Abstract
Background
The risk factors for central venous access device-related thrombosis (CRT) in children are not fully understood. We used evidence-based medicine to find the risk factors for CRT by pooling current studies reporting risk factors of CRT, aiming to guide clinical diagnosis and treatment.
Methods
A systematic search of PubMed, Web of Science, Embase, Cochrane Library, Scopus, CNKI, Sinomed, and Wanfang databases was conducted. RevMan 5.4 was employed for data analysis.
Results
The review included 47 studies evaluating 262,587 children with CVAD placement. Qualitative synthesis and quantitative meta-analysis identified D-dimer, location of insertion, type of catheter, number of lumens, catheter indwelling time, and central line-associated bloodstream infection as the most critical risk factors for CRT. Primarily due to observational design, the quality of evidence was regarded as low certainty for these risk factors according to the GRADE approach.
Conclusion
Because fewer high-quality studies are available, larger sample sizes and well-designed prospective studies are still needed to clarify the risk factors affecting CRT. In the future, developing pediatric-specific CRT risk assessment tools is important. Appropriate stratified preventive strategies for CRT according to risk assessment level will help improve clinical efficiency, avoid the occurrence of CRT, and alleviate unnecessary suffering of children.
Impact
-
This is the latest systematic review of risk factors and incidence of CRT in children.
-
A total of 47 studies involving 262,587 patients were included in our meta-analysis, according to which the pooled prevalence of CRT was 9.1%.
-
This study identified several of the most critical risk factors affecting CRT in children, including D-dimer, insertion location, type of catheter, number of lumens, catheter indwelling time, and central line-associated bloodstream infection (CLABSI).
Similar content being viewed by others
Introduction
Central venous access device (CVAD) is an infusion device inserted through different parts to make the tip of the catheter to the vena cava. In the clinic, CVAD is mainly divided into the following four categories: tunneled central venous catheter (CVC), nontunneled CVC, peripherally inserted central catheter (PICC), and totally implantable venous access port (TIVAP).1 Pediatric patients often require stable, multifunctional, and comfortable long-term vascular access due to factors such as poor puncture cooperation, small vessel diameter, poor peripheral venous visibility and tolerance, high water content in the body leading to easy dehydration, and easy changes in condition after diseases.2 The application of CVAD can significantly reduce the frequency of venipuncture, relieve the stimulation of drugs on the venous blood vessels, alleviate the pain and fear of the children, improve their medication compliance, ensure the effectiveness of intravenous infusion, and improve the quality of disease treatment.3,4,5 Therefore, CVAD is widely used in pediatric clinics and has become an indispensable aspect of complex medical care for children with severe and chronic diseases.
Although CVAD has become an important tool in the pediatric treatment and nursing process, there are also risks of complications related to it, including CVAD-related thrombosis (CRT), phlebitis, fluid and blood leakage at the puncture point, catheter displacement, catheter obstruction, central line-associated bloodstream infection (CLABSI) and so on.6,7 Among these, CRT is one of the most common and serious complications. The prevalence of CRT in children varies significantly by country, age, disease, and medical institution, ranging from 2 to 81%,4,8,9,10 while in Chinese children without prophylactic treatment ranges from 20 to 66%.11,12 CRT has no obvious clinical symptoms in the early stage, but it may still cause serious side effects, not only increasing the patient pain and medical costs but also delaying treatment timing, affecting prognosis and quality of life, and in severe cases, may even lead to thromboembolism, endangering life.13,14,15
Identifying risk factors and incidence of CRT facilitates clinical practitioners in the early identification of high-risk patients, designing specific preventive strategies, treatment regimens, and management plans, thereby effectively reducing the incidence of CRT in hospitalized children and alleviating unnecessary patient suffering. However, most current research on CRT involves only small-scale groups in isolated nursing units or specific disease types. To date, no up-to-date systematic review provides pooled estimates of the risk factors and prevalence of CRT in children. Therefore, this study had a dual purpose: 1. to explore potential risk factors for CRT in children and to determine a pooled level of CRT prevalence; and 2. to provide evidence-based recommendations to improve the recognition, control, and treatment of CRT in children, as well as better nursing management for CRT.
Methods
This review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.16 The detailed research protocol can be accessed on the PROSPERO website (registration number: CRD42023421353).
Search strategy
Eight electronic databases were utilized to conduct a thorough literature search: PubMed, Web of Science, Embase, Cochrane Library, Scopus, China National Knowledge Infrastructure (CNKI), Sinomed, and Wanfang. The search in these databases was conducted from the earliest records available up to January 31st, 2024. The search strategy used a combination of Mesh terms and free words. The following Mesh terms and free words were mainly used: “child,” “children,” “adolescent,” “infant,” “pediatrics,” “central venous access device-related thrombosis,” “CRT,” “catheter-related thrombosis,” “catheter-related venous thrombosis,” “CVC-related thrombosis,” “risk factors,” “protective factors,” “predictors,” “causality,” “influencing factors”. The full search strategy for each database is available in the Supplementary Materials. In addition, we screened the reference lists of all included studies for relevant studies that met the criteria. Grey literature was searched as well. Some authors were contacted through email to gather more information or clarify any uncertainties.
Inclusion criteria
-
The study population was hospitalized children aged ≤18 years.
-
The primary research objective was to explore the risk factors for CRT.
-
The study results have at least one statistically significant predictor.
-
Case-control studies or cohort studies.
-
Published in English or Chinese.
