Abstract
Objective
Functional electrical stimulation (FES) can enhance motor learning of hand fine motor skills in neurological diseases with upper motoneuron lesions. Nevertheless, FES is rarely applied in patients with chronic Guillan-Barré syndrome (GBS) with preserved deep tendon reflexes allowing for stimulation via nerve. This single case report documents the results of an FES-supported, task-oriented grasp training to regain hand closure and pinch grip.
Study design
Single-subject repeated measures study.
Setting
International FES Centre®, Swiss Paraplegic Centre Nottwil.
Methods
Three individually defined goals were formulated and scored by using the goal attainment scale. With a focus on these goals, FES was applied bilaterally to improve hand closure and pinch grip. Based on principles of motor learning FES was executed together with task-oriented movements. The hand closure distance (cm) between the tip of the middle finger and the palmar side of the hand was measured and the achievement of personal, predefined goals evaluated.
Results
After 16 weeks of daily stimulation, hand closure could be voluntarily performed. Regained opposition of the thumb to the index finger enabled improved individually defined fine motor control. Restored function remained unchanged in the follow-up at 6 months without stimulation.
Conclusion
Improving fine motor skills in chronic GBS with intact deep tendon reflexes was possible utilizing FES combined with task-oriented grasp training. These improvements were maintained over time indicating the combination was effective in promoting functionally meaningful motor gains.
Similar content being viewed by others
Introduction
The Guillain–Barré syndrome (GBS) is a rare disease with a median incidence of 1.11 per 100.000 people per year. It is subdivided into various subtypes; acute motor axonal neuropathy and acute inflammatory demyelinating polyneuropathy [1]. GBS is a dramatic event that leads to flaccid paralysis or muscle weakness in all four limbs and trunk within hours and typically reaches its culmination after two to four weeks [2, 3]. It is mostly characterized by hyporeflexia or areflexia and a decrease or absence of deep tendon reflexes [4]. In some cases of the motor axonal form of the disease, even hyperreflexia has been observed [5]. Two-thirds of the affected people reported a preceding infection of the respiratory or gastrointestinal tract. The few patients who showed the pure motor axonal form with preserved or increased deep tendon reflexes, suffered from a gastrointestinal infection caused by campylobactus jeuni within four weeks before the onset of symptoms e.g., muscle weakness [5]. This course of the disease is associated with a more severe neurological impairment and a delayed and incomplete motor recovery because of the mostly irreversible axonal degeneration, widespread along the nerve fiber length [3]. The treatment with the best-documented efficacy is plasma exchange and intravenous immunoglobulin therapy [6]. Improvement of motor functions can be expected within the first year, but even after three or more years, some motor function recovery was observed [7].
The clinical presentation of GBS patients resembles that of incomplete tetraplegia. Consequently, the physio and occupational therapies are similar in their aims and methods.
Electrical stimulation (ES) might be considered in the acute, sub-acute, and even chronic phases of GBS. Despite some promising results in the literature, the use of ES remains controversial in clinical practice [8,9,10,11,12,13]. In general, GBS is characterized by lower motoneuron damage. Hence denervated muscles will undergo degeneration. As a consequence, loss of viscoelasticity and contractures will occur in the muscles. In some cases, the excitability of the nerves remains unaffected but muscle weakness and disuse atrophy will still arise. Furthermore, paralyzed muscles lose their motor cortex representation within two months [14]. During neurological recovery after incomplete cervical spinal cord injury (cSCI), the maintenance of the representation of hand function, for example, hand opening and closing, may support the relearning process without compensatory movement strategies. Popovic and colleagues demonstrated that even in subacute incomplete cSCI the combination of task-oriented grasping with FES support is superior to task-oriented grasp training alone [15]. Clinical observations have shown that in subacute and even in chronic stages after cSCI an FES-supported task-oriented training could improve and maintain voluntary hand function.
Neuromuscular electrical stimulation (NMES)/(FES) or direct muscle stimulation may be indicated in acute and subacute critical illness neuropathy and chronic conditions with associated muscle atrophy or weakness [10, 16]. ES generates an electrical field under the electrodes causing a depolarization of the cell membrane of a neuron or in the case of direct muscle stimulation, an elicitation of muscle fiber action potentials. There is a major difference in the stimulation threshold between nerve and muscle fibers. Nerve fibers are excitable from 50 µs (0.05 ms) pulse duration whereas muscle fibers above 10 ms.
