilirubin levels can result in chronic sequelae in form of kernicterus spectrum disorders (KSD).1,2 In contrast, extremely preterm neonates can develop KSD, even at low to moderate plasma bilirubin concentrations.3 KSD in children born late preterm or at term is rare in the industrial world. In Denmark the incidence is reported as 1.2 per 100,000 late preterm and term neonates born alive,4 but the incidence is much higher in the developing world.5,6
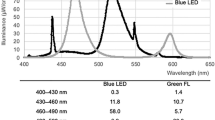
Currently, in Scandinavia, 2–5% of all late preterm and term neonates require phototherapy,7,8 and the treatment is essential for a great proportion of neonates who are born very preterm.9 Phototherapy using blue light with a spectral range 400–530 nm and peak of 450–460 nm, matching the absorption spectrum of the bilirubin–albumin complex, is based on in vitro investigations performed during the last 60 years.10,11 This light quality was recommended by the American Academic Society (AAP)1 and has been standard treatment worldwide, though exceptions exist.12 Fluorescent light sources emitting blue-green light with peak at 525 nm [introduced in order to avoid exposing the neonates to light in the wavelength range 400–450 nm,13,14,15 shown in vitro and homozygous Gunn-rats to be the most cyto- and geno-toxic light16,17,18] were not widely accepted, likely due to their lower efficacy.14,15
The phototherapy action spectrum, defined as the relationship between bilirubin removal by light as a function of its wavelength, has been intensively studied in vitro. In these studies where the bilirubin–albumin complex was exposed to light of wavelength 400–530 nm, only light in the blue-green spectrum range of 500 ± 10 nm demonstrated an action spectrum peak.19,20,21,22,23
The phototherapy action spectrum for the bilirubin–albumin complex in the circulating blood in the vessels near the skin surface of the newborn may be expected to be different from the in vitro environment, as it is affected by backscattering of the light and absorption by oxyhemoglobin, des-oxyhemoglobin, and melanin. Using models of the in vivo optical properties of the skin, the action spectrum was calculated by Pratesi et al.24 and Agati et al.25 applying modified Kubelka–Munk models, while Lamola et al.26,27 used a semi-empirical model. The peaks of the action spectra were found to be at 480, 490 ± 10, and 476 ± 4 nm, respectively. Based on these data, we hypothesize that the peak wavelength of the in vivo clinical action spectrum should be longer than the 460 nm in vitro peak absorption of the bilirubin–albumin complex.
The aim of this study is to review and assess the clinical evidence for in vivo action spectrum peak of phototherapy and to propose the ideal peak emission wavelength for devices intended for neonatal treatment.
Methods
In this study we included only investigations comparing the effect of 24 h of exposure to blue-green light (490/497/478 nm) (intervention groups) with the effect of blue light (452/459/459 nm) (control groups) on neonates with hyperbilirubinemia. The level of irradiances should be equal and measured by a spectrometer with equal sensitivity across the entire phototherapeutic light emission spectrum (400–530 nm). Furthermore, the exposed patient body surface areas should be equivalent.
This study is a secondary analysis of three earlier clinical randomized controlled trials.28,29,30 During the years of 2007–2020 we have produced three randomized controlled phototherapy trials which fulfill the inclusion criteria for this study.28,29,30 The goal of each of the investigations was to find the most efficient light quality represented by wavelength range and peak, between the two light sources investigated for treatment of neonatal hyperbilirubinemia. In our first investigation, the light sources emitted broad-spectrum fluorescent light with peaks of the blue-green and blue light at 490 and 452 nm, respectively.28 In the second and third investigations the light sources used were narrow-spectrum LED lamps.29,30 The emission peaks for the second investigation were 497 and 459 nm,28 and for the third investigation the peaks were 478 and 459 nm.30 Irradiances were measured either with a broad-spectrum photodiode power meter (EG&G, model 460, Salem, MA) with constant sensitivity over the range of 380–780 nm,28 or a UV/Vis fiberoptic spectrometer (USB 2000+, Dunedin, FL) with a flat sensitivity response over the 350–1100 nm range.29,30 Irradiance was measured either as energy of the photons (µW/cm2/nm)28 or photon fluence rate (photons/cm2/s).29,30
Because different types of light sources were used in study 1 (fluorescent tubes) and 2 and 3 [light-emitting diode (LED) tubes], and because the neonates included in investigation 1 were not identical with those in investigations 2 and 3, we normalized the results from each study by calculating the ratio between the decrease of TSB in neonates exposed for 24 h to blue-green light and the decrease of TSB in neonates exposed to blue light and graphing the ratios versus its respective peak wavelength. With this plot we define the clinical action spectrum for phototherapy of jaundiced newborns.