Exclusion criteria
-
Catheter-related infection, catheter dysfunction, or other catheter complications as the primary outcome indicators.
-
Repeated published research.
-
Case reports, study designs, or clinical trials.
-
Reviews, editorials, letters, and conference abstracts.
-
In vitro or animal research.
-
Data were incomplete and could not be extracted.
-
Unable to find the original article.
Data extraction
Data from each eligible study were independently extracted by two reviewers using a pre-designed data collection form. Any disagreements were resolved by discussions among all authors. Data on the following characteristics were obtained from all included studies (see Supplementary Table S1 for details):
-
(1)
Basic information: first author, country, year of publication, study duration, and study design.
-
(2)
Demographic characteristics: study population, sample size, number of CRT, and CRT rate.
-
(3)
Catheter-related features: catheter type, CRT type, and diagnostic method.
-
(4)
Potential risk factors for CRT: odds ratios (OR) or relative risks (RR) values and 95% confidence interval (CI) were extracted for each risk factor. If the study did not provide specific values, it was calculated by constructing a 2 × 2 contingency table.
Quality assessment
Two reviewers evaluated the quality of each study independently using the Risk of Bias Assessment for Nonrandomized Studies tool,17 with any differences settled via group discussion. The tool assessed six domains of risk of bias: participant selection, confounding variables, exposure measurement, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting. If all six domains were rated as low risk, the overall risk of bias for the study was low. The overall risk of bias was moderate if at least one domain was rated as unclear risk, and no domain was rated as high risk, and high if one or more domains were rated as high risk.
To ensure the accuracy of the assessment results, a third reviewer randomly selected five studies to check the data extraction and quality assessment.
Qualitative synthesis and quantitative meta-analysis
Qualitatively classify each risk factor as definite, likely, unclear, or not a risk factor based on the total number of studies with low and moderate bias risks and the proportion of studies demonstrating positive association (Box 1 in the supplementary material). If a risk factor was reported by more than two studies with low or moderate risk of bias, and the definition and reference range were sufficiently consistent, a quantitative meta-analysis was performed to estimate the combined OR.
Data were analyzed using Revman 5.4 software. In the meta-analysis of risk factors and CRT rate, the generic inverse variance method was applied, which only required effect estimate and standard error (SE).18 The SE was obtained by inverse transforming the 95% CI applying the standard normal distribution. Heterogeneity tests were performed on the studies included in the Meta-analysis to examine for the combinability of the results of each independent study. P ≥ 0.05 and I-squared (I2) < 50% considered less heterogeneity between studies and therefore a fixed-effects model was chosen for the analysis, conversely, P < 0.05 or I2 ≥ 50% considered greater heterogeneity, and a random-effects model was chosen.
Certainty of the evidence
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method was used to assess the certainty of the evidence. In this method, observational studies were initially classified as low-quality evidence and then downgraded and upgraded according to five downgrading and three upgrading principles. The 5 downgrading factors included risk of bias, inconsistency, indirectness, imprecision, and publication bias, and the 3 upgrading factors included the magnitude of an effect, dose-response gradient, and effect of plausible residual confounding. Based on these considerations, the overall certainty of each piece of evidence was rated as one of four levels: high, moderate, low, or very low.
Results
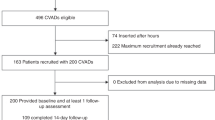
The initial search of the databases extracted a total of 4193 articles, of which 1656 were duplicates and removed. The titles and abstracts of the remaining 2537 articles were screened according to the inclusion criteria and 142 were selected for full-text search. After a rigorous eligibility review, 45 articles met the inclusion criteria. In addition, two articles were found to meet the eligibility criteria in a search of the reference lists of the selected articles and grey literature. In the end, a total of 47 articles were included in this review, of which 43 contributed to the qualitative synthesis and quantitative meta-analysis (Fig. 1).
Of the 47 studies, 19 were prospective4,13,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35 and the rest were retrospective,9,12,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61 of which 10 were multicenter4,9,13,21,23,26,27,28,49,59 and 37 were single-center.12,19,20,22,24,25,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,50,51,52,53,54,55,56,57,58,60,61 The sample sizes ranged from 47 to 158,299, with the two largest being 71,78213 and 158,299,59 respectively. In addition, three studies constructed clinical prediction models.22,28,47 Table 1 lists the summary characteristics of the included studies.
Study populations and CRT rates in included studies
These studies investigated a series of hospitalized children of different ages and departments, of which 12 studies with all hospitalized children as the study population, 12 studies with PICU hospitalized children as the study population, six studies with NICU hospitalized children as the study population, one study with all ICU hospitalized children as the study population, four studies with leukemia children as the study population, two studies with infants under 1-year-old as the study population, and the other ten studies with children with a specific disease as the study population.
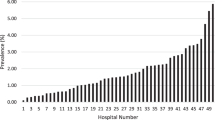
The combined CRT rate was 9.1% (95% CI: 5.7–14.5%) with a high degree of heterogeneity (I2 = 100%). The combined CRT rate was 11.5% (95% CI: 5.7–23.1%; I2 = 99%) in both male and female children. The frequency of CRT in PICU and NICU was available from 13 articles with 234,464 children and 7 articles with 6093 infants, which combined CRT rates were 10.7% (95% CI: 3.8–23.7%; I2 = 100%), 2.9% (95% CI: 1.0–6.5%; I2 = 96%), respectively. The combined CRT rate of children with leukemia was 13.0% (95% CI: 2.9–38.3%; I2 = 98%) (Supplementary Material Figs. S1–6)
Quality of the CRT studies
The methodological quality of the included studies varied (Fig. 2 and Supplementary Material Fig. S7). Nine studies had a low overall risk of bias, as all six domains were categorized as low risk. Four studies had a high overall risk of bias, three of which were associated with confounding variables and one to participant selection. The remaining 34 studies had a moderate overall risk of bias, with at least one of the six domains having an unclear risk.
