Abstract
In Hong Kong, newly diagnosed multiple myeloma (NDMM) receives bortezomib-based triplet induction. Upfront autologous stem cell transplant (ASCT) is offered to transplant eligible (TE) patients (NDMM ≤ 65 years of age), unless medically unfit (TE-unfit) or refused (TE-refused). Data was retrieved for 448 patients to assess outcomes. For the entire cohort, multivariate analysis showed that male gender (p = 0.006), international staging system (ISS) 3 (p = 0.003), high lactate dehydrogenase (LDH) (p = 7.6 × 10−7) were adverse predictors for overall survival (OS), while complete response/ near complete response (CR/nCR) post-induction (p = 2.7 × 10−5) and ASCT (p = 4.8 × 10−4) were favorable factors for OS. In TE group, upfront ASCT was conducted in 252 (76.1%). Failure to undergo ASCT in TE patients rendered an inferior OS (TE-unfit p = 1.06 × 10−8, TE-refused p = 0.002) and event free survival (EFS) (TE-unfit p = 0.00013, TE-refused p = 0.002). Among TE patients with ASCT, multivariate analysis showed that age ≥ 60 (p = 8.9 × 10−4), ISS 3 (p = 0.019) and high LDH (p = 2.6 × 10−4) were adverse factors for OS. In those with high-risk features (HR cytogenetics, ISS 3, R-ISS 3), ASCT appeared to mitigate their adverse impact. Our data reaffirmed the importance of ASCT. The poor survival inherent with refusal of ASCT should be recognized by clinicians. Finally, improved outcome with ASCT in those with high-risk features warrant further studies.
Similar content being viewed by others
Background
High dose melphalan followed by autologous stem cell transplant (ASCT) has been the standard of care for eligible patients with newly diagnosed multiple myeloma (NDMM) for over two decades [1]. Even when novel agents are used in first line treatment, addition of upfront ASCT has consistently shown to improve progression free survival (PFS) [2,3,4,5] and overall survival (OS) [6, 7]. Nonetheless, MM treatment is still challenged by relapses, precluding long-term remissions and hence cure of disease. Factors such as lack of response, International Staging System (ISS) stage, and high-risk cytogenetics have been linked to poor outcome in patients undergoing ASCT [8,9,10,11,12,13,14,15,16,17]. Other additional factors such as age, comorbidities, and cognitive/physical conditions have also been described to affect survival in ASCT patients [18,19,20,21].
In Hong Kong, bortezomib based triplet induction therapy (VTD/VCD) is given to patients with NDMM and upfront ASCT is generally offered to all consenting myeloma patient aged ≤65 years. Patients >65 years are considered transplant-ineligible (TIE). Upfront ASCT is offered to all TE patients ≤65 years, unless patients are medically unfit (TE-unfit) or refused (TE-refused). Utilizing our database on patients receiving bortezomib-based induction therapy and information on ASCT status, risk factors for survival were analyzed for the entire cohort of NDMM patients comprising TE and TIE patients in addition to those who had undergone ASCT. Moreover, the impact of TE-unfit and TE-refused ASCT on clinical outcomes in a real-world context was studied. Furthermore, in patients with complete cytogenetic data, the impact of ASCT to overcome high risk features including high risk fluorescence in situ hybridization test (HR FISH), ISS 3 and Revised International Staging System (R-ISS) 3 were analyzed.
Methods
Patients and data collection
Symptomatic NDMM patients treated in seven hematology centers, including Queen Mary Hospital, Princess Margaret Hospital, Tuen Mun Hospital, Pamela Youde Nethersole Eastern Hospital, United Christian Hospital, Tseung Kwan O Hospital and Queen Elizabeth Hospital, from January 2006 to January 2020 in Hong Kong were included in this retrospective study. Demographics and disease characteristics at the time of diagnosis, as well as treatment and transplant information were retrieved from the electronic medical records with approval from the Institutional Review Board of the University of Hong Kong.
Patient treatment
All patients received induction treatment containing bortezomib. The combination of choice with bortezomib was mainly determined by the drug availability at the time of diagnosis, patient tolerability and affordability.
Regarding patient selection in the TE and TIE groups, TE patients were recruited into the database consecutively over time as reimbursement for upfront bortezomib in TE patients is provided by our medical care. On the other hand, reimbursement for upfront bortezomib in TIE patients was not made available till 2019, and thus most TIE patients that could afford self-financed bortezomib upfront were included in this database. Patients deemed TE by their respective hematology centers received high dose melphalan 200 mg/m2 for their ASCT. None of the patients received reduced dose melphalan for conditioning.