The purpose of the single case report was to evaluate how task-oriented functional electrical stimulation (FES) improved fine motor skills in the chronic phase of GBS.
Participant description
The study participant was a 78-year-old male patient diagnosed with GBS in November 2008 (at age 68). Previously he had gastritis caused by an infection with campylobactus jejuni. Within 14 days, progressive complete paralysis developed. It began distally in the feet and hands that ended in a respiratory failure requiring mechanical ventilation. Therefore, a tracheostomy was performed. The respirator dependence lasted from November 2008 to January 2009. In addition, he showed GBS-related clinical features as pain in all paralyzed joints, autonomic dysfunction as urinary retention, and hypertension. Due to the involvement of the cranial nerves, mainly the glossopharyngeal nerve, the ability to swallow was reduced. Consequently, a PEG probe was inserted. He underwent the recommended therapeutic approach to GBS as intravenous immunoglobulin therapy and plasma exchange. In the acute phase, he received three times a day various therapeutical interventions for airway management including secretion clearance, two times physiotherapy for maintenance of joint mobility, and once occupational therapy taking care of positioning the upper limbs including splinting the hands to avoid contractures. Neurological testing showed preserved sensory function as evidenced by pinprick and light touch but a complete flaccid paralysis of the muscles of the upper and lower limbs and the trunk but with associated hyper-reflexic deep tendons.
The British Medical Research Council Scale for manual muscle testing (MRC) was used to assess voluntary muscle activity. First muscle activity was confirmed in the gluteus (MRC 1) and quadriceps (MRC 1) muscles in December. The muscles of the erector spinae reinnervated shortly afterwards. In January 2009 voluntary muscle activity was observed in the upper limbs (MRC 1 in triceps brachii and extensor pollicis). The patient was mobilized with a chin-controlled electric wheelchair for the first seven months. Thereafter he used a power wheel-controlled manual wheelchair. Despite active-assistive and passive movements of the hands including splinting, the patient developed contractures in the interphalangeal joints of both hands. Furthermore, the thumb showed impaired opposition and adduction. This led to the loss of fine motor skills as tying shoes, shirt buttoning, and writing. The cause of the insufficient hand closure was attributed to a muscular dysbalance between finger extensors and the weaker finger flexors and the intrinsic muscles.
In general, the neurological recovery of motor function was very slow (Fig. 1). At discharge in December 2009, he was able to manage the sit-to-stand transfer independently. Walking with two ankle-foot orthoses (AFO) was possible in a domestic setting. For short distances, he used a manual wheelchair. In the upper limbs, the patient managed to move the arms in a three-dimensional space against gravity. Performing overhead activities led to compensatory activation of the trapezius muscle. The motor function of the hands stagnated, the imbalance from extensors to flexors remained, so that grasping with grip reinforcement and aids for self-care and grooming were necessary.
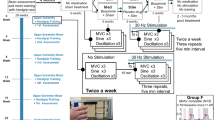
From December 2009 to June 2010 the patient received physio-, occupational, and hydrotherapy two times a week for 45 min. The physiotherapy focused on the function of the lower limbs and trunk, the occupational therapy on all activities of the upper limbs, mainly fine motor skills. During aquatic therapy, the full range of motion of all joints was enhanced.
In June 2010 the patient used a walker and AFOs instead of a manual wheelchair for short distances (up to 1 km). The function of the upper limbs, mainly the hands, remained unchanged. He continued his therapy with the same intensity. In March 2011, walking distances above one km could only be pursued with AFOs. There was no progress in the upper limb function. The outpatient therapy was continued as described previously. In 2014 the patient was able to walk with ankle footwear without AFOs 5 km to 6 km. The MRC scores in the hands remained unchanged, the range of motion in shoulder and elbow joints was normal. In contrast, the hand contractures in the interphalangeal joints persisted. He saw his quality of life as severely limited. An important goal for him was to be able to wear a shirt that could be buttoned by himself. Likewise, the manipulation of coins, as well as credit cards were desired skills. Per his own request and with self-imposed goals, FES was initiated in November 2018 to improve hand closure and thumb to index finger opposition.
