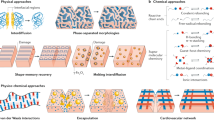
Abstract
Among various approaches to create self-healing polymers, the introduction of dynamic bonds to polymers is one of the most powerful approaches. Macroscopic failure of such polymers is usually accompanied by the cleavage of dynamic bonds at the broken surfaces, which can then reform due to their reversible nature and repair the failure. However, since the reformation of dynamic bonds requires molecular mobility, autonomous healing at room temperature is almost completely limited to polymers with good molecular mobility, such as gels and soft elastomers. Mechanical strength usually conflicts with a high molecular mobility, as well as an autonomous healing ability. In this review, we first overview recent successful approaches to overcome this limitation. These approaches include combining careful dynamic bond chemistry choices and smart designs of the environment around the dynamic bonds. In the latter part of this review, attempts to design mechanically robust polymers that can heal with the assistance of ubiquitous stimuli are summarized. Such a healing process is a suboptimal choice for practical, valuable healing materials.
Similar content being viewed by others
Introduction
Among various approaches to develop self-healing polymers, the introduction of dynamic bonds to chain molecules is one of the most powerful approaches [1]. In 2002, X. Chen et al [2] introduced this type of self-healing polymer by showing that a network polymer made by a Diels–Alder (DA) reaction between tris-maleimide and tetra-furan can be healed by heating. In this paper, a number of studies have reported healing polymers with various dynamic bonds, including reversible covalent bonds, such as DA adducts [2], disulfide bonds [3], urea bonds [4], siloxane bonds [5], acylhydrazone bonds [6], ester bonds with a catalyst for transesterification [7], C = C double bonds with a catalyst for olefin metathesis [8], hemiaminal linkages [9], alkoxyamine linkages [10], and diarylbibenzofuranone linkages [11], and intermolecular interactions, such as hydrogen bonds [12], ionic bonds [13], metal-ligand interactions [14], host–guest interactions [15, 16], and π−π interactions [17].
Macroscopic failure of polymers is usually accompanied by molecular chain cleavages at the broken surfaces. In polymers containing dynamic bonds, these cleavages are likely compensated for by the dissociation of dynamic bonds, while other covalent bonds are preserved. If the molecular structure of dynamic polymers is controlled by thermodynamics, the dynamic bonds as many as were broken by failure can autonomously reform and bridge the broken surfaces, which results in a full recovery of the mechanical properties on a macroscopic scale. Thus, any polymer with a sufficient number of dynamic bonds has the potential to self-heal at room temperature. However, the slow dynamics of polymer chains often prevent dynamic bonds from reforming. Furthermore, even when kinetic constraints do not hinder the recovery of dynamic bonds, some of these bonds form within a fractured surface. Shuffling of the bonded pairs is required for efficient bridging between the fractured surfaces. Therefore, autonomous and complete healing is limited to polymers with good molecular mobility, such as gels and soft elastomers containing highly dynamic bonds at room temperature.
A high molecular mobility usually conflicts with the mechanical strength of polymer materials. The addition of an autonomous healing ability to a polymer with superior mechanical robustness is a challenging issue. In this review, after providing an overview of the research on combing mechanical strength with an autonomous healing ability in polymer materials, attempts to design mechanically robust polymers with a self-healing ability via the assistance of ubiquitous stimuli are summarized (Fig. 1). Recently, many papers have reported self-healing polymers exhibiting remarkable healing behavior even though their mechanical strength is not high. Please refer to previous excellent reviews on these healing polymers [18,19,20].
