Abstract
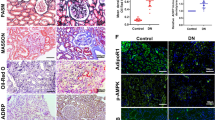
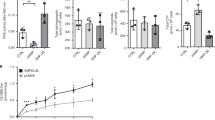
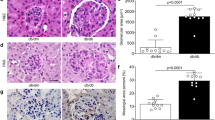
Podocyte lipotoxicity mediated by impaired cellular cholesterol efflux plays a crucial role in the development of diabetic kidney disease (DKD), and the identification of potential therapeutic targets that regulate podocyte cholesterol homeostasis has clinical significance. Coiled-coil domain containing 92 (CCDC92) is a novel molecule related to metabolic disorders and insulin resistance. However, whether the expression level of CCDC92 is changed in kidney parenchymal cells and the role of CCDC92 in podocytes remain unclear. In this study, we found that Ccdc92 was significantly induced in glomeruli from type 2 diabetic mice, especially in podocytes. Importantly, upregulation of Ccdc92 in glomeruli was positively correlated with an increased urine albumin-to-creatinine ratio (UACR) and podocyte loss. Functionally, podocyte-specific deletion of Ccdc92 attenuated proteinuria, glomerular expansion and podocyte injury in mice with DKD. We further demonstrated that Ccdc92 contributed to lipid accumulation by inhibiting cholesterol efflux, finally promoting podocyte injury. Mechanistically, Ccdc92 promoted the degradation of ABCA1 by regulating PA28α-mediated proteasome activity and then reduced cholesterol efflux. Thus, our studies indicate that Ccdc92 contributes to podocyte injury by regulating the PA28α/ABCA1/cholesterol efflux axis in DKD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The proteomics data presented in this study are openly available in ProteomeXchange with identifier PXD036050. Other data that support the findings of this study are available from the corresponding author upon reasonable request.
References
John S. Complication in diabetic nephropathy. Diabetes Metab Syndr. 2016;10:247–9.
Fu Y, Sun Y, Wang M, Hou Y, Huang W, Zhou D, et al. Elevation of JAML promotes diabetic kidney disease by modulating podocyte lipid metabolism. Cell Metab. 2020;32:1052–62.e8.
DeFronzo RA, Reeves WB, Awad AS. Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat Rev Nephrol. 2021;17:319–34.
Wahl P, Ducasa GM, Fornoni A. Systemic and renal lipids in kidney disease development and progression. Am J Physiol Ren Physiol. 2016;310:F433–45.
Brinkkoetter PT, Bork T, Salou S, Liang W, Mizi A, Ozel C, et al. Anaerobic glycolysis maintains the glomerular filtration barrier independent of mitochondrial metabolism and dynamics. Cell Rep. 2019;27:1551–66.e5.
Pedigo CE, Ducasa GM, Leclercq F, Sloan A, Mitrofanova A, Hashmi T, et al. Local TNF causes NFATc1-dependent cholesterol-mediated podocyte injury. J Clin Invest. 2016;126:3336–50.
Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res. 2014;55:561–72.
Ducasa GM, Mitrofanova A, Mallela SK, Liu X, Molina J, Sloan A, et al. ATP-binding cassette A1 deficiency causes cardiolipin-driven mitochondrial dysfunction in podocytes. J Clin Invest. 2019;129:3387–400.
Huang LO, Rauch A, Mazzaferro E, Preuss M, Carobbio S, Bayrak CS, et al. Genome-wide discovery of genetic loci that uncouple excess adiposity from its comorbidities. Nat Metab. 2021;3:228–43.
Vujkovic M, Keaton JM, Lynch JA, Miller DR, Zhou J, Tcheandjieu C, et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat Genet. 2020;52:680–91.
Zhao W, Rasheed A, Tikkanen E, Lee JJ, Butterworth AS, Howson JMM, et al. Identification of new susceptibility loci for type 2 diabetes and shared etiological pathways with coronary heart disease. Nat Genet. 2017;49:1450–7.
Klarin D, Zhu QM, Emdin CA, Chaffin M, Horner S, McMillan BJ, et al. Genetic analysis in UK Biobank links insulin resistance and transendothelial migration pathways to coronary artery disease. Nat Genet. 2017;49:1392–7.
Ren L, Du W, Song D, Lu H, Hamblin MH, Wang C, et al. Genetic ablation of diabetes-associated gene Ccdc92 reduces obesity and insulin resistance in mice. iScience. 2023;26:105769.
Wang Z, Jiang T, Li J, Proctor G, McManaman JL, Lucia S, et al. Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes. 2005;54:2328–35.
Fornoni A, Merscher S, Kopp JB. Lipid biology of the podocyte—new perspectives offer new opportunities. Nat Rev Nephrol. 2014;10:379–88.
Nijholt DA, de Graaf TR, van Haastert ES, Oliveira AO, Berkers CR, Zwart R, et al. Endoplasmic reticulum stress activates autophagy but not the proteasome in neuronal cells: implications for Alzheimer’s disease. Cell Death Differ. 2011;18:1071–81.
Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403.
Collins GA, Goldberg AL. The logic of the 26 S proteasome. Cell. 2017;169:792–806.
Lesne J, Locard-Paulet M, Parra J, Zivkovic D, Menneteau T, Bousquet MP, et al. Conformational maps of human 20 S proteasomes reveal PA28- and immuno-dependent inter-ring crosstalks. Nat Commun. 2020;11:6140.