Methods
Treatment
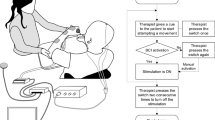
The testing of motor points with ES of the key actuators for hand closure and thumb to index finger opposition showed an efficient muscle contraction by excitation via nerve. The stimulation parameters used were 300 µs pulse duration with an amplitude between 20–30 mA and a frequency of 35 Hz. The ES testing was performed on the first dorsal interosseus muscle (IOD1), adductor pollicis (ADP), and flexor digitorum profundus (FDP). The muscle response was deemed efficient if the full range of motion was the result of the intended electrically stimulated muscle, without contribution from the neighboring muscles.
The MRC score was used to classify the response of the stimulated muscle as innervated, partially denervated, and denervated. Hereby the range of motion evoked by ES served as a surrogate. If no muscle contraction could be elicited, the muscle was considered denervated. In the case of an ES evoked contraction and/or a movement of an incomplete range of motion, the muscle was graded as partially denervated. Muscles were classified as innervated in the case of ≥3 MRC during ES, including the full range of motion. According to the testing, the stimulation parameters for treatment were 300 µs pulse duration, 50 Hz, and 20 mA. The index finger to thumb opposition reference electrode was placed on the hypothenar whereas the active electrode was located on the thenar. For finger flexion performed by FDP, the reference electrode was placed on the medial epicondyle in respect to the muscles innervated by the median nerve and the active electrode over the calculated motor point in the middle of the palmar forearm [27]. In accordance with the principles of motor learning the task to be learned was accompanied by a task-oriented exercise. The patient was asked to squeeze a foam ball, sized 6.5 cm in diameter, during ES exercise for FDP. The size and texture of the ball allowed a precise execution of the finger flexion without compensation with regard to hyperextension in the metacarpophalangeal joints. The latter would facilitate an undesirable claw hand. The spatial stimulus on the palmar side of the hand, which is given by the ball in combination with FES and the voluntary movement, ensures the initial flexion in the metacarpophalangeal joints, which is required for a balanced fist closure. By performing the index finger to thumb opposition, the patient was asked to press on a clothespin.
Stimulation protocol
The clothespin handle was widened, and non-slip coated so that it could be firmly held during ES exercise. The duty-cycle of the stimulation was individually adjusted to guarantee a precise movement without compensatory movements.
To avoid early fatigue, the ratio between stimulation time and pause was fixed at 1:2. The plateau phase with the maximum stimulation intensity has proven decisive for muscle fatigue [26].
The final ES stimulation duty-cycle setting was: 3 s ramp up, 6 s plateau, and 3 s ramp down with the current quality mentioned above, followed by a pause of 12 s. For stimulation, a two-channel neurostimulator (Microstim® Krauth&Timmermann, Hamburg Germany) with 3.2 cm round self-adhesive electrodes was used. The patient was instructed to exercise for each muscle function for 20 min twice a day, with at least 2 h rest between training sessions. One training session lasted for 60 min. The stimulation sessions for the FDP were carried out simultaneously for both hands. The opposition was stimulated on one hand after the other, for this movement is more complex and requires major attention. After instruction, the patient was able to perform the ES-supported exercises in the domestic setting.
Evaluations
Follow-ups were executed 12 and 16 weeks after onset of stimulation and after further 6 months without stimulation. To assess the stimulation outcome the distance of the tip of the middle finger to the palmar side of the hand was measured. The goal attainment scale (GAS) (Table 1) was used to assess the patient´s perceived changes in the predetermined goals.
Statistics
Descriptive statistics were used to summarize data for demographic, clinical, and operative characteristics. The distances are expressed in cm at baseline, 12 weeks, and 16 weeks for hand closure. The GAS assessed the result of the intervention based on the individual, patient-specific goal. The attainment of these goals was mapped and plotted on a pre-specified ordinal scale.
Statement of ethics
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Results
Distance between the tip of the middle finger and the palmar side of the hand decreased after 12 weeks of stimulation from 0.9 cm to light touch in the right hand and from 3.8 cm to 1.2 cm in the left hand. After 16 weeks the active voluntary hand closure was possible (Fig. 2a–d). The opposition of the thumb to index finger allowed the patient to button a shirt (Fig. 3) and manipulate coins, as well as inserting the credit card into the cash machine and the parking ticket in the payment terminal. The GAS was scored for button a shirt +2, manipulation of coin money, and handling cards +1 (Table 2). Voluntary function remained unchanged at 6 months follow-up after ES completion.
Discussion
The results of this case demonstrate that fine motor skills can improve following a 16-week intense treatment of FES combined with task-oriented functional training in an individual with chronic GBS with hyperreflexia. After finishing FES, the function persisted, controlled, and confirmed in the annual follow-ups performed by the responsible therapist.
