Abstract
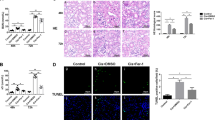
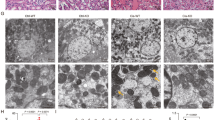
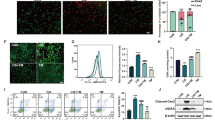
Acute kidney injury (AKI) is defined as sudden loss of renal function characterized by increased serum creatinine levels and reduced urinary output with a duration of 7 days. Ferroptosis, an iron-dependent regulated necrotic pathway, has been implicated in the progression of AKI, while ferrostatin-1 (Fer-1), a selective inhibitor of ferroptosis, inhibited renal damage, oxidative stress and tubular cell death in AKI mouse models. However, the clinical translation of Fer-1 is limited due to its lack of efficacy and metabolic instability. In this study we designed and synthesized four Fer-1 analogs (Cpd-A1, Cpd-B1, Cpd-B2, Cpd-B3) with superior plasma stability, and evaluated their therapeutic potential in the treatment of AKI. Compared with Fer-1, all the four analogs displayed a higher distribution in mouse renal tissue in a pharmacokinetic assay and a more effective ferroptosis inhibition in erastin-treated mouse tubular epithelial cells (mTECs) with Cpd-A1 (N-methyl-substituted-tetrazole-Fer-1 analog) being the most efficacious one. In hypoxia/reoxygenation (H/R)- or LPS-treated mTECs, treatment with Cpd-A1 (0.25 μM) effectively attenuated cell damage, reduced inflammatory responses, and inhibited ferroptosis. In ischemia/reperfusion (I/R)- or cecal ligation and puncture (CLP)-induced AKI mouse models, pre-injection of Cpd-A1 (1.25, 2.5, 5 mg·kg−1·d−1, i.p.) dose-dependently improved kidney function, mitigated renal tubular injury, and abrogated inflammation. We conclude that Cpd-A1 may serve as a promising therapeutic agent for the treatment of AKI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders HJ. Acute kidney injury. Nat Rev Dis Prim. 2021;7:51.
Xu Y, Ma H, Shao J, Wu J, Zhou L, Zhang Z, et al. A role for tubular necroptosis in cisplatin-induced AKI. J Am Soc Nephrol. 2015;26:2647–58.
Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol. 2017;13:697–711.
Peerapornratana S, Manrique-Caballero CL, Gómez H, Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96:1083–99.
See EJ, Jayasinghe K, Glassford N, Bailey M, Johnson DW, Polkinghorne KR, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019;95:160–72.
Grams ME, Sang Y, Coresh J, Ballew S, Matsushita K, Molnar MZ, et al. Acute kidney injury after major surgery: a retrospective analysis of veterans health administration data. Am J Kidney Dis. 2016;67:872–80.
Kaushal GP, Shah SV. Challenges and advances in the treatment of AKI. J Am Soc Nephrol. 2014;25:877–83.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72.
Wu ZH, Tang Y, Yu H, Li HD. The role of ferroptosis in breast cancer patients: a comprehensive analysis. Cell Death Discov. 2021;7:93.
Bao WD, Pang P, Zhou XT, Hu F, Xiong W, Chen K, et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021;28:1548–62.
Wu X, Li Y, Zhang S, Zhou X. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics. 2021;11:3052–9.
Chen C, Wang D, Yu Y, Zhao T, Min N, Wu Y, et al. Legumain promotes tubular ferroptosis by facilitating chaperone-mediated autophagy of GPX4 in AKI. Cell Death Dis. 2021;12:65.
Li P, Jiang M, Li K, Li H, Zhou Y, Xiao X, et al. Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity. Nat Immunol 2021;22:1107–17.
Ni L, Yuan C, Wu X. Targeting ferroptosis in acute kidney injury. Cell Death Dis. 2022;13:182.
Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–91.
Müller T, Dewitz C, Schmitz J, Schröder AS, Bräsen JH, Stockwell BR, et al. Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cell Mol Life Sci. 2017;74:3631–45.
Qiongyue Z, Xin Y, Meng P, Sulin M, Yanlin W, Xinyi L, et al. Post-treatment with irisin attenuates acute kidney injury in sepsis mice through anti-ferroptosis via the SIRT1/Nrf2 pathway. Front Pharmacol. 2022;13:857067.
Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, et al. Synchronized renal tubular cell death involves ferroptosis. Proc Nat Acad Sci USA. 2014;111:16836–41.
Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, et al. Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc. 2014;136:4551–6.
Wang JN, Wang F, Ke J, Li Z, Xu CH, Yang Q, et al. Inhibition of METTL3 attenuates renal injury and inflammation by alleviating TAB3 m6A modifications via IGF2BP2-dependent mechanisms. Sci Transl Med. 2022;14:eabk2709.