Risk factors of CRT in included studies
The 47 included studies reported 61 statistically significant risk factors for CRT (Table 1). These factors were classified into three categories: patient-related risk factors (37.7%, 23/61); CVAD-related risk factors (34.4%, 21/61), and treatment-related risk factors (27.9%, 17/61).
Based on the qualitative synthesis, six variables were considered to be definite risk factors for CRT, including D-dimer, location of insertion, type of catheter, number of lumens, catheter indwelling time, and CLABSI. Eleven variables were considered likely associated with CRT, including gastrointestinal diseases, history of catheterization, thrombophilia, geographic location of line placement, catheter dysfunction, number of catheters, insertion length (cm), catheter to vein ratio, dialysis, hypertonic liquid, and cardiac catheterization. For 42 variables, the relationship with CRT was deemed unclear due to conflicting results from studies assessed as having low and moderate risk of bias, or because they were positively associated in only one study. Additionally, birth weight and gestational age were considered non-risk factors (Table 2).
Meta-analyses were implemented for risk factors that were reported by at least two low or moderate risk of bias studies with a consistent definition and reference range (Table 3 and Figs. 3–6).
Forest plots of odds ratios (OR) that were included in the quantitative meta-analysis and the associated overall OR. For each OR, the size of the red square region is proportional to the corresponding study weight. Diamond shape intervals represent the overall OR. I2 represents the fraction of variability among the individual OR that cannot be explained by sampling variability.
Forest plots of odds ratios (OR) that were included in the quantitative meta-analysis and the associated overall OR. For each OR, the size of the red square region is proportional to the corresponding study weight. Diamond shape intervals represent the overall OR. I2 represents the fraction of variability among the individual OR that cannot be explained by sampling variability.
Forest plots of odds ratios (OR) that were included in the quantitative meta-analysis and the associated overall OR. For each OR, the size of the red square region is proportional to the corresponding study weight. Diamond shape intervals represent the overall OR. I2 represents the fraction of variability among the individual OR that cannot be explained by sampling variability.
Forest plots of odds ratios (OR) that were included in the quantitative meta-analysis and the associated overall OR. For each OR, the size of the red square region is proportional to the corresponding study weight. Diamond shape intervals represent the overall OR. I2 represents the fraction of variability among the individual OR that cannot be explained by sampling variability.
GRADE assessment of evidence
Supplementary Table S2 shows GRADE assessments for the certainty of evidence. Due to the design of the observational studies, all evidence was initially rated as low certainty. Based on five downgrading and three upgrading principles, 17 pieces of evidence were still rated as low certainty, and the remaining 44 pieces of evidence were downgraded to very low certainty for serious inconsistency and imprecision.
Discussion
Our study is the latest systematic review of risk factors and the incidence of CRT in hospitalized children. Based on 47 studies included in the current meta-analysis, which involved a total of 262,587 patients, the pooled prevalence of CRT is 9.1%. We conducted a qualitative synthesis analysis of 61 predictive factors and a quantitative meta-analysis of 38 factors, identifying six definite factors, 11 likely factors, and 42 unclear factors associated with CRT. Definite predictors included being of D-dimer, location of insertion, type of catheter, number of lumens, catheter indwelling time and CLABSI. The findings of our systematic review provide the latest comprehensive evidence summary that can inform the early identification of children at risk for CRT and the development of intervention measures to prevent and reduce CRT.
Implantable and temporary medical devices such as CVAD are exposed to blood for weeks to years depending on the type of CVAD in place. Since CVAD is an artificial surface and lacks an endothelial layer that inhibits platelet coagulation and adhesion, it is thought to potentially activate the contact pathways, ultimately leading to thrombosis. Assembly of artificial surface contact systems might be part of the host defense mechanism against foreign substances, but it can lead to kinin and thrombin generation, and complement activation.62 This eventually promotes thrombosis and inflammation. The presence of CVAD is the most common risk factor for venous thromboembolism (VTE). CRT accounts for 10% of deep vein thrombosis (DVT) in adults and 50–80% in children.10,55,63 The incidence of CRT in hospitalized children has increased significantly by 30–70% over the past 20 years,64,65 which may cause serious medical complications besides increasing healthcare expenditures and length of stay.