Definitions and clinical outcome variables
Clinical staging was based on the ISS and R-ISS. Complete remission (CR) was defined as complete resolution of disease with absent paraprotein, as evidenced by a negative serum protein electrophoresis (SPE) and immunofixation, and <5% plasma cells in the bone marrow. Near-complete remission (nCR) was defined as a negative SPE but positive immunofixation. An event included reappearance of the paraprotein on immunofixation in CR patients, recurrent paraproteinemia in the nCR patients, ≥25% paraprotein increase and/or appearance of new bone lesions. For patients with light chain myeloma, CR was defined as normalization of the level and ratio of serum free light chain, and negative serum and urine immunofixation. Herein, CR and nCR were grouped together as CR/nCR as immunofixation and bone marrow exam were not performed on all patients after reaching negative SPE.
Fluorescence in situ hybridization (FISH)
Detection of cytogenetic aberrations was performed on myeloma cells in the bone marrow sample by FISH. Enrichment for myeloma cells was achieved by sorting with CD138 immunomagnetic beads (MiniMACS, Miltenyi Biotec, Auburn, CA). The FISH probes comprised of the IGH/FGFR3 dual color dual fusion translocation probe for detection of t(4;14) (p16;q32), the IGH/MAF dual color dual fusion translocation probe for detection of t(14;16)(q32;q23) and the TP53/CEP17 deletion color probe for the detection of p53 deletion. At least 200 nuclei were analyzed. The cut-off for positivity was above 10% for fusion or break apart probes and 20% for numerical abnormalities in accordance with 2012 European Myeloma Network interphase FISH consensus [22]. HR FISH was defined according to International Myeloma Working Group (IMWG)-defined HR FISH alterations [7], including t(4;14), t(14;16) or del(17p) [23]. As complete FISH data was only available in 277 patients, the prognostic impact of HR FISH and R-ISS would be analyzed in univariate but not multivariate analysis.
Statistical analysis
Statistical analysis was conducted using IBM SPSS Statistics Version 26. Overall survival (OS) was measured from the date of treatment to the date of death or last follow-up. Event-free survival (EFS) was calculated from the date of treatment to the date of progression, relapse, death or last follow-up. The survival curves for OS and EFS were plotted using Kaplan-Meier method and compared by log-rank test. Multivariate Cox regression was performed to analyze the impact of risk factors on OS including diagnostic clinical parameters including gender, age, ISS stage, lactate dehydrogenase (LDH), immunoglobulin isotype, treatment response (post-induction CR/nCR) and ASCT for entire cohort, while ASCT was removed for transplanted cohort. Data on high-risk FISH was only available in 277 patients (61.8%), hence included in univariate but not multivariate analysis. A p-value of <0.05 was considered statistically significant and all p-values were two-sided.
Results
The entire NDMM cohort comprised 448 patients, 331 TE and 117 TIE patients. In our entire cohort, the majority of patients received bortezomib/ thalidomide/ dexamethasone (VTd) treatment (n = 348, 77.7%). Others received bortezomib/ doxorubicin/ dexamethasone (PAd) (n = 30, 6.7%), bortezomib/ cyclophosphamide/ dexamethasone (VCd) (n = 26, 5.8%), bortezomib/ dexamethasone (Vd) (n = 23, 5.1%), bortezomib/ melphalan/ prednisolone (VMP) (n = 12, 2.7%) and bortezomib/ lenalidomide/ dexamethasone (VRd) (n = 9, 2%). There were no differences in the demographics and proportion of high-risk MM (HR MM) (ISS 3, high LDH, IgG isotype, HR FISH and R-ISS 3) between TE and TIE MM patients (Table 1).
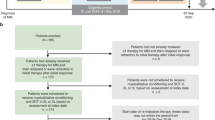
The median age was 59.5 years old. One-hundred and forty-four (32.5%) patients achieved CR/nCR after induction and 189 (42.7%) patients achieved VGPR. Among TE patients, 260 (78.5%) underwent ASCT. Of those that received ASCT, 252 ASCT were performed after upfront bortezomib based induction and eight ASCT as consolidation after salvage therapy (Fig. 1). Among the 277 patients with complete FISH data, 74 (26.7%) patients had at least one HR FISH.