Combining an autonomous healing ability and mechanical strength
There have already been many reports on autonomously healable polymers at room temperature, although most of the polymers are gels and soft elastomers with tensile strengths of less than 1 MPa. Strengths higher than 2 MPa have been achieved only in several reports (Table 1). Cordier et al. [12] developed a supramolecular elastomer constructed by multiple hydrogen bonds among several low-molecular-weight compounds. When freshly cut surfaces of this elastomer were kept in contact at room temperature, the elastomer recovered its strength (tensile strength after healing, σfh = 2.8 MPa) and stretchability (fracture strain after healing, εfh = 530%), and the values were close to the values of the virgin sample (σfv = 3.3 MPa, εfv = 600%). Das et al [21] demonstrated that a commercially available bromobutyl rubber could be converted into a healing material by an imidazole modification. Due to the physical crosslinks by an ionic association between imidazolium bromide molecules, this polymer exhibited good elastic properties (σfv = 9 MPa, εfv = 1000%) as well as an autonomous healing ability (σfh = 5.2 MPa, εfh = 960%). Two other examples that have successfully combined an autonomous healing ability and mechanical strength were network polymers based on a DA reaction between anthracene and maleimide [22] and dual crosslinking [23]. The former was created from an anthracene-terminated aliphatic prepolyester and a tris-maleimide, which recovered σfh = 4.4 MPa and εfh = 350% compared to the virgin properties of σfv = 26 MPa and εfv = 1080%. The latter, containing both permanent covalent-bond crosslinks and transient hydrogen-bond crosslinks exhibited σfh = 4 MPa after healing, which was 30% of its original value. Although the healing efficiency of the polymers in refs. 22 and 23 are poor, the recovered strengths are still high among those reported for autonomous healing polymers.
For the healing polymers mentioned so far, the reason why the autonomous healing ability and mechanical strength were successfully combined was not clearly explained. Y. Chen et al [24, 25] proposed that a multiphase design was a good strategy to combine these properties. They introduced intermolecular hydrogen bonds to the soft segment of brush [24] and block [25] copolymers to form soft-hard multiphase systems. These polymers exhibited an autonomous healing ability with a high recoverability. For example, a block-type copolymer regained its initial stiffness (Young’s modulus Eh ≈ Ev = 77 MPa), as well as strength (the fracture strength after healing, σfh = 5 MPa, compared to the initial maximum strength of σmv = 6 MPa). Seemingly, the hard domains in these polymers ensure the mechanical strength, while the soft matrices with dynamic bonds facilitate the healing ability. Interestingly, the introduction of dynamic bonds into the hard segment instead of the soft segment also provided a tough and autonomously healable elastomer [26]. An aromatic disulfide, which is well known to undergo an efficient metathesis reaction, was embedded into the hard segments of a polyurethane together with the carefully chosen sophorone diisocyanate, which assisted in maintaining a sufficient chain mobility for dynamic bond exchange while retaining the remarkable mechanical properties of the polymer. As a result of this molecular design, the polyurethane autonomously recovered its tensile strength, σfh, of 6.0 MPa and toughness, which was defined by the area under the stress–strain curve, Th, of 21 MJ m−3 within 2 h at room temperature (virgin: σfv = 6.8 MPa, Tv = 27 MJ m−3). Compared with that observed in multiphase brush/block copolymer systems, smaller polyurethane domain sizes might allow the dynamic nature of the aromatic disulfide bonds within the hard domain to be maintained, leading to a good healing ability. The same hard segment structure was also applied to organic–inorganic polyurethane hybrids, which autonomously recovered σfh = 3.8 MPa [27].
He et al [28] reported a unique self-healing hydrogel. A double networked hydrogel was prepared from a cationic polyelectrolyte, crosslinked anionic polyelectrolyte and poly(vinyl alcohol) (PVA) and compounded with Au nanoparticles prepared in situ. During the tensile test, this hydrogel first extended with a modulus of ~0 MPa to a strain of 60–80% and then became less extensible with a rapid increase in the modulus to 7.3 MPa at the break point (εfv = 100%). The extremely low modulus at a small strain suggests that the molecules in the unstretched state have a remarkably high mobility, which might help heal the hydrogel by reforming the metal−ligand coordination between the Au nanoparticles and the OH group of PVA, as well as the electrostatic interaction between the polyelectrolyte chains. As a result, the gel autonomously recovered a strength, σfh, of 6.7 MPa (virgin: σfv = 7.3 MPa) and its conductivity in 20 min.