With respect to the decrease in TSB during the treatment we compared the percentage decreases, which is independent of the initial TSB in contrast to the decrease expressed in µmol/L.
Results
Demographic and clinical data of neonates included in investigations 1–3 are shown in Table 1. Investigation 1 included preterm neonates with gestational ≥28 and ≤36 weeks, and birth weight ≥ 1000 g, while investigation 2 and 3 included neonates healthy except for hyperbilirubinemia with gestational age ≥33 weeks and birth weight ≥ 1800 g. Neonates in investigation 1 differed from neonates in investigations 2 and 3 with regard to gestational age, birth weight, postnatal age, initial bilirubin level, and presumably also hemoglobin concentration, as this increases with increasing gestational age.31 Neonates suffering from hemolytic disease were not included in any of the investigations.
The results are shown in Table 2. For the three investigations the ratios between the decrease of TSB between the intervention and control groups were 1.31, 1.18, and 1.04 for light with emission spectrum peaks at 478, 490, and 497 nm, respectively. A ratio of 1.31 means that light with peak at 478 nm is 31% more efficient than light with peak at 459 nm. The results are also shown graphically in Fig. 1, which demonstrates the course of the phototherapy action spectrum in neonates with hyperbilirubinemia. The data show that the treatment efficacy ratio increases from 452/459 nm to a maximum at 478 nm from which it decreases to 490 nm and finally to 497 nm. The data also demonstrate that 478 nm LED light is the most efficacious wavelength for clinical phototherapy. However, the decrease in TSB as result of exposure to light of 490 nm was still significantly greater than that at 452 nm,28 but the decrease in TSB as a result of exposure to light of 497 was not significant different from that of 459 nm29 (Table 2).
Discussion
This study reviews and expands on the action spectrum research from the in vitro absorption spectrum studies of the bilirubin–albumin complex, the in vitro action spectrum studies, the theoretical action spectrum studies using sophisticated in vivo optical skin models to the present final clinical determination of the action spectrum in neonates with hyperbilirubinemia.
The three investigations analyzed were comparable because the neonates in each of the investigations were randomized, and the results in each investigation were normalized by calculating the ratio between the decrease in TSB in neonates exposed to blue-green light and the decrease in TSB in those exposed to blue light.
The practical clinical significance of this study is that for an increase in the rate of decline of TSB with an optimized phototherapy device, the duration of the treatment time can be shortened relative to the time needed with a conventional device (peak ̴480 versus 460 nm). This can benefit the health of patients through more rapid decrease of dangerously high bilirubin levels, thereby possibly avoiding the need for blood exchange transfusion, establishment of a well-structured parent–neonate attachment and breast-feeding, make nursing access and observations less cumbersome, as well as reduce hospital stay and, consequently, the total cost of hospitalization. Finally, the shorter the neonates are exposed to an elevated TSB, the less the risk of bilirubin encephalopathy. Especially extremely preterm neonates should be exposed to as few photons as possible, as was demonstrated for sick, ventilated hyperbilirubinemic neonates with birth weight 500–750 g, treated aggressively with phototherapy. This category of newborns showed a higher mortality compared with neonates treated conservatively.32
We demonstrated that the clinical action spectrum of phototherapy in hyperbilirubinemic neonates with a wavelength range from 452 to 497 nm has a maximum efficacy at 478 nm. This clinical finding is in close agreement with the suggestion of the AAP33 that light within the range of 460–490 nm with peak at 475 nm may be more effective than light with peak at 460 nm. Later, Lamola et al.26,27 also recommended the use of light with peak emission at 476 nm. This peak of the action spectrum is located at an approximately 20 nm longer wavelength than the peak of the absorption spectrum of the bilirubin–albumin complex in plasma. The peak of the clinical in vivo action spectrum is almost identical with the theoretical peaks of the models of the clinical action spectrum,23,24,25,26,27 and the peak of the in vitro action spectrum is located at a 20 nm longer wavelength than the peak of the clinical in vivo action spectrum.20,21,22,23