Response after Induction and ASCT in transplanted MM
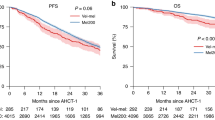
Among ASCT group, 85 (33.7%) patients attained CR/nCR after induction treatment and 171 (67.9%) patients achieved CR/nCR post ASCT. Two hundred and five (81.3%) patients attained VGPR or better after induction treatment and 238 (94.4%) patients achieved VGPR or better post ASCT (Fig. 1). After ASCT, the OS and EFS were superior in those that reached ≥CR/nCR (OS p = 0.035, EFS p = 0.019) or ≥VGPR (OS p = 0.000049, EFS p = 0.003) compared with those that failed to reach these end points after ASCT (Figs. 2 and 3).
Predictors of EFS and OS in the entire cohort
The median OS was 113 months and median EFS 40 months for the entire cohort. Adverse risk factors for both EFS and OS include male gender (EFS p = 0.017, OS p = 0.003), ISS 3 (EFS p = 0.003, OS p = 2.2 × 10−8), R-ISS stage 3 (EFS p = 0.000001, OS p = 7.8 × 10−13), high LDH (EFS p = 0.000149, OS p = 2.8 × 10−9) (Table 2). Multivariate analysis (excluding HR FISH as data was unavailable in 171 patients within the entire cohort) showed that male gender (p = 0.006), ISS 3 (p = 0.003) and high LDH (p = 7.6 × 10−7) were negative predictors for OS. Achievement of post induction CR/nCR was a predictor of improved EFS and OS in univariate (EFS p = 7.3 × 10−7, OS p = 0.001) and multivariate analysis (OS p = 2.7 × 10−5). Patients who underwent ASCT had superior EFS (p = 0.000007) and OS (p = 7 × 10−14) in univariate and multivariate analysis (OS p = 4.8 × 10−4) (Tables 2 and 3) compared to those that did not receive ASCT (i.e. TIE, TE-refused and TE-unfit). Among the 277 patients with complete FISH data, the presence of HR FISH negatively impacted OS (p = 0.001) and EFS (p = 0.015) (Table 2).
Predictors of EFS and OS in the ASCT cohort
A total of 260 patients received ASCT after bortezomib based induction with median OS of 153 months and median EFS 53 months. There were significant worsening of OS and EFS among those with ISS 3 (EFS p = 0.015, OS p = 0.000293), R-ISS stage 3 (EFS p = 0.000014, OS p = 9.5 × 10−9), age ≥60 (EFS p = 0.073, OS p = 0.002) and high LDH (EFS p = 0.027, OS p = 0.000025) (Table 4). Multivariate analysis (excluding HR FISH as there was incomplete data in 94 patients) showed age ≥60 (p = 8.9 × 10−4), ISS 3 (p = 0.019) and high LDH (p = 2.6 × 10−4) were adverse factors for OS (Table 5). Post induction CR/nCR correlated with superior EFS (EFS p = 0.006) but not OS (p = 0.154) in ASCT patients (Table 4). Among the 166 patients with complete FISH data post ASCT, the presence of HR FISH negatively impacted on OS (p = 0.023) but not EFS (p = 0.207) (Table 4).
We further investigated the baseline characteristic and treatment responses of ASCT patients <60 and ≥60 years old, to explain for the survival difference within a narrow age spectrum. No differences were observed for gender, ISS, R-ISS, LDH, presence of IMWG-defined HR FISH, post induction CR/nCR, post induction VGPR, post ASCT CR/nCR and post ASCT VGPR between the two age groups (Table 6).
Survival analysis in the TE group
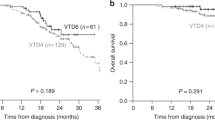
Among the TE patients, ASCT was not performed in 63, due to being medically unfit (TE-unfit) (N = 41; 12.4%) or patient refusal (TE-refused) (N = 22; 6.6%). Major reasons patients were deemed unfit included renal impairment (n = 15, 36.6%), early disease progression (n = 12, 29.2%) and cardiac disease (n = 7, 17.1%) (Fig. 1). Compared with those transplanted MM, failure to undergo ASCT rendered a much inferior OS in TE-unfit (p = 1.06 × 10−8) and TE-refused (p = 0.002) and EFS (TE-unfit p = 0.00013, TE refused p = 0.002), with poor EFS and OS comparable to that of TIE patients (OS p = 0.576, EFS p = 0.614) (Fig. 4).