We discover that a higher level of D-dimer is an independent risk factor for CRT in hospitalized children, consistent with the results of adult studies.66 D-dimer is a soluble fibrin degradation product deriving from the plasmin-mediated degradation of cross-linked fibrin that is increased or positive in secondary hyperfibrinolysis, such as hypercoagulable states, disseminated intravascular coagulation, and thrombolytic therapy.67,68 Increased D-dimer suggests an association with thrombotic disorders in the body of various origins and an increase in fibrinolytic activity. D-dimer has been extensively investigated for excluding the diagnosis of VTE and is used routinely for this indication.67,69 Therefore, for early recognition and to reduce the incidence of CRT, D-dimer levels should be closely monitored before and after catheterization. However, the elevated D-dimer test results cannot fully explain the cause and location of CRT formation and must be analyzed in conjunction with clinical and other test results. Inherited thrombophilia, caused by genetic defects leading to a deficiency or abnormality in associated proteins, including protein C, protein S, antithrombin, the coagulation factor V Leiden mutation, and factor II mutation G20210A,70 is considered a potential risk factor for CRT. The prevalence of thrombophilia varies widely among different populations, with a reported prevalence of 10% to 59% in pediatric VTE patients.71 Children with gastrointestinal diseases like short bowel syndrome (SBS) and inflammatory bowel disease (IBD) have an increased risk of developing CRT during hospitalization. The precise mechanism behind this association is still uncertain according to current research. It may be attributed to the heightened inflammation levels during catheterization, particularly in patients with active IBD episodes or admissions during surgery, which leads to a period of increased inactivity.55 This suggests that delaying placement during the most active period of inflammation may reduce the rate of thrombosis.
A narrative review pointed out that age is one of the most significant risk factors for VTE. In children, CRT shows a bimodal distribution, with the highest incidence rate in infancy and adolescence.10 The higher incidence in infancy may be due in part to the smaller diameter of the vein, making insertion difficult and requiring multiple attempts. However, whether age is a risk factor for CRT is still highly controversial. The study by Chojnacka et al. did not find a statistically significant difference,39 although a trend toward a similar bimodal distribution was found in the study population. Cancer, cardiovascular disease, sepsis, asphyxia, and neurological diseases are also considered unclear factors for CRT. Pediatric patients diagnosed with leukemia have multiple risk factors for VTE formation, such as the presence of hypercoagulable blast cells, the pro-thrombotic nature of the cancer itself, and treatment with steroids and L-asparaginase. Chen et al.38 and Jaffray et al.4 concluded that children with leukemia are more likely to develop CRT. Sepsis causes the coagulation mechanism to become fragile, which in turn activates the coagulation system and creates thrombosis.72 However, a study by Onyeama et al.52 showed that sepsis was significantly associated with a reduced incidence of CRT, and the exact mechanism is currently unknown.
The location of insertion and type of catheter are critical risk factors for CRT. The incidence of CRT is higher in femoral vein catheterizations compared to subclavian and jugular vein catheterizations in children, which is contrary to findings in adult patients.73 The femoral location is a larger vessel and allows placement of a larger size catheter. Femoral CVAD is prioritized in urgent and emergency situations. In such cases, the patients tend to be more critically ill and often immobilized, further exacerbating the low-flow state. In addition, there may be vein compression and kinking beneath the inguinal ligament with leg movement, which may increase the risk of CRT.27 PICC catheters provide a reliable medium to long-term route to intravenous therapy for children, but compared with other types of catheters, the risk of CRT is higher. We speculate that the long tunnel length and relatively large lumen size of the PICC, compared to the diameter of the vessel at the insertion site, may lead to increased blood flow obstruction.52 Additionally, patients with PICC may be more likely to be diagnosed with symptomatic VTE than tunneled lines (TLs) because PICC is often placed in smaller vessels and journeys through the arm or leg causing limb pain and swelling, whereas TLs are located in the chest.
The risk of CRT increases with the number of lumens. A possible explanation for this finding is that multilumen catheters tend to have larger catheter sizes and thus occupy more area within the vessel lumen, leading to obstruction of normal blood flow within the veins. The relationship between CRT and CLABSI is bidirectional. Following catheter insertion, a fibrin sheath forms around the catheter. Microorganisms, especially staphylococcus aureus, easily adhere to the fibrin sheaths, and may lead to CLABSI.74 Conversely, CLABSI can trigger inflammatory reactions, leading to further progression of thrombosis. CVAD duration is positively associated with the risk of CRT. Catheter placement may cause mechanical injury to the vein. As the indwelling duration increases, many damaged smooth muscle and endothelial cells become embedded within the fibrin, resulting in thrombus formation. In addition, prolonged indwelling increases the chance of platelet contact with the vessel lining, activating coagulation factors and thrombin, increasing the risk of thrombosis.22 Therefore, nurses should perform routine maintenance of the catheter in children who require long-term CVAD indwelling. The duration of CVAD should be monitored, the necessity of its indwelling should be assessed daily, and the catheter should be removed as early as possible while ensuring treatment.
As obstruction of venous blood flow from the CVAD is considered an essential causative mechanism for the development of VTE, a high ratio between catheter size and vein diameter could be a risk factor for CRT. The 2012 international guidelines on pediatric CVC insertion recommend that the ratio between the catheter’s external diameter and the cannulated vein’s diameter should not exceed 0.33.75 However, this suggestion is only based on expert opinions and currently lacks relevant clinical data support. Therefore, further research is still needed to verify it. Catheter dysfunction is mainly caused by small clots or fibrous sheaths wrapping around the tip of the catheter. Prolonged accumulation may lead to incomplete or complete blockage of blood vessels, becoming a gathering point for thrombosis.74 Journeycake et al. observed that the risk of VTE was highest in pediatric cancer patients with multiple episodes of catheter dysfunction.76 A study of pediatric brain tumor patients reported that VTE was more common in patients with catheter dysfunction.77 Thus, these studies and the current data support the need to consider catheter dysfunction as a possible risk factor for CRT and to design further screening and intervention studies for early identification and prevention of catheter dysfunction.
