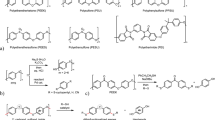
Abstract
Non-ionic aliphatic polymers containing ester and sulfonyl moieties, poly(ester-sulfones) were found to show anode-selective electrophoresis under electrophoretic deposition (EPD) conditions. Various polyesters containing a sulfide linkage were prepared via low-temperature polycondensation using scandium trifluoromethanesulfonate [Sc(OTf)3] as a catalyst in order to clarify the relationship between the chemical structure and the electrophoresis. Subsequently, oxone oxidation was carried out in order to obtain the expected saturated and unsaturated poly(ester-sulfone)s, and electrophoretic deposition (EPD) of the poly(ester-sulfone) onto stainless steel was performed. For unsaturated poly(ester-sulfone) onto stainless steel, subsequent UV irradiation cured the deposited film, improving the peeling strength of the coating.
Graphical Abstract
Various polyesters containing a sulfide linkage were prepared via low-temperature polycondensation using scandium trifluoromethanesulfonate [Sc(OTf)3] as a catalyst in order to clarify the relationship between the chemical structure and the electrophoresis. Subsequently, oxone oxidation was carried out in order to obtain the expected saturated and unsaturated poly(ester-sulfone)s, and electrophoretic deposition (EPD) of the poly(ester-sulfone) onto stainless steel was performed. For unsaturated poly(ester-sulfone) onto stainless steel, subsequent UV irradiation cured the deposited film, improving the peeling strength of the coating.

Similar content being viewed by others
Introduction
The design of new functional polyesters and the development of new properties of these polyester-based frontier materials are expanding in both academia and industry [1,2,3,4,5,6,7]. Polyesters are usually produced by condensation of a diol with a dicarboxylic acid or hydroxylcarboxylic acid [8,9,10,11,12], or by the ring-opening polymerization of a lactone [13,14,15]. Over the past decade, our group has investigated the low-temperature polycondensation of diols and dicarboxylic acids [16,17,18,19,20,21,22,23,24], which has allowed us to use thermally labile monomers containing functional groups [25,26,27,28]. The recent growth of click chemistry [29] prompted us to investigate a new synthetic approach to functional polyesters via click polymerization (polyaddition), and we have already reported polyesters containing triazole units [30, 31] and sulfide linkages [32] in the main chain via Cu(I)-catalyzed click chemistry [33, 34] and thiol-ene click chemistry [35,36,37,38,39,40,41], respectively. We have also reported that oxone oxidation of poly(ester-sulfide) prepared via thiol-ene-based click polymerization affords the corresponding poly(ester-sulfone), and unexpectedly found that the non-ionic poly(ester-sulfone) undergoes anode-selective electrophoretic deposition (EPD) on stainless steel (Figs. 1, 2) [32]. This enabled us to coat both bioactive glass [32] and titanium (IV) oxide (TiO2) [42] anode selectively, and further, to fabricate electrophoretic poly(ester-sulfone) gels [43]. In turn, this prompted us to explore why the poly(ester-sulfone) is deposited onto the anode, and what part of the sulfone-containing polyester structure is responsible for this unusual electrophoretic behavior. Recently, we synthesized a new poly(2-oxazoline) [44, 45] and a new poly(alkyl methacrylate) [46, 47] containing pendent sulfones (for transparent coating applications) via ring-opening polymerization and atom transfer radical polymerization (ATRP) procedures, and we investigated the electrophoretic behavior. A dispersion of these polymers in N,N-dimethylformamide (DMF)/ethanol also moved to the anode, suggesting that the sulfonyl groups are key to this phenomenon, and we concluded that partial charge separation of protic alcohols is induced by the pendent sulfonyl group at the interface under the influence of the electric field [48]. Now, a series of poly(ester-sulfone)s must be synthesized in order to clarify the relationship between the chemical structure and electrophoretic behavior, and polycondensation as a synthetic strategy is likely to be a reliable approach.
Electrophoresis is employed in numerous engineering applications such as in the separation of polyelectrolytes and in coating via EPD. Smoluchowski presented the most well-known model wherein he argued that the mobility of a charged spherical colloid is given by μ = εζ/(4πη) in the thin double-layer limit (where μ is the mobility, ε is the dielectric constant of the solution, ζ is the electrostatic potential at slip surfaces, and η is the viscosity of the solution) [49, 50].