Impact of ASCT in high-risk MM with IMWG-defined HR FISH, ISS3 or R-ISS
Among the entire cohort, 204 patients were ISS 3, of which 96 received ASCT. Compared to the ISS 1/2 patients with ASCT, the OS (p = 0.000293) and EFS (p = 0.015) were significantly inferior for those with ISS 3 and ASCT (Fig. 5). HR FISH abnormalities were found in 74 patients throughout the entire cohort, of which 44 received ASCT. Compared with standard risk FISH patients who underwent ASCT, the OS (p = 0.023) was still significantly worse in those with HR FISH despite receiving ASCT, though the EFS (p = 0.207) was comparable (Fig. 6). One hundred and seven patients from the entire cohort were R-ISS 3, of which 45 had ASCT done. Again, compared with R-ISS 1/2 patients who had ASCT, the OS (p = 9.5 × 10−9) and EFS (p = 0.000014) were significantly worse for those with R-ISS 3 even after ASCT (Fig. 7). The EFS and OS were the worst among those with either ISS stage 3, HR FISH and R-ISS stage 3 that did not receive ASCT (Figs. 5–7).
Discussion
Our data reaffirms the real-world survival benefit of ASCT as well as the adverse impact of ISS 3, elevated LDH, HR FISH, transplant ineligibility and failure of achieving deep responses such as CR/nCR. These findings are consistent with previous publications on predictors of survival in NDMM patients [4, 5, 8,9,10,11,12,13,14,15, 24]. The EMN02/ HO95 trial using an induction regimen of VMP similar to ours showed significant improvement in PFS and OS among those transplanted [2]. Importantly, ASCT can deepen post induction response. There was a substantial increase in patients reaching CR/nCR and VGPR after ASCT that translated to significant improvement in EFS and OS among those reaching CR/nCR or VGPR post ASCT in our cohort. Therefore, a deep response is pivotal to superior survival. In this connection, minimal residual disease (MRD) may pose as a more important end-point of MM treatment [25, 26]. Indeed, MRD-negativity has been shown to render superior survivals [27,28,29]. Addition of a CD38 antibody has been shown to yield a higher rate of MRD negativity CR that translated into superior PFS [30,31,32]. Indeed, triple negative CR including immunofixation, MRD and acetate positron emission tomography negative CR maybe the next goal and definition of CR in MM treatment [33]. Moreover, to enhance the rate of deep response in non-transplant candidates, incorporation of CD38 antibody has been shown to render a higher rate of MRD negative CR that translated into superior PFS and OS than the control arm [34]. In a resource restricted setting, three weekly daratumumab is a cost-effective yet efficacious option compared to the weekly and biweekly loading regimen [35]. Despite age difference between TE and TIE patients, our data confirmed similar frequency of HR factors in both groups, showing that these factors in myeloma patients are independent of transplant eligibility. Irrefutably, comorbidities and frailty also play an important role in the response and overall prognosis of TIE patients. Myeloma specific comorbidity index and frailty score can be used more widely to predict mortality and treatment toxicities in older myeloma patients in clinical practice [19, 36].
Secondly, ASCT appears to mitigate but not abolish the adverse impact of HR FISH, with improvements with EFS but the OS remained inferior to SR MM undergoing ASCT. The mitigation of adverse prognosis in HR FISH patients is dependent not only on whether ASCT was performed but also the cumulative number of unfavorable risk factors present and the induction regimen given. Our induction regimen consisted mainly of VTd/ VCd before daratumumab was available, which in this day and age, is clearly not potent enough for high-risk patients. The FORTE trial comparing carfilzomib lenalidomide dexamethasone (KRd) plus ASCT versus carfilzomib cyclophosphamide dexamethasone (KCd) plus ASCT versus KRd alone showed a favorable impact of KRd plus ASCT in patients with one HR FISH compared with the other two arms. In double hit myeloma, the PFS and OS were significantly worse compared with SR and one HR FISH groups regardless of the treatment arms [4]. Potent induction regimen with Dara KRd plus ASCT in the single arm phase 2 MASTER trial also showed improvements in sustained MRD negativity and progression free survival in patients with one HR FISH but not for those with two or more HR FISH [37]. ASCT seems to overcome the adverse prognosis of one HR FISH when paired with a potent induction regimen including an anti-CD38 antibody, second generation proteosome inhibitor and second generation immunomodulator. Dedicated trials for high-risk patients, in particular ultra-high-risk patients and plasma cell leukemia patients, are eagerly awaited [38, 39]. Therefore, the role of ASCT in HR MM warrants further study in prospective trials.