The patient showed a slow but continuous recovery of motor function in the lower limbs over years but none in the upper limbs, mainly in the hands. He focused on improvement in hand function, hence his compliance and motivation were high to define functional goals and to perform the stimulation twice a day. The improvement of hand closure and pinch grip was small but critical for performing tasks in daily life. Even after several years of GBS, there was no denervation but disuse atrophies. The latter only occurs if the LMN is intact. Hence disuse atrophy can be treated by FES that excites the conduction of the action potentials via the nerve. The therapeutical application of FES is meant to be a short-term treatment modality. After training, the patient is expected to perform the addressed tasks voluntarily without FES.
FES tackled two specific aspects: (1) motor relearning including skill training to improve hand functions that were lost due to disuse and superimposed compensation movements during rehabilitation and (2) strengthening of the intrinsic and extrinsic hand, finger, and thenar muscles involved in hand closure and pinch grip. Motor learning includes many facets e.g., intrinsic motivation, repetitions, task specificity, and the recognition of previously learned and automatized movements [15, 17, 18]. The personal driving force is most important in the learning process. Success by performing a movement that has its origin in individual expectancies facilitates motor learning supported by dopamine release for the consolidation of the learned and the development of neural pathways [17]. In addition, an external focus directs the attention to the task goal [17]. The individually defined goal that yields as the outcome ensures the recognition effect and the task specificity according to the principles of motor learning.
Skill training, including fine motor movements, is defined as the acquisition of movements that are specified and new. It takes place in the motor cortex that is organized in neural connections controlling and coordinating movements across joints like that of hands and fingers. Learning by this training is initiated by changes in the connectivity of neural accumulations. The changes consist of alterations in the number of cortical synapses and the representation of movements evoked by stimulation [19]. The added FES onto the sensory afferents facilitate changes in motor control by activity-dependent plasticity of the motor cortex [20]. In the current case, the principles of motor learning were applied by individually expressed targets, determined by the goal attainment scale. The targets were fine motor movements that did not have to be learned anew but had to be relearned. Presumably, they disappeared from the motor cortex due to disuse and compensation to perform the desired task in a different manner [21]. Because of the self-perceived limitation of the quality of life, the incentive to achieve the desired hand function was very high, wherefore the required training dose, 60 min twice a day, was accepted.
In addition to the motor relearning process, the effect of the treatment on muscle weakness has to be taken into account. Multiple studies have proven the effect of FES on strengthening muscles in UMN and LMN lesions [22,23,24,25]. The effect depends on the type of the lesion (UMN/LMN), the extent (motor–complete/motor–incomplete), and the time after disease regarding muscle atrophy and consequently muscle fatigue [26]. The exercises with the clothespin and the foam ball fulfilled the criteria in muscle strengthening and task specificity.
However, the interpretation of the results should consider the single case character of the study. Still, it could be an impulse for further clinical research particularly in cases of diseases with a small incidence as this subgroup of GBS patients with preserved tendon reflexes [5]. It is still unknown whether the current results can be replicated and transferred to a larger population of GBS patients. Future studies should focus on potential changes in the motor cortex through FES-supported task-oriented training and what the weighting of therapy intensity should be.
In conclusion, task-oriented functional training supported by FES can lead to long-term improvements in the fine motor skills of the upper extremity even in chronic conditions after GBS with preserved deep tendon reflexes.
Data availability
The dataset generated and analyzed during the study is available from the corresponding author on reasonable request.
References
Hughes RA, Cornblath DR. Guillain-Barré syndrome. Lancet. 2005;366:1653–66.
Doorn PAV, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. Lancet Neurol. 2008;7:939–50.
Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016;388:717–27.
Sejvar JJ, Kohl KS, Gidudu J, Amato A, Bakshi N, Baxter R, et al. Guillain–Barré syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29:599–612.
Uncini A, Notturno F, Kuwabara S Hyper-reflexia in Guillain-Barré syndrome: systematic review. J Neurol Neurosurg Psychiatry. 2020;91:jnnp-2019-321890.
Esposito S, Longo MR. Guillain–Barré syndrome. Autoimmun Rev. 2017;16:96–101.
Vanhoutte EK, Faber CG, Merkies ISJ.group P study96th ENMC international workshop: outcome measures in inflammatory peripheral neuropathies 8–10 February 2013, Naarden, The Netherlands. Neuromuscul Disord. 2013;23:924–33.