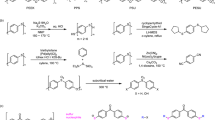
In this article, we report the low-temperature dehydration polycondensation of a series of sulfur-containing dicarboxylic acids and diols to produce the corresponding poly(ester-sulfide)s (Scheme 1). Successive sulfone oxidation leading to the corresponding saturated poly(ester-sulfone) enabled us to perform anode-selective EPD from DMF/alcohol dispersions. Low-temperature polycondensation enabled us to use unsaturated dicarboxylic acid to afford an unsaturated poly(ester-sulfone) without cross-linking. After the EPD, the coated film was cured by ultraviolet (UV) irradiation. Properties of the coatings were evaluated via a tape test, in which a cross-cut mark (1 mm × 1 mm) was applied to the coating surface to evaluate peeling.
Experimental procedure
Materials and methods
Chemicals were obtained from commercial sources and used without further purification. 1H NMR spectra were acquired at 27 °C using a Bruker Analytik DPX200 spectrometer (200 MHz) or a Bruker Analytik DPX400 spectrometer (400 MHz). Tetramethylsilane was used as the internal standard (0 ppm). FT-IR spectra of the polyesters in KBr disks were obtained using a JASCO/IR430 spectrometer. The number average molecular weight (Mn) and the polydispersity index (Mw/Mn) of each polymer were determined by size exclusion chromatography using a Tosoh DP8020 pump system, an RI (Tosoh RI-8020) detector, a TSK-Gel supermultiporehz-M column (eluent, chloroform; flow rate, 0.35 ml/min; temperature, 40 °C) or a TSK-GEL α-3000 column (eluent, 0.05% (w/v) LiBr/DMF; flow rate, 0.5 ml/min; temperature, 40 °C; Tosoh Corp.). The columns were calibrated with polystyrene standards. Differential scanning calorimetry (DSC) was performed from –90 to 180 °C to –90 °C, at 10 °C/min on a DSC6220S calorimeter (Seiko Instruments Inc., Chiba, Japan). The instrument was calibrated with indium and tin. Each polyester sample weighed between 5 and 8 mg, contained in covered aluminum pan in the calorimeter. The glass-transition temperature (Tg) was taken as the inflection point of the DSC heat capacity jump. The melting temperature (Tm) was defined as the minimum point in the endothermic trough. Zeta potential (ζ) measurements were performed using a Zetasizer Nano ZS (Malvern Instruments, UK). The measurement principle was electrophoretic light scattering, and literature η values were used.
Synthesis of poly(ester-sulfide)s by polycondensation
A typical procedure for bulk polycondensation at relatively low temperature was performed using various monomers; an example (Table 2, entry 3) follows. Sc(OTf)3 49 mg (0.1 mmol), 2,2′-thiodiglycolic acids (TDGA) 1.50 g (10 mmol), and 3-methyl-1,5-pentanediol (MPD) 1.18 g (10 mmol) were mixed and then stirred at 80 °C until a homogeneous state was observed. The pressure was gradually decreased to 0.3–3 mm Hg for 10 h. For purification, the products were dissolved in CHCl3, reprecipitated with diethyl ether, and dried under reduced pressure. The product, poly(TDGA-alt-MPD), was characterized by 1H NMR spectroscopy. 1H NMR (200 MHz in CDCl3, δ): 4.18 [t, OCH2CH2CH(CH3), 4H, 6.0 Hz], 3.39 (s, O = CCH2SCH2, 4H), 1.83–1.62 [brm, OCH2CH2CH(CH3) and OCH2CH2CH(CH3), 3H], 1.62–1.40 [brm, OCH2CH2CH(CH3), 2H], 0.97 [d, OCH2CH2CH(CH3)CH2, 3H, 6.0 Hz].