The rationale for studying the relationship between the insertion side of CVAD and the risk of CRT is based on the anatomy of the upper body venous system. The left brachiocephalic vein is longer and courses more horizontally than the right side, thus entering the superior vena cava at a sharper angle. The right jugular vein is the most direct and shortest route for the CVAD to enter the heart. By contrast, the CVAD located in the left jugular vein has a greater distance to the heart and passes through 2 angles in the venous system, which may cause endothelial damage and increase the likelihood of blood flow obstruction and venous wall adhesion.26 However, our meta-analysis did not find a statistically significant increase in the risk of CRT with left-sided placement compared to right-sided placement. The ideal location for the catheter tip is the junction of the superior vena cava and the right atrium. This location is preferred because of the higher blood flow rate, which may be protective against thrombosis.43 Currently, the pediatric literature on the effect of optimal tip position on CRT is scarce and inconclusive. In addition, catheter tips do not always remain in that position after initial placement. Therefore, tip movement should be a significant concern in pediatric patients, especially active, growing, and requiring long-term catheter use.
Providing renal replacement therapy is a lifelong task for pediatric end-stage renal disease (ESRD) patients. Although successful transplantation can be achieved even in young patients, the lifespan of the graft is limited. Consequently, many transplant recipients may be put back on dialysis as part of their ESRD treatment.78 CVC remains the main vascular access for hemodialysis in children. Long-term reliance on CVC is related to a high incidence of catheter dysfunction and failure. The frequent need for recurrent CVC placement in such patients leads to an elevated risk of central vein stenosis and CRT. Cardiac catheterization is also a possible risk factor for CRT. Appropriate anticoagulation is required during catheterization, without which the risk of thrombosis is up to 40%. However, the use of unfractionated heparin in pediatric patients is challenging because the coagulation system and heparin response are different from that of adults.79 There’s a need for further research to determine if children are receiving adequate doses of heparin during cardiac catheterization to prevent thrombosis without increasing the risk of bleeding complications. The incidence of VTE in adult patients who are chronically bedridden and braked is 3.59 times higher than in patients with normal activity levels.80 In critically ill or surgical children, mechanical ventilation is often performed in the early stages, requiring continuous use of multiple sedative or inotropic drugs to reduce cardiac load and protect pulmonary function. During sedation, the child is in a braked state, limb activity is reduced or even inactive, blood flow slows down, and blood stagnates in the veins, increasing the chance of platelet adhesion to the endothelium, which may increase the risk of CRT. Therefore, passive movements such as limb abduction, internal rotation, elbow flexion and elbow extension should be performed appropriately when the child’s condition permits.
Nutritional support is an important part of critical illness treatment, including enteral and parenteral nutrition (PN). CVAD is the supply channel for total parenteral nutrition (TPN), and some children may even need this method to provide calories for a long time. High glucose and calcium concentrations in PN are both possible triggers of CRT, and PN has been shown to upregulate the extrinsic coagulation cascade, especially with long-term use.60 Diamanti et al. reported that the incidence rate of TPN complicated with CRT was 20%.81 Mannitol or glycerol fructose are widely used as hypertonic drugs in clinical practice, which can increase plasma osmolality to dehydrate tissues after entering the body. At the same time, it may cause a cellular stress response, induce apoptosis, and can activate inflammatory cytokines and coagulation pathways to induce thrombosis. Jiang et al.22 found vasoactive drugs to be a risk factor for CRT. The possible reason is that vasoactive drugs can cause strong vasoconstriction, endothelial function damage or impairment, and promote fibrinogen synthesis. However, this is contrary to the findings of Marquez et al.28 and Faustino et al.21 Therefore, larger prospective studies are still needed to assess this risk factor more precisely.
The strengths of this study include the systematic identification of all relevant studies of risk factors for CRT in hospitalized children and the classification of risk factors into three categories, patient-related risk factors, CVAD-related risk factors, and treatment-related risk factors, to offer a logical progression of the possible causes of CRT in children. However, several limitations of this systematic review should be stated. Firstly, as most of the studies originate from Western countries, extrapolating these results to Eastern populations is questionable. Second, significant heterogeneity was encountered in our analysis, potentially stemming from variations in regimen, duration, population enrolled, and center setting, among other factors. This diversity necessitates a cautious interpretation of the results. In addition, only a few high-quality studies with a low risk of bias, and many of the studies suffer from significant sources of bias. Furthermore, the effect in many occasions was assessed by very few studies. Therefore, the evidence to support it is low, which needs to be validated in future studies. Finally, risk factors for CRT could not be made causal assertions since the majority of studies were retrospective.
Conclusions
In conclusion, we have identified several critical factors that affect CRT, including D-dimer, location of insertion, type of catheter, number of lumens, catheter indwelling time, and CLABSI. Nevertheless, none of the included studies considered the impact of socio-demographic factors on CRT, such as parental education level, occupation, and family economic status. Therefore, larger sample sizes and well-designed prospective studies are still needed to clarify the predictors affecting CRT in the future. In addition, there is a lack of pediatric-specific CRT risk assessment tools, which need to be further developed and validated. Machine learning (ML), as a method for designing risk assessment models that help to efficiently explore and mine useful information, has been widely used in recent years to solve a variety of challenging medical problems. Likewise, the application of ML in CRT risk diagnosis may contribute to a more precise assessment. In clinical practice, it is necessary to take appropriate stratified preventive measures according to the level of CRT risk assessment of children, to improve the efficiency of clinical work, reduce the burden of clinical work, and minimize the occurrence of CRT under the premise of ensuring the safety of children.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Yeow, M. et al. A systematic review and network meta-analysis of randomized controlled trials on choice of central venous access device for delivery of chemotherapy. J. Vasc. Surg. Venous Lymphat. Disord. 10, 1184–91.e8 (2022).