Ideally, for efficient photo-alteration of bilirubin, the emission spectrum of the phototherapy device must correspond to the in vivo absorption spectrum of bilirubin in the body. This has been the guiding principle for the design of phototherapy devices in terms of the wavelength of the emitted light. In reality, the in vitro absorption spectrum of the plasma bilirubin–albumin complex has been used as a surrogate. The assumption has been that the more energy is absorbed, the greater the rate of photo-degradation of bilirubin. Thus, traditionally, phototherapy devices have been produced with lamps (fluorescent, metal halide, and even LED) that emit light with a peak wavelength of 460 ± 10 nm.34
However, as mentioned, the in vitro studies showed an action spectrum peak for bilirubin degradation of 500 ± 10 nm,19,20,21,22,23 and using the models of the optical properties of the skin, optimum wavelength for phototherapy should be 476-490 nm, again 20–30 nm longer than the bilirubin absorption peak.24,25,26,27 Finally, our three clinical studies (as discussed in this report) demonstrated an in vivo action spectrum peak of nearly 480 nm (478 nm), 20 nm distant from the absorption peak of 460 nm.
We propose the following explanation as to why the in vitro and in vivo action spectrum results deviate from the bilirubin absorption spectrum: bilirubin photo-alteration yields lower molecular weight photo-oxidation and photo-cyclization (lumirubin) products via irreversible reactions, while configurational E,Z isomers are interconvertible, and even are convertible to lumirubin20 (Fig. 2). Thus, it is not only a matter of energy absorption but also the formation of an irreversible product that is wavelength-dependent. The photo-cyclization reactions that lead to lumirubin formation are the most important reactions therapeutically20 and there exist specific wavelength relationships, which cause increased lumirubin production at wavelengths ≥460 nm.34 Both the in vitro lumirubin production rate and the in vitro action spectrum for bilirubin photo-alteration peak at 500 nm.21,22 If the efficacy of phototherapy is primarily dependent on the process of lumirubin formation, the energy for photo-oxidation and Z,E isomerization diminishes as lumirubin formation peaks. Any further increase in light wavelength >500 nm causes a decrease in lumirubin formation (that ceases altogether at approximately 530–550 nm). It follows that lumirubin formation in vivo also drives the bilirubin degradation process. As the wavelength of the light increases beyond 460 nm, photo-oxidation and isomerization product yields decrease while lumirubin production is still increasing, reaching an equilibrium at 480 nm. Contributing to the equilibrium of this reaction are the physical properties of the skin and the presence of other light-absorbing compounds.24,25,26,27,30,35
Formation of bilirubin isomers during phototherapy of hyperbilirubinemic neonates. When the native Z,Z-bilirubin in the skin absorbs a photon, it can be transformed reversibly to the configurational isomers Z,E- and E,Z-bilirubin and irreversibly to the structural isomers E-Z- and E,E-lumirubin (photo-cyclization).
We have shown with the present studies the value of LEDs as a light source for phototherapy. We would not have been able to carry out our studies without the use of LEDs. No other technology allows for the production of light sources that emit a selected wavelength range, desired emission intensity, and surfaces that produce the desired light footprint. LED technology emits narrow-spectrum light, more precisely targeted radiation, hence causes fewer possible side effects. Furthermore, although LED lamps produce some infrared light (heat), it is significantly less than the light from fluorescent or metal halide lamps and they produce no ultraviolet radiation. Finally, LED light can be focused, thus producing less stray light that negatively affects nursing personnel.36
Possible alterations in the potential risk of side effects of phototherapy using light with longer wavelengths than is presently in use, i.e. blue-green LED light with peak at 478 nm versus blue LED light with peak at 459 nm, are shown in Table 3: decreased risks of cyto- and geno-toxicity,17,18,37,38,39 and cancer40 as well as increased risk of childhood allergic diseases,41,42 the degree of riboflavin deficiency may be altered,43 as is the degree of accumulation of bronze pigments.44 Together, these alterations also seem to favor the use of longer wavelength lights.