Oxone oxidation of poly(ester-sulfide)s to poly(ester-sulfone)s
An example (Table 2, entry 3) of the oxidation of a poly(ester-sulfide) to a poly(ester-sulfone) follows. The poly(ester-sulfide), poly(TDGA-alt-MPD), (232 mg, 1.0 mmol repeating unit), and oxone (1.23 g 2.0 mmol) in DMF (20 ml) were added to a 20 ml round-bottom flask. After stirring at room temperature for 24 h, the mixture was filtered, and the solvent was evaporated under reduced pressure. For purification, the product was then dissolved in DMF, reprecipitated by addition of H2O, filtered, and dried under reduced pressure. The product was characterized by 1H NMR spectroscopy. 1H NMR (200 MHz in CDCl3, δ): 4.34 (s, O = CCH2SO2CH2, 4H), 4.31–4.17 [brm, OCH2CH2CH(CH3), 4H], 1.96–1.64 [brm, OCH2CH2CH(CH3) and OCH2CH2CH(CH3), 3H], 1.64–1.42 [brm, OCH2CH2CH(CH3), 12H], 0.97 [d, OCH2CH2CH(CH3)CH2, 3H, 4.7 Hz].
Preparation of poly(ester-sulfone)/DMF dispersions and EPD on stainless steel electrodes
The poly(ester-sulfone)s were stirred in DMF. Then, a poor solvent was added to the solution, which was then ultrasonically agitated for 5 min. To deposit the poly(ester-sulfone)s onto the stainless steel electrodes via EPD, the distance between the electrodes was 6.5 mm, and the deposition time was 90 s at a voltage of 200 V. The depositions were carried out on 0.2 × 15 × 25 mm stainless steel SUS 301 electrodes obtained from the Japan Metal Service (Saitama, Japan).
Photo-induced photo-curing of coated film (unsaturated poly(ester-sulfone)) on a stainless steel anode
UV curing of the unsaturated poly(ester-sulfone) was performed using longer-wavelength UV light (in the near visible region, above 430 nm). The curing performance was evaluated via a tape test (peeling test), in which a cross-cut mark (1 mm × 1 mm) was observed on the coating surface after UV curing, while the mark disappeared on the uncured unsaturated poly(ester-sulfone)-coated stainless surface. The resultant images are shown in Fig. 4. A cross-cut mark was applied onto the coating surface to evaluate the effectiveness of the curing. This cut mark appears even after the tape test, indicating that UV curing was successful.
Results and discussion
Synthesis of the poly(ester-sulfide)
Polycondensation of TDGA with MPD was used to compare the effectiveness of two catalysts (Table 1, runs 1 and 2; conditions: 80 °C, 5 h reaction time, 0.5 mol% catalyst/monomer). The Mn values of the polyester when bis(nonafluorobutanesulfonyl)imide (Nf2NH) [23] was used as the catalyst were less than when Sc(OTf)3 was used, although Nf2NH produced a polyester with a similar molecular weight distribution. When the reaction time was extended from 5 to 8 h using Sc(OTf)3 as the catalyst, the Mn value of the polyester increased to 14.5 kDa. These results suggest the use of Sc(OTf)3 as an effective catalyst in the polycondensation of TDGA with MPD.
Next, the Sc(OTf)3-catalyzed polycondensations of TDGA and 3,3′-thiodipropionic acid (TDPA) with several diols including MPD, 2-methyl-propanediol (MPrD), 2,2-dimethyl-1,3-propanediol (DMPrD), and 1,4-cyclohexanedimethanol (CHDM) were investigated at 80–90 °C (Table 2). The results are summarized in Table 2, which clearly shows that polyesters of greater molecular weights (Mn = 14.5 kDa) were prepared (run 3). Polycondensation of TDGA with CHDM did not proceed at 80 °C because CHDM is not miscible in solid TDGA at that temperature. When the polycondensation of TDGA with CHDM was carried out at 90 °C, a polyester with an Mn value of 19.5 kDa was obtained.
Synthesis of the poly(ester-sulfone)
Successive oxone oxidation of the poly(ester-sulfide)s was then performed, producing the corresponding poly(ester-sulfone)s (Table 2). According to our reported procedure [32], 2 equiv. of oxone per sulfide quantitatively converted the sulfides to sulfones (Supporting Information, Supplementary Figs. S1, S2). The Mns of the poly(ester-sulfone)s were similar to those of the parent poly(ester-sulfone)s (Table 2).