The strength of the dynamic bond seems to be a critical factor for polymer healing [29]. Garcia et al. [30] claimed that when the lifetime of molecular flowing, τb, which is determined by the crossover frequency of the storage and loss moduli, was between 10 and 100 s, the polymer exhibited a good combination of a healing ability and mechanical robustness. After this paper, however, the same group showed that the relaxation process related to the branches in the polymers, which was faster than this criterion, led to effective healing [31]. Polyetherimides were synthesized from aromatic dianhydride and a fatty diamine with two long-chain aliphatic (length ≈ 8 carbons) branches. When the dianhydride/diamine molar ratio was optimized, the polymer with Efv = 54 MPa, σfv = 5.7 MPa, and εfv = 440% recovered its mechanical properties almost completely at room temperature without any external stimuli. The authors assumed that weak but numerous van der Waals interactions between the alkyl branches formed a transient supramolecular network, which enabled the combination of relatively good mechanical properties and an autonomous healing ability. By taking advantage of the relaxation process that was much faster than τb, Yanagisawa et al [32] also developed an autonomously healable poly(ether-thiourea) with an exceptionally high mechanical robustness. In this polymer that combined thiourea with a triethylene glycol spacer, a tight yet rearrangeable network structure was formed by dense yet exchangeable hydrogen-bonded pairs of thiourea. The segmental motion accompanying the exchange of the thiourea pairs enabled the healing process to proceed by interpenetration of polymer chains to bridge the fractured surfaces, even at temperatures slightly lower than the glass transition temperature (Tg = 27 °C). Consequently, the polymer recovered an extremely high strength (Efh > 1.2 GPa, Efv = 1.4 GPa, σfh ≈ σfv = 39 MPa) within 6 h upon compression at 24 °C.
As shown in this section, research on rigid healing polymers has gradually progressed, but we still need to continue to develop widely applicable strategies to combine a spontaneous healing ability with mechanical robustness in various polymers. Alternatively, the utilization of mild and ubiquitous stimuli is a valuable approach for healing polymers. In the following sections, healing polymers with the assistance of mild stimuli, such as heat, light, solvent, chemicals, and water, will be reviewed.
Thermally triggered healing
Heat treatment is a viable approach for healing polymers because dynamic bonds are easier to dissociate and more dynamic at higher temperatures. Using a carefully chosen thermal treatment, dynamic polymers have a good chance to recover a mechanical strength not achieved by healing at room temperature (Table 2). The groundbreaking healing polymer by X. Chen et al [2] was a material that could be repaired with the assistance of a heat treatment. The polymer was a densely crosslinked network polymer made by a DA reaction between tetra-furan and M3. Despite the rigid nature with σfb = 68 MPa, the DA polymer recovered 57% of its original fracture load upon heating to 150 °C and cooling to room temperature. At 150 °C, the equilibrium of this DA reaction favors the dissociation of the DA adduct, while the equilibrium shifts toward adduct formation again at temperatures lower than ca. 80 °C. The dissociation of dynamic bonds in dynamic polymers is often accompanied by macroscopic collapse, which makes the realization of self-healing without a shape change difficult. However, this was not the case for this DA polymer, which was probably because the retro-DA reaction only partially proceeded due to the densely packed DA crosslinks. Therefore, efficient crosslink reshuffling proceeded without a macroscopic deformation.
Macroscopic shape changes during thermal healing can also be avoided by performing the treatment at a temperature lower than the dissociation temperature. As mentioned in the introduction, dynamic bonds are believed to preferentially break at the fractured surfaces during crack formation. If the liberated functional groups at the fractured surfaces completely reconnect again, the crack is repaired without passing through the dissociation temperature of the dynamic bond. In fact, Yoshie et al. [33] achieved healing in an aliphatic polyester loosely crosslinked with the furan-maleimide DA adduct at 60 °C, which is above the melting temperature of this polymer (47 °C) and below the dissociation temperature of the DA adducts. The material recovered σfh = 18 MPa and εfh = 510% compared with the original values of σfv = 27 MPa and εfv = 750%, respectively. Surprisingly, this polymer also partially recovered its mechanical properties (σfh = 6 MPa, εfh = 80%) by prompt heating at 60 °C followed by maintaining at room temperature. The characteristic low crystallization rate of this polymer provided enough time to reform the dynamic bonds in its supercooled state.