Cellini, M. et al. Guidelines of the Italian Association of Pediatric Hematology and Oncology for the management of the central venous access devices in pediatric patients with onco-hematological disease. J. Vasc. Access 23, 3–17 (2022).
Ares, G. & Hunter, C. J. Central venous access in children: indications, devices, and risks. Curr. Opin. Pediatr. 29, 340–346 (2017).
Jaffray, J. et al. Peripherally inserted central catheters lead to a high risk of venous thromboembolism in children. Blood 135, 220–226 (2020).
Zhang, J. J. et al. Factors affecting mechanical complications of central venous access devices in children. Pediatr. Surg. Int. 38, 1067–1073 (2022).
Akhtar, N. & Lee, L. Utilization and Complications of Central Venous Access Devices in Oncology Patients. Curr. Oncol. 28, 367–377 (2021).
Ullman, A. J., Marsh, N., Mihala, G., Cooke, M. & Rickard, C. M. Complications of Central Venous Access Devices: A Systematic Review. Pediatrics 136, e1331–e1344 (2015).
Östlund, Å. et al. Erratum to ‘Incidence of and risk factors for venous thrombosis in children with percutaneous non-tunnelled central venous catheters’ (Br J Anaesth 2019; 123: 316-24). Br. J. Anaesth. 123, 918 (2019).
McLaughlin, C. M. et al. Symptomatic catheter-associated thrombosis in pediatric trauma patients: Choose your access wisely. Surgery 166, 1117–1121 (2019).
Citla Sridhar, D., Abou-Ismail, M. Y. & Ahuja, S. P. Central venous catheter-related thrombosis in children and adults. Thromb. Res. 187, 103–112 (2020).
Zhou, X. et al. A retrospective analysis of risk factors associated with catheter-related thrombosis: a single-center study. Perfusion 35, 806–813 (2020).
Li, S. et al. Risk factors for central venous catheter-related thrombosis in hospitalized children: a single-center a retrospective cohort study. Transl. Pediatr. 11, 1840–1851 (2022).
Patel, N., Petersen, T. L., Simpson, P. M., Feng, M. & Hanson, S. J. Rates of Venous Thromboembolism and Central Line-Associated Bloodstream Infections Among Types of Central Venous Access Devices in Critically Ill Children. Crit. Care Med. 48, 1340–1348 (2020).
Timsit, J. F. et al. A state of the art review on optimal practices to prevent, recognize, and manage complications associated with intravascular devices in the critically ill. Intensive Care Med. 44, 742–759 (2018).
Ullman, A. J. et al. Pediatric central venous access devices: practice, performance, and costs. Pediatr. Res. 92, 1381–1390 (2022).
Hutton, B. et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann. Intern Med. 162, 777–784 (2015).
Kim, S. Y. et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J. Clin. Epidemiol. 66, 408–414 (2013).
Borenstein, M., Hedges, L. V., Higgins, J. P. & Rothstein, H. R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 1, 97–111 (2010).
Beck, C., Dubois, J., Grignon, A., Lacroix, J. & David, M. Incidence and risk factors of catheter-related deep vein thrombosis in a pediatric intensive care unit: a prospective study. J. Pediatr. 133, 237–241 (1998).
Dubois, J. et al. Incidence of deep vein thrombosis related to peripherally inserted central catheters in children and adolescents. Cmaj 177, 1185–1190 (2007).
Faustino, E. V. et al. Incidence and acute complications of asymptomatic central venous catheter-related deep venous thrombosis in critically ill children. J. Pediatr. 162, 387–391 (2013).
Jiang, W. et al. Construction and validation of a risk prediction model for central venous catheter-associated deep venous thromboses in children with congenital heart disease after surgery. Chin. J. Nurs. 57, 2217–2224 (2022).
Faustino, E. V. et al. Factor VIII May Predict Catheter-Related Thrombosis in Critically Ill Children: A Preliminary Study. Pediatr. Crit. Care Med. 16, 497–504 (2015).
Jones, S., Butt, W., Monagle, P., Cain, T. & Newall, F. The natural history of asymptomatic central venous catheter-related thrombosis in critically ill children. Blood 133, 857–866 (2019).
Kim, E. H. et al. Central venous catheter-related thrombosis in pediatric surgical patients: A prospective observational study. Paediatr. Anaesth. 32, 563–571 (2022).
Male, C. et al. Central venous line-related thrombosis in children: association with central venous line location and insertion technique. Blood 101, 4273–4278 (2003).
Male, C., Julian, J. A., Massicotte, P., Gent, M. & Mitchell, L. Significant association with location of central venous line placement and risk of venous thrombosis in children. Thromb. Haemost. 94, 516–521 (2005).
Marquez, A., Shabanova, V. & Faustino, E. V. Prediction of Catheter-Associated Thrombosis in Critically Ill Children. Pediatr. Crit. Care Med. 17, e521–e528 (2016).
Menéndez, J. J. et al. Incidence and risk factors of superficial and deep vein thrombosis associated with peripherally inserted central catheters in children. J. Thromb. Haemost. 14, 2158–2168 (2016).