Preparation of poly(ester-sulfone)/DMF dispersions and EPD on stainless steel electrodes
According to our reported procedure [32], n-BuOH was first used as the poor solvent for the poly(ester-sulfone)/DMF dispersions. In all cases, stable dispersions were obtained, and all of the dispersions visibly moved to anode, but deposition of the poly(ester-sulfone) was not observed for the reaction of TDGA and MPrD (Run 1, Table 3) and TDPA and DMPrD (Run 6, Table 3). We considered that the resulting selective affinity of the ions (charge-separated alcohol) to this poly(ester-sulfone) prepared via room-temperature polycondensation may also lead to finite electrophoretic mobility, which coincides well with Uematsu’s new theory [50] that electrophoresis of electrically neutral porous spheres can be induced by selective affinity of ions.
The results described above indicated that the sulfonyl groups in poly(ester-sulfone) main are key functionality in the electrophoretic behavior. Therefore we synthesized the poly(ester-sulfone) containing higher sulfonyl content in the main chain by polycondensation of 1,2-ethylenebis(dithioglycolic acid) (EBGA) with MPD and subsequent oxone oxidation (Table 2, run 7) (see also Supporting Information, Supplementary Fig. S3). In the EPD, much deposition was confirmed (run 8, Table 3), compared with the results using other poly(ester-sulfone) (Table 3). The composite thickness (run 8, Table 3) was 56 μm on average (Fig. 3), which is thicker than that of poly(ester-sulfone) prepared via polycondensation of TDGA and MPD (run 3 in Table 3 and please see also Supplementary Fig. S4). The results well-coincided with that a poly(ester-sulfone) colloidal suspension has formed and that the sulfone moieties were responsible for anode-selective deposition.
Scanning electron micrographs of poly(ester-sulfone) films prepared via polycondensation of EBGA with MPD (run 8 in Table 3). Thickness of the composite was estimated from a cross section of the composite (×800 magnification) (Color figure online)
Next, MeOH was used as the poor solvent (Table 4). Stable dispersions were also obtained in all cases. All of the dispersions were again visibly observed to move toward the anode, but resulting in deposition of only two poly(ester-sulfone)s consisting of TDGA and MPD (run 3, Table 4), and TDPA and CHDM (run 4, Table 4) [13,14,15,16,17,18,19,20,21,22,23,24]. These results indicate that the EPD behavior is influenced by both the type of poly(ester-sulfone) as well as the poor solvent. The thickest EPD film that obtained a higher molecular weight poly(ester-sulfone) was used; [16,17,18,19,20,21,22,23,24] however, the composite films cracked (runs 4 and 5, Table 3; run 4, Table 4). These results indicate that the molecular weight of the poly(ester-sulfone)s also influences the properties of the EPD-coated film.
Zeta potential measurements of the poly(ester-sulfone) emulsions
To characterize the electrophoretic behavior of the poly(ester-sulfone), its zeta potential was measured in different mixtures of DMF and n-BuOH or MeOH (Tables 3, 4). The negative values measured support the anode-selective deposition, and the larger the negative values were, the more stable the suspensions obtained were (runs 3, 4, and 7 in Table 4), when we used MeOH as the poor solvent for the preparation of dispersions. From the results above, we concluded that negative zeta potential coincided with the anode-selective EPD behavior, in which we visibly observed that the electrophoresis mobility is correlate with the absolute value of zeta potential, but the deposition mechanism is influenced by both the structure of poly(ester-sulfone) as well as the solvent of dispersion.
Synthesis of unsaturated poly(ester-sulfone) by room-temperature polycondensation
Next urgent subject in a series of these studies is to strengthen as well as increase the coated film, in which photo-induced cross-linking is the most accessible approach. Recently, we reported a synthetic pathway via acyclic diene metathesis (ADMET) polymerization [51]. As another synthetic procedure, room-temperature polycondensation [25,26,27,28] is the most reliable way in polymer science. We already reported unsaturated polyesters using several unsaturated dicarboxylic acid. In this work, we demonstrated ternary polycondensation of TDGA, MPD, and itaconic acid (IA) (Scheme 2) in which the polycondensation proceeded smoothly at 80 °C to give an expected unsaturated poly(esfter-sulfide), poly[(TDGA-alt-MPD)-co-(IA-alt-MPD)] with Mn of 10.1 × 103 (Mw/Mn = 2.9). Subsequent oxone oxidation afforded the expected poly(ester-sulfone) with Mn of 9.8 × 103 (Mw/Mn = 2.8) (Scheme 2).