Thirdly, our results highlight the poor survival in those TE-unfit or refusing ASCT. Our study is unique in showcasing the poor survival outcomes of TE-refused patients that has not been reported before. Since upfront ASCT is a free service offered to all NDMM among TE patients in Hong Kong, ASCT refusal is most likely due to personal reasons or preference. Indeed, many socioeconomical factors closely shape and influence treatment choices of patients [18,19,20,21]. When patients refuse upfront ASCT, there should be an in-depth discussion of alternative treatment strategies and options. First, use of potent quadruplet induction regimens to increase the likelihood of achieving CR and MRD negativity in first remission should be explored [30, 31]. The results of the FORTE and the GMMG HD7 trials have shown that there may be a role of carfilzomib with the addition of an anti CD38 antibody for induction to enhance the depth of response even in non-transplant candidates [4, 40]. Second, the option of delayed ASCT can be discussed and if possible, early collection and storage of stem cells for future use [41]. Though upfront ASCT is associated with improved PFS compared to delayed ASCT, delayed ASCT does not compromise OS [42, 43] and should remain an option when upfront ASCT for personal or logistic reasons is not feasible [44]. Nonetheless, treating physicians should be aware that up to one-third of patients may be unable to receive ASCT at relapse due to development of new comorbidities, decline in performance status or rapid progression of disease at relapse [45].
Interestingly, our results showed that among ASCT recipients, OS was inferior in those ≥60 years than those <60. The cut-off at 60 years old was based on a previous retrospective analysis looking at whether age could affect outcomes of transplanted myeloma patients (≤66 years). In that study, there was a higher risk of death in those ≥60 years old, mainly due to the higher percentage of ISS 2/3 stages [46]. By contrast, IMWG-defined HR FISH was not over-represented in our patients ≥60 years of age [46,47,48,49].
For TE-unfit (renal) patients, the median creatinine was 368 umol/L, ranging from 125 to 1046 umol/L. Though there is increasing evidence ASCT can be safety performed in patients with severe renal impairment leading to similar benefits of PFS and OS as in patients with adequate renal function [50,51,52,53], the majority of our patients within this cohort received treatment before such practice was widely adopted with support of literature. The advancements in induction therapy with novel agents, use of melphalan 140 mg/m2 and improvements in peri-transplant supportive measures have undoubtedly played a major role in maximizing the effectiveness while minimizing the toxicities of ASCT for patients with renal impairment [54]. From a culture perspective, Chinese patients have a heavy stigma on renal dialysis and would avoid deterioration of renal function for fear of dialysis at all cost. Furthermore, it would be helpful to gather collective real-world data to evaluate whether the addition of an anti CD38 antibody on induction can salvage a higher percentage of renal impairment before ASCT is performed.
This study is limited by its retrospective design. As the decision for ASCT is based on the discretion of individual hematology centers, we do not have details of the exact medical comorbidities that precluded ASCT [55, 56]. As immunofixation was not performed routinely in all centers or SPE negative samples, distinction of CR and nCR was not possible in all cases, hence CR and nCR were grouped together for analysis. Indeed, deep responses of stringent CR conferred prognostic implications in both post induction and post ASCT settings [57].
Apart from reaffirming the role of ASCT, pretransplant risk factors and post-transplant responses, our study is the first to show the poor outcome of those refusing ASCT among the TE. The adverse impact of age ≥60-66 years among the transplanted was unaccounted for in our analysis. Finally, the impact of ASCT on HR MM warrants further randomized controlled studies.
Data availability
The datasets analyzed for this study are available from the corresponding author upon reasonable request.
References
Costa LJ, Zhang MJ, Zhong X, Dispenzieri A, Lonial S, Krishnan A, et al. Trends in utilization and outcomes of autologous transplantation as early therapy for multiple myeloma. Biol Blood Marrow Transpl. 2013;19:1615–24.
Cavo M, Gay F, Beksac M, Pantani L, Petrucci MT, Dimopoulos MA, et al. Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study. Lancet Haematol. 2020;7:e456–e68.
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N Engl J Med. 2017;376:1311–20.
Gay F, Musto P, Rota-Scalabrini D, Bertamini L, Belotti A, Galli M, et al. Carfilzomib with cyclophosphamide and dexamethasone or lenalidomide and dexamethasone plus autologous transplantation or carfilzomib plus lenalidomide and dexamethasone, followed by maintenance with carfilzomib plus lenalidomide or lenalidomide alone for patients with newly diagnosed multiple myeloma (FORTE): a randomised, open-label, phase 2 trial. Lancet Oncol. 2021;22:1705–20.