Rubio Longo, M. C. et al. Catheter-related deep vein thrombosis in newborn infants. Arch. Argent. Pediatr. 119, 32–38 (2021).
Östlund, Å. et al. Incidence of and risk factors for venous thrombosis in children with percutaneous non-tunnelled central venous catheters. Br. J. Anaesth. 123, 316–324 (2019).
Sol, J. J. et al. Chronic Complications After Femoral Central Venous Catheter-related Thrombosis in Critically Ill Children. J. Pediatr. Hematol. Oncol. 37, 462–467 (2015).
van Rooden, C. J. et al. Infectious complications of central venous catheters increase the risk of catheter-related thrombosis in hematology patients: a prospective study. J. Clin. Oncol. 23, 2655–2660 (2005).
Zeng, X., Zhang, C. & Shi, Y. Analysis of risk factors for complicated catheter-related thrombosis in children. Chin. J. Emerg. Med. 29, 719–723 (2020).
Wei, Y. et al. The incidence and risk factors of catheter-related-thrombosis during induction chemotherapy in acute lymphocytic leukemia children. Chin. J. Hematol. 38, 313–317 (2017).
Deng, G. & Liao, Q. Analysis of risk factors for venous thrombosis after PICC placement in critically ill children. Int. I Nurs. 39, 775–777 (2020).
Badheka, A. V. et al. Catheter related thrombosis in hospitalized infants: A neural network approach to predict risk factors. Thromb. Res. 200, 34–40 (2021).
Chen, K. et al. Risk factors for central venous catheter-related thrombosis in children: a retrospective analysis. Blood Coagul. Fibrinol. 27, 384–388 (2016).
Chojnacka, K., Krasiński, Z., Wróblewska-Seniuk, K. & Mazela, J. Catheter-related venous thrombosis in NICU: A case-control retrospective study. J. Vasc. Access 23, 88–93 (2022).
Derderian, S. C., Good, R., Vuille-Dit-Bille, R. N., Carpenter, T. & Bensard, D. D. Central venous lines in critically ill children: Thrombosis but not infection is site dependent. J. Pediatr. Surg. 54, 1740–1743 (2019).
Diamond, C. E. et al. Catheter-Related Venous Thrombosis in Hospitalized Pediatric Patients with Inflammatory Bowel Disease: Incidence, Characteristics, and Role of Anticoagulant Thromboprophylaxis with Enoxaparin. J. Pediatr. 198, 53–59 (2018).
Noailly Charny, P. A. et al. Increased Risk of Thrombosis Associated with Peripherally Inserted Central Catheters Compared with Conventional Central Venous Catheters in Children with Leukemia. J. Pediatr. 198, 46–52 (2018).
Gnannt, R. et al. Increased risk of symptomatic upper-extremity venous thrombosis with multiple peripherally inserted central catheter insertions in pediatric patients. Pediatr. Radio. 48, 1013–1020 (2018).
Gray, B. W. et al. Characterization of central venous catheter-associated deep venous thrombosis in infants. J. Pediatr. Surg. 47, 1159–1166 (2012).
Haddad, H. et al. Routine surveillance ultrasound for the management of central venous catheters in neonates. J. Pediatr. 164, 118–122 (2014).
Lambert, I., Tarima, S., Uhing, M. & Cohen, S. S. Risk Factors Linked to Central Catheter-Associated Thrombosis in Critically Ill Infants in the Neonatal Intensive Care Unit. Am. J. Perinatol. 36, 291–295 (2019).
Li, H. et al. Prediction of central venous catheter-associated deep venous thrombosis in pediatric critical care settings. BMC Med. Inf. Decis. Mak. 21, 332 (2021).
Lovett, M. E. et al. Catheter-associated deep vein thrombosis in children with severe traumatic brain injury: A single-center experience. Pediatr. Blood Cancer 70, e30044 (2023).
MacLean, J. et al. Need for tissue plasminogen activator for central venous catheter dysfunction is significantly associated with thrombosis in pediatric cancer patients. Pediatr. Blood Cancer 65, e27015 (2018).
Noonan, P. J., Hanson, S. J., Simpson, P. M., Dasgupta, M. & Petersen, T. L. Comparison of Complication Rates of Central Venous Catheters Versus Peripherally Inserted Central Venous Catheters in Pediatric Patients. Pediatr. Crit. Care Med. 19, 1097–1105 (2018).
Pei, L. et al. Clinical characteristics and risk factors of symptomatic central venous catheter-related deep vein thrombosis in children. Chin. Pediatr. Emerg. Med. 23, 450–454 (2016).
Onyeama, S. N. et al. Central Venous Catheter-associated Venous Thromboembolism in Children With Hematologic Malignancy. J. Pediatr. Hematol. Oncol. 40, e519–e524 (2018).
Shah, S. H. et al. Clinical risk factors for central line-associated venous thrombosis in children. Front. Pediatr. 3, 35 (2015).
Shin, H. S., Towbin, A. J., Zhang, B., Johnson, N. D. & Goldstein, S. L. Venous thrombosis and stenosis after peripherally inserted central catheter placement in children. Pediatr. Radio. 47, 1670–1675 (2017).
Smitherman, A. B. et al. The incidence of catheter-associated venous thrombosis in noncritically ill children. Hosp. Pediatr. 5, 59–66 (2015).
Steen, E. H. et al. Central Venous Catheter-Related Deep Vein Thrombosis in the Pediatric Cardiac Intensive Care Unit. J. Surg. Res 241, 149–159 (2019).