Photo-induced photo-curing of coated film (unsaturated poly(ester-sulfone)) on a stainless steel anode
UV curing of the unsaturated poly(ester-sulfone) was performed using longer-wavelength UV light (in the near visible region, above 430 nm), in which the UV irradiation was confirmed by decrease of IR signal assigned to stretching signal of C = C vibration at 1620/cm (Supporting Information, Supplementary Fig. S5). The curing performance was evaluated via a tape test (peeling test), in which a cross-cut mark (1 mm × 1 mm) was observed on the coating surface after UV curing, while the mark disappeared on the uncured unsaturated poly(ester-sulfone)-coated stainless surface. The resultant images are shown in Fig. 4. A cross-cut mark was applied onto the coating surface to evaluate the effectiveness of the curing. This cut mark appears even after the tape test, indicating that UV curing was successful.
Conclusion
Non-ionic saturated and unsaturated polyesters containing sulfide groups linkages on the polymer backbone were synthesized via dehydration polycondesation using Sc(OTf)3 as the more ideal dehydration catalyst. Subsequent oxone oxidation was carried out, affording the corresponding poly(ester-sulfone)s, and these poly(ester-sulfone)s were coated onto stainless steel via anode-selective EPD. The EPD behavior was influenced by both the type of poly(ester-sulfone) as well as the poor solvent used. For unsaturated poly(ester-sulfone), coating strength was evaluated via a tape test, in which a cross-cut mark was observed on the coating surface after UV curing, while the mark disappeared on the uncured unsaturated poly(ester-sulfone)-coated stainless surface. The fundamental results indicated that room-temperature polycondensation as well as ADMET polymerization are the beneficial synthetic procedures for electrophoretic unsaturated polyesters.
References
Vert M. Aliphatic polyesters: great degradable polymers that cannot do everything. Biomacromolecules 2005;6:538–46.
Takwa M, Xiao Y, Simpson N, Malmström E, Hult K, Koning CE, Heise A, Martinelle M. Lipase catalysed HEMA initiated ring-opening polymerization: in-situ formation of mixed polyester methacrylates by transesterification. Biomacromolecules 2008;9:704–10.
Misra SK, Valappil SP, Roy I, Boccaccini AR. Polyhydroxyalkanoate (PHA)/inorganic phase composites for tissue engineering applications. Biomacromolecules 2006;7:2249–58.
Seyednejad H, Vermonden T, Fedorovich NE, Eijik R, Steenbergen MJ, Dhert WJA, Nostrum CF, Hennink WE. Synthesis and characterization of hydroxyl-functionalized caprolactone copolymers and their effect on adhesion, proliferation, and differentiation of human mesenchymal stem cells. Biomacromolecules 2009;10:3048–54.
Naolou T, Busse K, Kressler J. Synthesis of well-defined graft copolymers by combination of enzymatic polycondensation and “click” chemistry. Biomacromolecules 2010;11:3660–7.
Zhang P, Wu H, Wu H, Lu Z, Deng C, Hong Z, Jing X, Chen X. RGD-conjugated copolymer incorporated into composite of poly(lactide-co-glycotide) and poly(L-lactide)-grafted nanohydroxyapatite for bone tissue engineering. Biomacromolecules 2011;12:2667–80.
Moglia RS, Holm JL, Sears NA, Wilson CJ, Harrison DM, Cosgriff-Hernandez E. Development of injectable polyHIPEs as high porosity bone grafts. Biomacromolecules 2011;12:3621–8.
Carothers WH, Dorough GL. Studies on polymerization and ring formation. IV ethylene succinates. J Am Chem Soc 1930;52:711–21.
Takiyama E, Hatano Y. High- molecular- weight unsaturated polyesters. Japan Patent 04183714 A 19920630, 1992.
Takahashi M, Ito S, Takiyama, E. Manufacture of unsaturated polyesters using recycled poly(ethylene terephthalate) Japan Patent 2000159878 A 20000613, 2000.
Buzin P, Lahcini M, Schwarz G, Kricheldord HR. Aliphatic polyesters by bismuth triflate-catalyzed polycondensations of dicarboxylic acids and aliphatic diols. Macromolecules. 2008;41:8491–5.
Garaleh M, Lahcini M, Kricheldorf HR, Weidner SM. Syntheses of aliphatic polyesters catalyzed by lanthanide triflates. J Polym Sci A Polym Chem 2009;47:170–7.