Integration of shape memory into self-healing polymers is an effective method to enhance the healing efficiency by thermal treatment. Rodriguez et al. [34] proposed this concept by blending a shape-memory polymer and crosslinked poly(ε-caprolactone) (cl-PCL) with linear poly(ε-caprolactone) (l-PCL). Upon increasing the temperature over the melting point of the PCL, open cracks were gradually closed by the shape recovery of the cl-PCL and bridged by the chain entanglement of l-PCL. The material entirely recovered its tensile yield strength (σyh ≈ σyv = 10 MPa).
Two other examples of healing polymers that recover their relatively high strength by a simple heat treatment are polyurethane extended by a DA reaction between furan and maleimide [35] and cis-1,4-polyisoprene dually crosslinked with weaker multiple hydrogen bonds and stronger Zn–triazole coordination [36]. The former recovered σfh = 20 MPa and εfh = 430% at 120 °C, while the latter recovered σfh = 15.5 MPa and Th = 42.8 MJ m−3 at 80 °C.
Another option to heal thermoresponsive dynamic polymers is adopting photothermal effects instead of direct heating. An advantage of this method is that light is easy to apply locally, remotely, and effectively to the damaged area [14]. Therefore, macroscopic deformation during healing can be mostly excluded. In addition, only a few seconds to a few minutes of irradiation is sufficient for healing with a high efficiency in most cases. For a good balance of healing ability and mechanical strength in dynamic polymers, fillers that efficiently absorb light and transform into heat energy work particularly well. A graphene-polyurethane composite [37] and carbon nanotube-epoxy composite [38] can be healed by IR light with a high recoverability. The former fully recovered its mechanical strength of σfh ≈ σfv ≈ 34 MPa, while the latter exhibited σfh = 20 MPa (original strength σfv = 24 MPa). Graphene also enabled the composite to fully recover its mechanical strength by electricity and electromagnetic wave [37]. Another filler for photothermal healing is gold nanoparticles. Their surface plasmon resonance was used for photoinduced heating of a healing polymer [39]. Furthermore, shape memory has also been combined with photothermal healing in some polymers and composites [39,40,41].
Solvent- and solution-triggered healing
Applying solvents onto damaged areas is a healing procedure applicable to a wide range of polymers with dynamic bonds (Table 3). Swelling of a solvent in the damaged areas helps enhance the mobility of the polymer chain, leading to a higher chance of dynamic bond reformation. Hong et al [42] reported that a polymer crosslinked with tridentate ligand-Zn2+ exhibited good mechanical properties, as well as a solvent-assisted, self-healing ability. After dropping a solvent on a deep notch every hour, the polymer completely recovered its high strength (σfh ≈ σfv = 9.5 MPa, εfh ≈ εfv = 1450%, Eh ≈ Ev = 3.5 MPa). To this polymer, spiropyran was also introduced to sense damage. When the polymer was stretched, it was colored by the transformation of spiropyran into its merocyanine form.
Application of solutions containing crosslinking agents further facilitates self-healing by adding crosslinks around the damaged area. Peterson et al. [43] examined the healing behavior of a furan-functionalized epoxy−amine thermoset by treatment with solvents and bismaleimide (M2) solutions. Unlike the DA polymers mentioned above, this polymer was a nonreversibly crosslinked thermoset containing free furan groups. The DA adducts formed as additional crosslinks only when the M2 solution was applied. For this thermoset with a flexural strength σflv of 73 MPa and interlaminar fracture toughness GIc of 920 Jm−2, the application of a solvent slightly assists the healing (recovery efficiency of the fracture load R(LCT) ∼30%) by forming physical crosslinks, such as chain entanglements, while the application of the M2 solution significantly enhances the healing ability. The healing efficiency increased with the concentration of the M2 solution and reached R(LCT) ∼70% at the optimal concentration. The authors also showed that this method is applicable to highly rigid fiber-reinforced composites of this polymer. Zeng et al [44] prepared bio-based healing polymers by DA reactions between poly(2,5-furandimethylene succinate) and M2 and demonstrated their healing with the assistances of M2 solutions. By keeping the maleimide to furan (M/F) ratio at less than one, the excess amount of furan in these polymers can be used for healing. The polymer with a low crosslink density (M/F = 1/6) was a soft elastomer with an autonomous healing ability, whereas the polymer with a high crosslink density (M/F = 1/2) hardly healed even with the M2 solution treatment. The maximum effect of the M2 solution was achieved with the polymer with a middle crosslink density (M/F = 1/4), which clearly indicated that even in a swollen state, polymer healing required a certain level of molecular mobility to activate the dynamic bonds. The authors also showed the remarkable effect of the chemical structure of M2 on the healing ability of these polymers [45].