Vartak T, Godson C, Brennan E. Therapeutic potential of pro-resolving mediators in diabetic kidney disease. Adv Drug Deliv Rev. 2021;178:113965.
Kim Y, Lim JH, Kim MY, Kim EN, Yoon HE, Shin SJ, et al. The adiponectin receptor agonist adiporon ameliorates diabetic nephropathy in a model of type 2 diabetes. J Am Soc Nephrol. 2018;29:1108–27.
Patel M, Wang XX, Magomedova L, John R, Rasheed A, Santamaria H, et al. Liver X receptors preserve renal glomerular integrity under normoglycaemia and in diabetes in mice. Diabetologia. 2014;57:435–46.
Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21:37–46.
Schelling JR. The contribution of lipotoxicity to diabetic kidney disease. Cells. 2022;11:3236.
Song Y, Liu J, Zhao K, Gao L, Zhao J. Cholesterol-induced toxicity: An integrated view of the role of cholesterol in multiple diseases. Cell Metab. 2021;33:1911–25.
Merscher-Gomez S, Guzman J, Pedigo CE, Lehto M, Aguillon-Prada R, Mendez A, et al. Cyclodextrin protects podocytes in diabetic kidney disease. Diabetes. 2013;62:3817–27.
Yang Q, Hu J, Yang Y, Chen Z, Feng J, Zhu Z, et al. Sirt6 deficiency aggravates angiotensin II-induced cholesterol accumulation and injury in podocytes. Theranostics. 2020;10:7465–79.
Wang N, Silver DL, Thiele C, Tall AR. ATP-binding cassette transporter A1 (ABCA1) functions as a cholesterol efflux regulatory protein. J Biol Chem. 2001;276:23742–7.
Oram JF, Lawn RM. ABCA1. The gatekeeper for eliminating excess tissue cholesterol. J Lipid Res. 2001;42:1173–9.
Pohl C, Dikic I. Cellular quality control by the ubiquitin-proteasome system and autophagy. Science. 2019;366:818–22.
Meyer-Schwesinger C. The ubiquitin-proteasome system in kidney physiology and disease. Nat Rev Nephrol. 2019;15:393–411.
Beeken M, Lindenmeyer MT, Blattner SM, Radon V, Oh J, Meyer TN, et al. Alterations in the ubiquitin proteasome system in persistent but not reversible proteinuric diseases. J Am Soc Nephrol. 2014;25:2511–25.
Goru SK, Kadakol A, Gaikwad AB. Hidden targets of ubiquitin proteasome system: to prevent diabetic nephropathy. Pharmacol Res. 2017;120:170–9.
Sharpe LJ, Cook EC, Zelcer N, Brown AJ. The UPS and downs of cholesterol homeostasis. Trends Biochem Sci. 2014;39:527–35.
Ogura M, Ayaori M, Terao Y, Hisada T, Iizuka M, Takiguchi S, et al. Proteasomal inhibition promotes ATP-binding cassette transporter A1 (ABCA1) and ABCG1 expression and cholesterol efflux from macrophages in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2011;31:1980–7.
Chaki M, Airik R, Ghosh AK, Giles RH, Chen R, Slaats GG, et al. Exome capture reveals ZNF423 and CEP164 mutations, linking renal ciliopathies to DNA damage response signaling. Cell. 2012;150:533–48.
Bernatik O, Pejskova P, Vyslouzil D, Hanakova K, Zdrahal Z, Cajanek L. Phosphorylation of multiple proteins involved in ciliogenesis by Tau Tubulin kinase 2. Mol Biol Cell. 2020;31:1032–46.
Yadranji Aghdam S, Mahmoudpour A. Proteasome activators, PA28alpha and PA28beta, govern development of microvascular injury in diabetic nephropathy and retinopathy. Int J Nephrol. 2016;2016:3846573.
de Haan W, Bhattacharjee A, Ruddle P, Kang MH, Hayden MR. ABCA1 in adipocytes regulates adipose tissue lipid content, glucose tolerance, and insulin sensitivity. J Lipid Res. 2014;55:516–23.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (T2321004, 91949202, 82090024, 81873614, 82090021, 81900621, 81970580, 82070753, 82170734, 81800645, 81800643, 22107058); Shandong Provincial Natural Science Foundation, China (ZR2019ZD40, ZR2019MH041, 2023HWYQ-020); The Taishan Scholars Program of Shandong Province, China (tsqn202306074) and Cutting Edge Development Fund of Advanced Medical Research Institute (GYY2023QY01).
Author information
Authors and Affiliations
Contributions
FWZ. conducted the in vivo and in vitro experiments, performed data analysis, and helped write the manuscript. ZYL, MWW, JYD, PZD and HRZ. contributed to the experimental design and performed the in vitro experiments. XJW performed the in vivo animal studies. YS and YZ helped design the experiments. JCW performed confocal microscopy. WT and YSX analyzed the data. FY, ZYW, and ML designed the experiments, interpreted the data, wrote the manuscript, and approved the final version of the manuscript for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zuo, Fw., Liu, Zy., Wang, Mw. et al. CCDC92 promotes podocyte injury by regulating PA28α/ABCA1/cholesterol efflux axis in type 2 diabetic mice. Acta Pharmacol Sin 45, 1019–1031 (2024). https://doi.org/10.1038/s41401-023-01213-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-023-01213-4