Richardson PG, Jacobus SJ, Weller EA, Hassoun H, Lonial S, Raje NS, et al. Triplet Therapy, Transplantation, and Maintenance until Progression in Myeloma. N Engl J Med. 2022;387:132–47.
Gay F, Oliva S, Petrucci MT, Conticello C, Catalano L, Corradini P, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015;16:1617–29.
Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371:895–905.
Chakraborty R, Muchtar E, Kumar S, Buadi FK, Dingli D, Dispenzieri A, et al. The impact of induction regimen on transplant outcome in newly diagnosed multiple myeloma in the era of novel agents. Bone Marrow Transpl. 2017;52:34–40.
Chanan-Khan AA, Giralt S. Importance of achieving a complete response in multiple myeloma, and the impact of novel agents. J Clin Oncol. 2010;28:2612–24.
Neben K, Lokhorst HM, Jauch A, Bertsch U, Hielscher T, van der Holt B, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119:940–8.
O’Shea D, Giles C, Terpos E, Perz J, Politou M, Sana V, et al. Predictive factors for survival in myeloma patients who undergo autologous stem cell transplantation: a single-centre experience in 211 patients. Bone Marrow Transpl. 2006;37:731–7.
Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol. 2015;33:2863–9.
Pourmoussa AM, Spielberger R, Cai J, Khoshbin O, Farol L, Cao T, et al. Predictive Factors for Early Relapse in Multiple Myeloma after Autologous Hematopoietic Stem Cell Transplant. Perm J. 2019;23:19.012.
Scott EC, Hari P, Sharma M, Le-Rademacher J, Huang J, Vogl D, et al. Post-Transplant Outcomes in High-Risk Compared with Non-High-Risk Multiple Myeloma: A CIBMTR Analysis. Biol Blood Marrow Transpl. 2016;22:1893–9.
van de Velde HJ, Liu X, Chen G, Cakana A, Deraedt W, Bayssas M. Complete response correlates with long-term survival and progression-free survival in high-dose therapy in multiple myeloma. Haematologica. 2007;92:1399–406.
Alvares CL, Davies FE, Horton C, Patel G, Powles R, Sirohi B, et al. Long-term outcomes of previously untreated myeloma patients: responses to induction chemotherapy and high-dose melphalan incorporated within a risk stratification model can help to direct the use of novel treatments. Br J Haematol. 2005;129:607–14.
Chakraborty R, Muchtar E, Kumar SK, Jevremovic D, Buadi FK, Dingli D, et al. Impact of Post-Transplant Response and Minimal Residual Disease on Survival in Myeloma with High-Risk Cytogenetics. Biol Blood Marrow Transpl. 2017;23:598–605.
Chamoun K, Firoozmand A, Caimi P, Fu P, Cao S, Otegbeye F, et al. Socioeconomic Factors and Survival of Multiple Myeloma Patients. Cancers. 2021;13:590.
Palumbo A, Bringhen S, Mateos MV, Larocca A, Facon T, Kumar SK, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125:2068–74.
Saad A, Mahindra A, Zhang MJ, Zhong X, Costa LJ, Dispenzieri A, et al. Hematopoietic cell transplant comorbidity index is predictive of survival after autologous hematopoietic cell transplantation in multiple myeloma. Biol Blood Marrow Transpl. 2014;20:402–8.e1.
Xu L, Wang X, Pan X, Wang X, Wang Q, Wu B, et al. Education level as a predictor of survival in patients with multiple myeloma. BMC Cancer. 2020;20:737.
Ross FM, Avet-Loiseau H, Ameye G, Gutiérrez NC, Liebisch P, O’Connor S, et al. Report from the European Myeloma Network on interphase FISH in multiple myeloma and related disorders. Haematologica. 2012;97:1272–7.
Fonseca R, Bergsagel PL, Drach J, Shaughnessy J, Gutierrez N, Stewart AK, et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia. 2009;23:2210–21.
Chim CS, Lie AKW, Chan EYT, Liu HSY, Lau CW, Yip SF, et al. Treatment outcome and prognostic factor analysis in transplant-eligible Chinese myeloma patients receiving bortezomib-based induction regimens including the staged approach, PAD or VTD. J Hematol Oncol. 2012;5:28.
Yao Q, Bai Y, Orfao A, Chim CS. Standardized Minimal Residual Disease Detection by Next-Generation Sequencing in Multiple Myeloma. Front Oncol. 2019;9:449.