Hiramatsu M, Hotchkiss RS, Karl IE, Buchman TG. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock. 1997;7:247–53.
Xie SS, Dong ZH, He Y, Chen ZW, Yang Q, Ma WX, et al. Cpd-0225 attenuates renal fibrosis via inhibiting ALK5. Biochem Pharmacol. 2022;204:115240.
Wang JN, Liu MM, Wang F, Wei B, Yang Q, Cai YT, et al. RIPK1 inhibitor Cpd-71 attenuates renal dysfunction in cisplatin-treated mice via attenuating necroptosis, inflammation and oxidative stress. Clin Sci. 2019;133:1609–27.
Lu B, Chen XB, Hong YC, Zhu H, He QJ, Yang B, et al. Identification of PRDX6 as a regulator of ferroptosis. Acta Pharmacol Sin. 2019;40:1334–442.
Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–8.
Song X, Zhu S, Chen P, Hou W, Wen Q, Liu J, et al. AMPK-mediated BECN1phosphorylation promotes ferroptosis by directly blocking system Xc(-) activity. Curr Biol. 2018;28:2388–99.
Brookes MJ, Hughes S, Turner FE, Reynolds G, Sharma N, Ismail T, et al. Modulation of iron transport proteins in human colorectal carcinogenesis. Gut. 2006;55:1449–60.
Yang Q, Zang HM, Xing T, Zhang SF, Li C, Zhang Y, et al. Gypenoside XLIX protects against acute kidney injury by suppressing IGFBP7/IGF1R-mediated programmed cell death and inflammation. Phytomedicine. 2021;85:153541.
Meng XM, Li HD, Wu WF, Ming-Kuen Tang P, Ren GL, Gao L, et al. Wogonin protects against cisplatin-induced acute kidney injury by targeting RIPK1-mediated necroptosis. Lab Invest. 2018;98:79–94.
Schumer M, Colombel MC, Sawczuk IS, Gobé G, Connor J, O’Toole KM, et al. Buttyan R: Morphologic, bio chemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. Am J Pathol. 1992;40:831–8.
Xia W, Li Y, Wu M, Jin Q, Wang Q, Li S, et al. Gasdermin E deficiency attenuates acute kidney injury by inhibiting pyroptosis and inflammation. Cell Death Dis. 2021;12:139.
Wang Y, Quan F, Cao Q, Lin Y, Yue C, Bi R, et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J Adv Res. 2020;28:231–43.
Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–82.
Yang WS, Stockwell BR. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 2016;26:165–76.
Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 2017;13:81–90.
Park E, Chung SW. ROS-mediated autophagy increases intracellular iron levels and ferroptosis by ferritin and transferrin receptor regulation. Cell Death Dis. 2019;10:822.
Stockwell BR. Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell. 2022;185:2401–21.
Zhang DL, Ghosh MC, Rouault TA. The physiological functions of iron regulatory proteins in iron homeostasis—an update. Front Pharmacol 2014;5:124.
Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol 2007;69:69–85.
Mapuskar KA, Wen H, Holanda DG, Rastogi P, Steinbach E, Han R, et al. Persistent increase in mitochondrial superoxide mediates cisplatin-induced chronic kidney disease. Redox Biol. 2019;20:98–106.
Acknowledgements
We acknowledge the Center for Scientific Research of Anhui Medical University and the Inflammation and Immune Mediated Diseases Laboratory of Anhui Province for their valuable help. This work was supported by the National Natural Science Foundation of China (No. 81970584), the promotion plan of basic and clinical cooperative research in Anhui Medical University (No. 2019xkjT014), the Project of Collaborative Innovation for Colleges of Anhui Province (No. GXXT-2021-070), the Major Projects of Science and Technology in Anhui Province (202103a07020013), the Natural Science Foundation of Anhui (2208085QH240), and the Graduate Research and Practice Innovation Project of Anhui Medical University (YJS20230059).
Author information
Authors and Affiliations
Contributions
YC designed the study, performed the cell experiments, analyzed the data and wrote the manuscript. MFW performed the cell experiments, analyzed the data and wrote the manuscript. MMX and YL. performed the cell experiments analyzed the data. CL, SSX, and WXM performed part of the animal experiments and histological analysis. MLJ, RH, ZHD, RBH, MMZ, HL, and LG performed part of the cellular experiments and histological analysis. JGW, JJ, and XWD provided a series of experimental instructions and help. JXC and XMM provided a series of experimental instructions and wrote the manuscript. All authors have revised and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Wu, Mf., Xie, Mm. et al. Cpd-A1 alleviates acute kidney injury by inhibiting ferroptosis. Acta Pharmacol Sin (2024). https://doi.org/10.1038/s41401-024-01277-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-024-01277-w