This study has several limitations. In the first investigation, the neonates were exposed to broad-spectrum fluorescent tube light, because LED lamps had not been developed yet. In the second and third investigations, narrow-spectrum LED lamps were used. This means that the peaks of the emission spectra of the blue lights in the control groups were not quite identical: in investigation 1 the peak was at 452 nm and in investigations 2 and 3 at 459 nm. Another limitation is that the neonates in the first investigation differed from the neonates in investigations 2 and 3. We have attempted to correct for these limitations through normalization of the results from the intervention and control groups. A third possible issue is the long-time interval of 15 years between studies, during which changes of procedures may have occurred. However, the neonatal cohorts in each investigation were normalized by randomization, and we compared the ratios.
The strengths of the present study are that the levels of irradiance of the intervention and control groups were equalized through use of specialized spectrometers. Also, the exposed body surface areas were equal, and the neonates were treated for 24 h, making comparisons between the different light sources appropriate. Finally, the investigations were all performed in the same neonatal department with uniform routines and skilled personnel, and the nursing procedures were found to have not changed significantly. We excluded two reported studies: one where the irradiance of the blue-green light (centered at 505 nm) was significantly underestimated due to the use of a clinical irradiance meter with maximum sensitivity at 460 nm,45 and the other where the level of irradiance of the light sources was not equal.46
Conclusions
We have demonstrated that the clinical in vivo action spectrum (effectiveness) of phototherapy of hyperbilirubinemic neonates occurs at approximately 478 nm. Thus, we recommend that, for optimal phototherapy (more rapid decrease of TSB or shorter treatment time), neonates be treated with narrow-spectrum LED light with ̴478 nm wavelength peak some 20 nm longer than is currently used (460 nm) in many phototherapy devices. Reductions in potential side effects also seems to favor the use of this wavelength.
References
American Academy of Pediatrics, Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 114, 297–316 (2004).
Pichon, J.-B., Riordan, S. M., Watchko, J. & Shapiro, S. The neurological sequelae of neonatal hyperbilirubinemia: definitions, diagnosis and treatment of the kernicterus spectrum disorders (KSDs). Curr. Pediatr. Rev. 13, 199–209 (2017).
Watchko, J. F. & Maisels, M. J. The enigma of low bilirubin kernicterus in premature infants: why does it still occur, and is it preventable? Semin. Perinatol. 38, 397–406 (2014).
Donneborg, M. L., Hansen, B. M., Vandborg, P. K., Rodrigo-Domingo, M. & Ebbesen, F. Extreme neonatal hyperbilirubinemia and kernicterus spectrum disorder in Denmark year 2000–2015. J. Perinatol. 40, 194–202 (2020).
Iskander, I. et al. Serum bilirubin and bilirubin/albumin ratio as predictors of bilirubin encephalopathy. Pediatrics 134, 1330–1339 (2014).
Farouk, Z. L. et al. Follow-up of children with kernicterus in Kano, Nigeria. J. Trop. Pediatr. 64, 176–182 (2018).
Alken, J., Haakansson, S., Ekeus, C., Gustafson, P. & Norman, M. Rates of extreme neonatal hyperbilirubinemia and kernicterus in children and adherence to national guidelines for screening, diagnosis, and treatment in Sweden. JAMA Netw. Open 2, e190858 (2019).
Toenne, A., Meberg, A. & Hager, H. B. Erindring i diagnostik og behandling av hyperbilirubinemi hos nyfødte. Tidskr. Nor. Legeforen. 130, 18–24 (2010). Article in Norwegian.
Mreihil, K., Benth, J. S., Stensvold, H. J., Nakstad, B. & Hansen, T. W. R. Phototherapy is commonly used for jaundice but greater control is needed to avoid toxicity in the most vulnerable infants. Acta Paediatr. 107, 611–619 (2018).