Yao Q, Bai Y, Orfao A, Kumar S, Chim CS. Upgraded Standardized Minimal Residual Disease Detection by Next-Generation Sequencing in Multiple Myeloma. J Mol Diagn. 2020;22:679–84.
Munshi NC, Avet-Loiseau H, Rawstron AC, Owen RG, Child JA, Thakurta A, et al. Association of Minimal Residual Disease With Superior Survival Outcomes in Patients With Multiple Myeloma: A Meta-analysis. JAMA Oncol. 2017;3:28–35.
Munshi NC, Avet-Loiseau H, Anderson KC, Neri P, Paiva B, Samur M, et al. A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma. Blood Adv. 2020;4:5988–99.
Perrot A, Lauwers-Cances V, Corre J, Robillard N, Hulin C, Chretien ML, et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood. 2018;132:2456–64.
Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394:29–38.
Voorhees PM, Kaufman JL, Laubach J, Sborov DW, Reeves B, Rodriguez C, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136:936–45.
Facon T, Kumar SK, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:1582–96.
Ngai C, Kumar S, Chi-lai Ho G, Chen S, Chim C-s. Case series: MRD negativity assessment using 11C-Acetate PET with 3-weekly daratumumab-based quadruplet induction in newly diagnosed multiple myeloma. Therapeutic Adv Hematol. 2021;12:20406207211030369.
Rodriguez C, Kaufman JL, Laubach J, Sborov DW, Reeves B, Chari A, et al. Daratumumab (DARA) + lenalidomide, bortezomib, and dexamethasone (RVd) in transplant-eligible newly diagnosed multiple myeloma (NDMM): A post hoc analysis of sustained minimal residual disease (MRD) negativity from GRIFFIN. J Clin Oncol. 2022;40:8011.
Chim CS, Kumar S, Wong VKC, Ngai C, Kwong YL. 3-weekly daratumumab-lenalidomide/pomalidomide-dexamethasone is highly effective in relapsed and refractory multiple myeloma. Hematology. 2021;26:652–5.
Monika E, Anne-Saskia D, Sandra Maria D, Gabriele I, Heike R, Alexander Z, et al. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica. 2017;102:910–21.
Costa LJ, Chhabra S, Medvedova E, Dholaria BR, Schmidt TM, Godby KN, et al. Daratumumab, Carfilzomib, Lenalidomide, and Dexamethasone With Minimal Residual Disease Response-Adapted Therapy in Newly Diagnosed Multiple Myeloma. J Clin Oncol. 2022;40:2901–12.
Kaiser MF, Hall A, Walker K, Sherborne A, Tute RMD, Newnham N, et al. Daratumumab, Cyclophosphamide, Bortezomib, Lenalidomide, and Dexamethasone as Induction and Extended Consolidation Improves Outcome in Ultra-High-Risk Multiple Myeloma. J Clin Oncol. 2023;41:3945–55.
Leypoldt LB, Tichy D, Besemer B, Hänel M, Raab MS, Mann C, et al. Isatuximab, Carfilzomib, Lenalidomide, and Dexamethasone for the Treatment of High-Risk Newly Diagnosed Multiple Myeloma. J Clin Oncol. 2024;42:26–37.
Goldschmidt H, Mai EK, Bertsch U, Fenk R, Nievergall E, Tichy D, et al. Addition of isatuximab to lenalidomide, bortezomib, and dexamethasone as induction therapy for newly diagnosed, transplantation-eligible patients with multiple myeloma (GMMG-HD7): part 1 of an open-label, multicentre, randomised, active-controlled, phase 3 trial. Lancet Haematol. 2022;9:e810–e21.
Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Gastineau DA, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035–42.
Kumar SK, Lacy MQ, Dispenzieri A, Buadi FK, Hayman SR, Dingli D, et al. Early versus delayed autologous transplantation after immunomodulatory agents-based induction therapy in patients with newly diagnosed multiple myeloma. Cancer. 2012;118:1585–92.
Dunavin NC, Wei L, Elder P, Phillips GS, Benson DM Jr, Hofmeister CC, et al. Early versus delayed autologous stem cell transplant in patients receiving novel therapies for multiple myeloma. Leuk Lymphoma. 2013;54:1658–64.
Soekojo CY, Kumar SK. Stem-cell transplantation in multiple myeloma: how far have we come? Ther Adv Hematol. 2019;10:2040620719888111.