Wang, J. & Ren, G. Peripherally inserted central catheter related venous thromboembolism in children with acute leukemia: a factorial analysis. Chin. J. Biomed. Eng. 27, 288–293 (2021).
Dubbink-Verheij, G. H. et al. Femoral Vein Catheter is an Important Risk Factor for Catheter-related Thrombosis in (Near-)term Neonates. J. Pediatr. Hematol. Oncol. 40, e64–e68 (2018).
Tran, M., Shein, S. L., Ji, X. & Ahuja, S. P. Identification of a “VTE-rich” population in pediatrics - Critically ill children with central venous catheters. Thromb. Res 161, 73–77 (2018).
Wisecup, S., Eades, S. & Turiy, Y. Characterizing the Risk Factors Associated With Venous Thromboembolism in Pediatric Patients After Central Venous Line Placement. J. Pediatr. Pharm. Ther. 20, 358–366 (2015).
Zhu, W., Zhang, H., Xing, Y. Clinical Characteristics of Venous Thrombosis Associated with Peripherally Inserted Central Venous Catheter in Premature Infants. Children 9, https://doi.org/10.3390/children9081126 (2022).
Ekdahl, K. N. et al. Innate immunity activation on biomaterial surfaces: a mechanistic model and coping strategies. Adv. Drug Deliv. Rev. 63, 1042–1050 (2011).
Takemoto, C. M. et al. Hospital-associated venous thromboembolism in children: incidence and clinical characteristics. J. Pediatr. 164, 332–338 (2014).
Boulet, S. L. et al. Trends in venous thromboembolism-related hospitalizations, 1994-2009. Pediatrics 130, e812–e820 (2012).
Raffini, L., Huang, Y. S., Witmer, C. & Feudtner, C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics 124, 1001–1008 (2009).
Lin, S., Zhu, N., YihanZhang, Du, L. & Zhang, S. Development and validation of a prediction model of catheter-related thrombosis in patients with cancer undergoing chemotherapy based on ultrasonography results and clinical information. J. Thromb. Thrombolysis 54, 480–491 (2022).
Johnson, E. D., Schell, J. C. & Rodgers, G. M. The D-dimer assay. Am. J. Hematol. 94, 833–839 (2019).
Favresse, J. et al. D-dimer: Preanalytical, analytical, postanalytical variables, and clinical applications. Crit. Rev. Clin. Lab Sci. 55, 548–577 (2018).
Weitz, J. I., Fredenburgh, J. C. & Eikelboom, J. W. A Test in Context: D-Dimer. J. Am. Coll. Cardiol. 70, 2411–2420 (2017).
Darlow, J. & Mould, H. Thrombophilia testing in the era of direct oral anticoagulants. Clin. Med. 21, e487–e491 (2021).
Monagle, P. et al. American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. 2, 3292–3316 (2018).
Meziani, F., Gando, S. & Vincent, J. L. Should all patients with sepsis receive anticoagulation? Yes. Intensive Care Med 43, 452–454 (2017).
Saber, W. et al. Risk factors for catheter-related thrombosis (CRT) in cancer patients: a patient-level data (IPD) meta-analysis of clinical trials and prospective studies. J. Thromb. Haemost. 9, 312–319 (2011).
Journeycake, J. M. & Buchanan, G. R. Thrombotic complications of central venous catheters in children. Curr. Opin. Hematol. 10, 369–374 (2003).
Lamperti, M. et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med 38, 1105–1117 (2012).
Journeycake, J. M. & Buchanan, G. R. Catheter-related deep venous thrombosis and other catheter complications in children with cancer. J. Clin. Oncol. 24, 4575–4580 (2006).
Deitcher, S. R., Gajjar, A., Kun, L. & Heideman, R. L. Clinically evident venous thromboembolic events in children with brain tumors. J. Pediatr. 145, 848–850 (2004).
Mandel-Shorer, N., Tzvi-Behr, S., Harvey, E. & Revel-Vilk, S. Central venous catheter-related venous thrombosis in children with end-stage renal disease undergoing hemodialysis. Thromb. Res 172, 150–157 (2018).
Chen, D., Långström, S., Petäjä, J., Heikinheimo, M. & Pihkala, J. Thrombin formation and effect of unfractionated heparin during pediatric cardiac catheterization. Catheter Cardiovasc Inter. 81, 1174–1179 (2013).
Reynolds, P. M. et al. Evaluation of Prophylactic Heparin Dosage Strategies and Risk Factors for Venous Thromboembolism in the Critically Ill Patient. Pharmacotherapy 39, 232–241 (2019).
Diamanti, A. et al. Prevalence of life-threatening complications in pediatric patients affected by intestinal failure. Transpl. Proc. 39, 1632–1633 (2007).
Funding
This study was supported by the Fundamental Research Funds for the Central Universities [grant numbers YCJJ20230244] and Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology Research Fund [grant numbers 2022C09].
Author information
Authors and Affiliations
Contributions
GY and YL framed the review questions on the basis of input from MF and QY. YY and XQ conducted the literature search. MF, WS, and QY screened and evaluated the identified papers. GY and YY performed data extraction and analysis. MF, WS, XQ and QY prepared the initial manuscript with revisions and comments from GY, YL, and XX. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fu, M., Yuan, Q., Yang, Q. et al. Risk factors and incidence of central venous access device-related thrombosis in hospitalized children: a systematic review and meta-analysis. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03225-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03225-0