Cremer, R. J., Perryman, P. W. & Richards, D. H. Influence of light on the hyperbilirubinemia of infants. Lancet 24, 1094–1097 (1958).
Ennever, J. F., McDonagh, A. F. & Speck, W. T. Phototherapy for neonatal jaundice: optimal wavelengths of light. J. Pediatr. 103, 295–299 (1983).
Vreman, H. J., Wong, R. J., Murdock, J. R. & Stevenson, D. K. Standardized bench method for evaluating the efficacy of phototherapy devices. Acta Paediatr. 97, 308–316 (2008).
Vecchi, C., Donzelli, G. P., Migliorini, M. G. & Sbrana, G. Green light in phototherapy. Pediatr. Res. 17, 461–463 (1983).
Tan, K. L. Efficacy of fluorescent daylight, blue, and green lamps in the management of nonhemolytic hyperbilirubinemia. J. Pediatr. 114, 132–137 (1989).
Amato, M. & Inaebnit, D. Clinical usefulness of high intensity green light phototherapy in the treatment of neonatal jaundice. Eur. J. Pediatr. 150, 274–276 (1991).
Parshad, R., Sanford, K. K., Jones, G. M. & Tarone, R. E. Fluorescent light-induced chromosome damage and its prevention in mouse cells in culture. Proc. Natl Acad. Sci. USA 75, 1830–1833 (1978).
Roll, E. B. & Christensen, T. Formation of photoproducts and cytotoxicity of bilirubin irradiated with turquoise and blue phototherapy light. Acta Paediatr. 94, 1448–1454 (2005).
Uchida, Y. et al. Phototherapy with blue and green mixed light is as effective against unconjugated jaundice as blue light and reduces oxidative stress in the Gunn rat model. Early Hum. Dev. 97, 381–385 (2015).
Gutcher, G. R., Yen, W. M. & Odell, G. B. The in vitro and in vivo photoreactivity of bilirubin: I. Laser-defined wavelength dependence. Pediatr. Res. 17, 120–123 (1983).
McDonagh, A. F., Agati, G., Fusi, F. & Pratesi, R. Quantum yields for laser photocyclization of bilirubin in the presence of human serum albumin. Dependence on quantum yield of excitation wavelength. Photochem. Photobiol. 50, 305–319 (1989).
Greenberg, J. W., Malhotra, V. & Ennever, J. F. Wavelength dependence of the quantum yield for the structural isomerization of bilirubin. Photochem. Photobiol. 46, 453–456 (1987).
Vreman, H. J. et al. The effect of light wavelength on in vitro bilirubin photodegradation and photoisomer production. Pediatr. Res. 85, 865–873 (2019).
Onishi, S., Itoh, S. & Isobe, K. Wavelength-dependence of the relative rate constants for the main geometric and structural photoisomerization of bilirubin IXα bound to human serum albumin. Biochem. J. 236, 23–29 (1986).
Pratesi, R. et al. Skin optics and phototherapy of jaundice. Photochem. Photobiol. 40, 77–83 (1984).
Agati, G., Fusi, F., Donzelli, G. P. & Pratesi, R. Quantum yield and skin filtering effects on the formation of lumirubin. J. Photochem. Photobiol. B 18, 197–203 (1993).
Lamola, A. A., Bhutani, V. K., Wong, R. J., Stevenson, D. K. & McDonagh, A. F. The effect of hematocrit on the efficacy of phototherapy for neonatal jaundice. Pediatr. Res. 74, 54–60 (2013).
Lamola, A. A. A pharmacologic view of phototherapy. Clin. Perinatol. 43, 259–276 (2016).
Ebbesen, F., Madsen, P., Stoevring, S., Hundborg, H. & Agati, G. Therapeutic effect of turquoise versus blue light with equal irradiance in preterm infants with jaundice. Acta Paediatr. 96, 837–841 (2007).
Ebbesen, F. et al. Effect of phototherapy with turquoise vs. blue LED light of equal irradiance in jaundiced neonates. Pediatr. Res. 79, 308–312 (2016).
Ebbesen, F., Rodrigo-Domingo, M., Moeller, A. M., Vreman, H. J. & Donneborg, M. L. Effect of blue LED phototherapy centered at 478 nm versus 459 nm in hyperbilirubinemic neonates: a randomized study. Pediatr. Res. 89, 598–603 (2021).