Kansagra A, Gonsalves WI, Gertz MA, Buadi FK, Dingli D, Dispenzieri A, et al. Analysis of Clinical Factors and Outcomes Associated with Nonuse of Collected Peripheral Blood Stem Cells for Autologous Stem Cell Transplants in Transplant-Eligible Patients with Multiple Myeloma. Biol Blood Marrow Transpl. 2018;24:2127–32.
Chretien ML, Hebraud B, Cances-Lauwers V, Hulin C, Marit G, Leleu X, et al. Age is a prognostic factor even among patients with multiple myeloma younger than 66 years treated with high-dose melphalan: the IFM experience on 2316 patients. Haematologica. 2014;99:1236–8.
Cordas Dos Santos DM, Saliba RM, Patel R, Bashir Q, Saini N, Hosing C, et al. Age Is a Prognostic Factor for the Overall Survival of Patients with Multiple Myeloma Undergoing Upfront Autologous Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transpl. 2020;26:1077–83.
Du C, Wang Y, Mao X-H, Yan Y, Liu J, Fan H, et al. The Relative Risk of Prognostic Factors of Age Stratified Multiple Myeloma. Blood. 2020;136:26–7.
Avet-Loiseau H, Hulin C, Campion L, Rodon P, Marit G, Attal M, et al. Chromosomal abnormalities are major prognostic factors in elderly patients with multiple myeloma: the intergroupe francophone du myélome experience. J Clin Oncol. 2013;31:2806–9.
Zhong H, Xie X, Xu G. Autologous Stem Cell Transplantation in Multiple Myeloma with Renal Failure: Friend or Foe? Stem Cells Int. 2019;2019:9401717.
Lazana I, Floro L, Christmas T, Shah S, Bramham K, Cuthill K, et al. Autologous stem cell transplantation for multiple myeloma patients with chronic kidney disease: a safe and effective option. Bone Marrow Transpl. 2022;57:959–65.
Garderet L, Ouldjeriouat H, Bekadja MA, Daguenet E, Bigot N, Vincent L, et al. Low non-relapse mortality and good haematological and renal responses after autologous haematopoietic stem cell transplantation in multiple myeloma patients with renal insufficiency at transplant: A prospective Société Francophone de Greffe de Moelle-Thérapie Cellulaire observational study. Br J Haematol. 2023.
Wang S, Tan M, Halim NAA, Soekojo C, Chen Y, Chng WJ, et al. P-186: Toxicity and survival outcomes of autologous stem cell transplant in Multiple Myeloma patients with renal impairment. Clin Lymphoma Myeloma Leuk. 2021;21:S139.
Li AY, Atenafu EG, Bernard RS, Masih-Khan E, Reece D, Franke N, et al. Toxicity and survival outcomes of autologous stem cell transplant in multiple myeloma patients with renal insufficiency: an institutional comparison between two eras. Bone Marrow Transpl. 2020;55:578–85.
Bodge MN, Reddy S, Thompson MS, Savani BN. Preparative regimen dosing for hematopoietic stem cell transplantation in patients with chronic kidney disease: analysis of the literature and recommendations. Biol Blood Marrow Transpl. 2014;20:908–19.
Badros A, Barlogie B, Siegel E, Roberts J, Langmaid C, Zangari M, et al. Results of autologous stem cell transplant in multiple myeloma patients with renal failure. Br J Haematol. 2001;114:822–9.
Kapoor P, Kumar SK, Dispenzieri A, Lacy MQ, Buadi F, Dingli D, et al. Importance of achieving stringent complete response after autologous stem-cell transplantation in multiple myeloma. J Clin Oncol. 2013;31:4529–35.
Acknowledgements
We thank Hong Kong Society of Myeloma (HKSOM) for facilitating inter-hospital collaboration.
Author information
Authors and Affiliations
Contributions
CS Chim designed the study, interpreted results, revised and approved the manuscript. HKK Tang, CS Chim, YL Kwong contributed to the management of patients, and writing and approval of the manuscript. CY Fung helped with clinical data retrieval and statistical analysis. All have contributed to management of patients, provision of clinical information and approval of the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, H.K.K., Fung, C.Y., Hwang, Y.Y. et al. Prognostic factors in 448 newly diagnosed multiple myeloma receiving bortezomib-based induction: impact of ASCT, transplant refusal and high-risk MM. Bone Marrow Transplant 59, 660–669 (2024). https://doi.org/10.1038/s41409-024-02227-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-024-02227-0