Wageck B, Nunes GS, Silva FL, Damasceno MCP, Noronha MD. Application and effects of neuromuscular electrical stimulation in critically ill patients: Systematic review. Med Intensiv Engl Ed. 2014;38:444–54.
Segers J, Hermans G, Bruyninckx F, Meyfroidt G, Langer D, Gosselink R. Feasibility of neuromuscular electrical stimulation in critically ill patients. J Crit Care. 2014;29:1082–8.
Maffiuletti NA, Roig M, Karatzanos E, Nanas S. Neuromuscular electrical stimulation for preventing skeletal-muscle weakness and wasting in critically ill patients: a systematic review. BMC Med. 2013;11:137.
Harbo T, Markvardsen LK, Hellfritzsch MB, Severinsen K, Nielsen JF, Andersen H. Neuromuscular electrical stimulation in early rehabilitation of Guillain‐Barré syndrome: a pilot study. Muscle Nerve. 2019;59:481–4.
Connolly B, O’Neill B, Salisbury L, Blackwood B. Physical rehabilitation interventions for adult patients during critical illness: an overview of systematic reviews. Thorax 2016;71:881–90.
Sachetti A, Carpes MF, Dias AS, Sbruzzi G. Segurança no uso da eletroestimulação neuromuscular em pacientes graves: revisão sistemática. Rev Bras Ter Intensiv 2018;30:219–25.
Ramachandran VS, Rogers-Ramachandran D. Phantom limbs and neural plasticity. Arch Neurol. 2000;57:317–20.
Popovic MR, Kapadia N, Zivanovic V, Furlan JC, Craven BC, McGillivray C. Functional electrical stimulation therapy of voluntary grasping versus only conventional rehabilitation for patients with subacute incomplete tetraplegia: a randomized clinical trial. Neurorehabil Neural Repair. 2011;25:433–42.
Jones S, Man WD, Gao W, Higginson IJ, Wilcock A, Maddocks M. Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease. Cochrane Database Syst Rev. 2016;10:1–10.
Wulf G, Lewthwaite R. Optimizing performance through intrinsic motivation and attention for learning: The OPTIMAL theory of motor learning. Psychon B Rev. 2016;23:1382–414.
Kitago T, Krakauer JW. Motor learning principles for neurorehabilitation. Handb Clin Neurol. 2013;110:93–103.
Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol. 2006;101:1776–82.
Fu M, Yu X, Lu J, Zuo Y. Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature 2012;483:92–5.
Kleim JA. Neural plasticity and neurorehabilitation: teaching the new brain old tricks. J Commun Disord. 2011;44:521–8.
James DC, Solan MC, Mileva KN. Wide-pulse, high-frequency, low-intensity neuromuscular electrical stimulation has potential for targeted strengthening of an intrinsic foot muscle: a feasibility study. J Foot Ankle Res. 2018;11:16.
Hartkopp A, Harridge SDR, Mizuno M, Ratkevicius A, Quistorff B, Kjaer M, et al. Effect of training on contractile and metabolic properties of wrist extensors in spinal cord-injured individuals. Muscle Nerve. 2003;27:72–80.
Coupaud S, Gollee H, Hunt KJ, Fraser MH, Allan DB, McLean AN. Arm-cranking exercise assisted by Functional Electrical Stimulation in C6 tetraplegia: a pilot study. J Technol Assess Health Care. 2008;16:415–27.
Carraro U, Kern H, Gava P, Hofer C, Loefler S, Gargiulo P, et al. Recovery from muscle weakness by exercise and FES: lessons from Masters, active or sedentary seniors and SCI patients. Aging Clin Exp Res. 2017;29:579–90.
Gorgey AS, Black PPCD, Elder PC, Dudley GA. Effects of electrical stimulation parameters on fatigue in skeletal muscIe. J Orthop Sports Phys Ther. 2009;39:684–92.
Bersch I, Koch-Borner S, Frid‚n J. Motor point topography of fundamental grip actuators in tetraplegia-implications in nerve transfer surgery. J Neurotrauma.2019;25:441–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bersch, I., Fridén, J. Long-term effect of task-oriented functional electrical stimulation in chronic Guillain Barré syndrome–a single-subject study. Spinal Cord Ser Cases 7, 53 (2021). https://doi.org/10.1038/s41394-021-00419-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41394-021-00419-0