Kricheldorf HR, Kreiser-Saunders I. Polylactones, 19. Anionic polymerization of L-lactide in solution. Macromol Chem Phys 1990;191:1057–66.
Lofgren A, Albertsson AC, Dubois P, Jerome R. Recent advances in ring-opening polymerization of lactones and related-compounds. J Macromol Sci Rev Macromol Chem Phys 1995;35:379–418.
Kim S, Han YK, Kim YH, Hong SI. Multifunctional initiation of lactide polymerization by stannous octoate/pentaerythritol. Macromol Chem Phys 1992;193:1623–31.
Takasu A, Oishi Y, Iio Y, Inai Y, Hirabayashi T. Synthesis of aliphatic polyesters by direct polyesterification of dicarboxylic acids with diols under mild conditions catalyzed by reusable rare-earth triflate. Macromolecules 2003;36:1772–4.
Takasu A, Iio Y, Oishi Y, Narukawa Y, Hirabayashi T. Environmentally benign polyester synthesis by room temperature direct polycondensation of dicarboxylic acid and diol. Macromolecules 2005;38:1048–50.
Takasu A, Iio Y, Mimura T, Hirabayashi T. Room-temperature polycondensation of dicarboxylic acids and diols catalyzed by water-stable Lewis acid. Polym J 2005;37:946–53.
Takasu A, Takemoto A, Hirabayashi T. Polycondensation of dicarboxylic acids and diols in water catalyzed by surfactant-combined catalysts and successive chain extension. Biomacromolecules 2006;7:6–9.
Takasu A, Narukawa Y, Hirabayashi T. Direct dehydration polycondensation of lactic acid catalyzed by water-stable Lewis acids. J Polym Sci Polym Chem 2006;44:5247–53.
Takasu A, Tsuruta H, Narukawa Y, Shibata Y, Oshimura M, Hirabayashi T. Dual catalytic system for combination of chain and step polymerizations: ring-opening polymerization of ε-caprolactone and successive dehydration polycondensation with dicarboxylic acid using the same catalyst. Macromolecules 2008;41:4688–93.
Takasu A, Makino T, Yamada S. Polyester synthesis at moderate temperatures via the direct polycondensation of dicarboxylic acid and diols catalyzed by rare-earth perfluoroalkanesulfonates and bis(perfluoroalkansulfonyl)imides. Macromolecules 2010;43:144–9.
Yamada S, Takasu A. Microwave-assisted low-temperature dehydration polycondensation of dicarboxylic acids and diols Polym J. 2011;43:1003–7.
T. Moyori T, Tang T, Takasu A. Dehydration polycondensation of dicarboxylic acids and diols using sublimating strong Brønsted acids. Biomacromolecules 2012;12:1240–3.
Takasu A, Shibata Y, Narukawa Y, Hirabayashi T. Chemoselective dehydration polycondensations of dicarboxylic acids and diols having pendant hydroxyl groups using the room temperature polycondensation technique. Macromolecules 2007;40:151–3.
Shibata Y, Takasu A. Synthesis of polyester having pendent hydroxyl groups via regioselective dehydration polycondensations of dicarboxylic acids and diols by low temperature polycondensation. J Polym Sci A Polym Chem 2009;47:5747–59.
Takasu A, Yamamoto K. Preparation of gelatinous reversible addition−fragmentation chain transfer agents “RAFT Gel” via chemoselective polycondensations of a dicarboxylic acid containing a mercapto group and diols. Macromolecules 2010;43:8519–23.
Sato Y, Takasu A. Conversion of diols to dithiols via a dehydration polycondensation with a dicarboxylic acid containing a disulphide and subsequent reduction. Polym J 2010;42:956–9.
Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed 2001;40:2004–21.
Nagao Y, Takasu A. Click polyester: synthesis of polyesters containing triazole units in the main chain by click chemistry and improved thermal property. Macromol Rapid Commun 2009;30:199–203.
Nagao Y, Takasu A. “Click polyester”: synthesis of polyesters containing triazole units in the main chain via safe and rapid “click” chemistry and their properties. J Polym Sci A Polym Chem 2011;48:4207–18.
Nagao Y, Takasu A, Boccaccini AR. Anode-selective electrophoretic deposition of a bioactive glass/sulfone-containing click polyester composite. Macromolecules 2012;45:3326–34.
Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A Stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed 2002;41:2596–9.