Jobling, J., Henry, E., Wiedmeier, S. E. & Christensen, R. D. Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: data from a multihospital health care system. Pediatrics 123, e333–e337 (2009).
Morris, B. H. et al. Aggressive vs. conservative phototherapy for infants with extremely low birth weight. N. Engl. J. Med. 359, 1885–1889 (2008).
American Academy of Pediatrics, Bhutani, V. K., The Committee on Fetus and Newborn. Phototherapy to prevent severe neonatal hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 128, e1046–e1052 (2011).
Plavskii, V. Y., Tretyakova, A. I. & Mostovnikova, G. R. Phototherapeutic systems for treatment of hyperbilirubinemia of newborns. J. Opt. Technol. 81, 341–348 (2014).
Donneborg, M. L., Vandborg, P. K., Hansen, B. M., Rodrigo-Domingo, M. & Ebbesen, F. The impact of hemoglobin on the efficacy of phototherapy in hyperbilirubinemic infants. Pediatr. Res. 82, 947–951 (2017).
Vreman, H. J., Wong, R. J. & Stevenson, D. K. Phototherapy: current methods and future directions. Semin. Perinatol. 28, 326–333 (2004).
Christensen, T., Kinn, G., Granli, T. & Amundsen, I. Cells, bilirubin and light: formation of bilirubin photoproducts and cellular damage at defined wavelengths. Acta Paediatr. 83, 7–12 (1994).
Cristensen, T., Reitan, J. B. & Kinn, G. Single-strand breaks in the DNA of human cells exposed to visible light from phototherapy lamps in the presence and absence of bilirubin. J. Photochem. Photobiol. B Biol. 7, 337–346 (1990).
Van der Schoor, L. W. E. et al. LED-phototherapy does not induce oxidative DNA damage in hyperbilirubinemic Gunn rats. Pediatr. Res. 85, 1041–1047 (2019).
Newman, T. B. et al. Retrospective cohort study of phototherapy and childhood cancer in Northern California. Pediatrics 137, e20151354 (2016).
Jasprova, J. et al. Neuro-inflammatory effects of photodegradative products of bilirubin. Sci. Rep. 8, 7444 (2018).
Gloria-Bottini, F. & Bottini, E. Is there a role of early neonatal events in susceptibility to allergy? Int. J. Biochem. Sci. 6, 8–12 (2010).
Rubaltelli, F. F., Allegri, G., Costa, C. & De Antoni, A. Urinary excretion of tryptophan metabolities during phototherapy. J. Pediatr. 85, 865–867 (1974).
Itoh, S., Okada, H., Kuboi, T. & Kusaka, T. Phototherapy for neonatal hyperbilirubinemia. Pediatr. Int. 59, 959–966 (2017).
Seidman, D. et al. A prospective randomized controlled study of phototherapy using blue and blue-green light-emitting devices, and conventional halogen-quartz phototherapy. J. Perinatol. 23, 123–127 (2003).
Kuboi, T. et al. Green light-emitting diode phototherapy for neonatal hyperbilirubinemia: randomized controlled trial. Pediatr. Intern. 61, 465–470 (2019).
Acknowledgements
We thank John Mahoney for review of the manuscript and Lene Pedersen for drafting Fig. 1.
Author information
Authors and Affiliations
Contributions
F.E. conceptualized and designed the study, drafted the initial manuscript, reviewed, and revised the manuscript. M.L.D. and P.K.V. reviewed and made recommendations towards improvement of the manuscript. H.J.V. made substantial contributions to the initial manuscript, reviewed, and made recommendations towards improvement of the final manuscript. All authors approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent and approval
Patient consent is not required, as the study does not contain any new data. It is a comparison of data published in our three previous articles. These three have all been approved by the Ethics Committee of North Jutland Region.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ebbesen, F., Donneborg, M.L., Vandborg, P.K. et al. Action spectrum of phototherapy in hyperbilirubinemic neonates. Pediatr Res 92, 816–821 (2022). https://doi.org/10.1038/s41390-021-01743-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01743-9