Chan TR, Hilgraf R, Sharpless KB, Fokin VV. Polytriazoles as copper(I)-stabilizing ligands in catalysis. Org Lett 2004;17:2853.
Wilderbeek HTA, Goossens JGP, Bastiaansen CWM, Broer DJ. Photoinitiated bulk polymerization of liquid crystalline thiolene monomers. Macromolecules 2002;35:8962–8.
Wilderbeek HTA, Meer MGM, Bastiaansen CWM, Broer DJ. Photo-initiated polymerization of liquid crystalline thiol-ene monomers in isotropic and anisotropic solvents. J Phy Chem B 2002;106:12874–83.
Killops KL, Campos LM, Hawker CJ. Efficient, and orthogonal synthesis of dendrimers via thiol-ene click chemistry. J Am Chem Soc 2008;130:5062–4.
Kwinsnek L, Nazarenko S, Hoyle CE. Effects of chemical modification of thiol-ene networks on enthalpy relaxation. Macromolecules 2009;42:7031–41.
Kloxin CJ, Scott TF, Bowman CN. Spatial and temporal control of thiol-michael addition via photocaged superbase in photopatterning and two-stage polymer networks formation. Macromolecules 2009;42:2551–6.
Shin J, Matsushima H, Chan JW, Hoyle CE. Segmented polythiourethane elastomers through sequential thiol-ene and thiol-isocyanate reactions. Macromolcules 2009;42:3294–301.
Lluch C, Ronda JC, Galia M, Lligadas G, Cádiz V. Rapid approach to biobased telequelics through two one-pot thiol-ene click reactions. Biomacromolecules 2010;11:1646–53.
Fukuoka T, Takasu A. Anode-selective coating of titanium oxide using electrophoretic sulfone-containing click polyester. RSC Adv 2014;31:15983–94.
Mano Y, Nagao Y, Takasu A. Synthesis of electrophoretic poly(ester-sulfone) gel via thiol-yne click gelation. Polym J 2014;46:682–7.
Hayashi T, Takasu A. Design of electrophoretic poly(2-oxazoline)s using sulfoneimides as the initiator and hybridization with bioactive glass. Polym. Prepr. (Am. Chem. Soc. Div. Polym.) POLY-6 2014.
Hayashi T, Takasu A. Design of electrophoretic and biocompatible poly(2-oxazoline)s initiated by perfluoroalkanesulfoneimides and electrophoretic deposition with bioactive glass. Biomacromolecules 2015;16:1259–66.
Kameyama T, Takasu A. Electrophoretic vinyl polymers via atom transfer radical polymerization of a methacrylate containing sulfide. Polym. Prepr. (Am. Chem. Soc. Div. Polym.) POLY-398 2014.
Kameyama T, Takasu A. A vinyl polymer having pendent sulfones prepared by atom-transfer radical polymerization of a sulfide-containing methacrylate and electrophoretic transparent coating on a stainless-steel anode. Polym Chem 2015;6:4336–42.
Yokoyama M, Takasu A. Alcohol dependence of anode-selective electrophoretic deposition of non-ionic poly(ester-sulfone). Polymer 2016;88:1–8.
Smoluchowski MZ. Drei vorträge über diffusion, brownsche molekularbewegung und koagulation von kolloidteilchen. Phys Chem. 1916; 17:557-71.
Uematsu Y. Electrophoresis of electrically neutral porous spheres induced by selective affinity of ions. Phys Rev 2015; 91:022303.
Kawarazaki I, Takasu A. Synthesis of unsaturated nonionic poly(ester-sulfones) via acyclic diene metathesis (ADMET) polymerization and anode-selective electrophoretic deposition. Macromol Chem Phys. 2016;217:2595-2600 10.1002/macp.201600277.
Acknowledgements
This work was funded by the Ministry of Education, Science, and Culture of Japan (Grant-in-Aid for Development Scientific Research, no. 22750104 and 15K04872). We are grateful to Prof. Masahiro Higuchi of the Nagoya Institute of Technology for valuable advice and fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Matsumoto, Y., Takasu, A. Synthesis of non-ionic poly(ester-sulfone) via low-temperature polycondensation for anode-selective electrophoretic deposition and subsequent photo cross-linking. Polym J 50, 187–196 (2018). https://doi.org/10.1038/s41428-017-0001-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-017-0001-y