Water- and aqueous solution-triggered healing
Healing induced by water swelling
Over the past decade, tremendous studies have been reported on self-healing hydrogels [46, 47]. Owing to the intrinsic water that maintains the molecular mobility of crosslinked polymers, various hydrogels with dynamic bonds exhibit fast and autonomous healing abilities. However, most research efforts on autonomous healing gels have concentrated on gels with fracture stress values from tens to hundreds of kPa because the dynamic nature of the polymer chain is restricted in hard and/or tough gels [47]. An autonomous self-healing hydrogel [28] that overcame this limitation was already discussed in section above.
However, the excellent healing ability of soft gels is valuable for designing healing plastic materials (Table 4). Xing et al. [48] focused on the mechanical property changes in hydrogels depending on their water content and utilized this swelling-deswelling transition to create a self-healing of a plastic material. Because of the dynamic crosslinks due to hydrogen bonding interactions, poly(2-acrylamido-2-methyl-1-propanesulfonic acid) (PAMPSA) forms a relatively weak but autonomously healable gel (σf = 25 kPa, E = 22 kPa at a water content of ~47 wt%), whereas PAMPSA becomes a hard but nonhealable plastic in the dry state (σfv = 20 MPa, Ev = 387 MPa at a water content of ~9 wt%). The dried, plastic PAMPSA recovered a high mechanical strength after healing under a 100% relative humidity and re-drying. The dried PAMPSA was also healed by spraying water onto the cut surfaces and keeping the material under 100% relative humidity. Kakuta et al. [49] applied the swelling-deswelling process to heal a xerogel possessing host (β-cyclodextrin, βCD) and guest (adamantane) molecules. The xerogel recovered σfh = 2.8 MPa (88% of the initial value) by dropping small amounts of water onto the fractured surfaces. The authors also achieved excellent adhesion ability with an adhesive strength of 5.1 MPa through this host–guest interaction. Additionally, the host–guest interaction between βCD and adamantane was also applied to a conductive composite containing single-walled carbon nanotubes [50]. With the assistance of a drop of water, this composite quantitatively recovered its mechanical properties and conductivity (σfh ≈ σfv = 4 MPa, conductivity: γh ≈ γv = 0.15 s m−1 at 50–60% relative humidity). Li et al [51] demonstrated that a catechol-containing polymer crosslinked by complexation between catechol and Ca2+ (or Mg2+) can be effectively healed in seawater rather than pure water. Because of intense swelling, the polymer was deformed and damaged in water, which prevented efficient healing. In contrast, the higher osmotic pressure of seawater led to a lower level of swelling, which was required to maintain the molecular mobility and reform the metal-catechol complexes. By applying seawater to the damaged point and drying for 1 day, this polymer healed and recovered σfh of 4.2 MPa and Th of 5.3 MJ m−3 with little shape change (virgin values: σfv = 3.5 MPa, Tv = 6.8 MJ m−3).
Autonomous healing of polymers submerged in water
Further swelling limitations have another advantage for healing polymers underwater-related conditions. If we can suppress the swelling ratio of a dynamic polymer to the level required to prevent structural deformation and maintain minimal mechanical property changes but still ensure molecular mobility for self-healing, we can use this material underwater, which is where the material displays an autonomous healing ability. Kim et al. [52] developed an undersea healing polymer by this strategy. A networked polyacrylate crosslinked by tetrahedral boronate ester bonds exhibited a low water absorption (~2 wt%) and relatively high strength (σfv = 2.3 MPa) under seawater with long-term stability. This polymer also displayed an in situ healing ability with a high efficiency (σfh ≈ σfv). These properties were not achieved by catechol-metal (Ca2+) coordination crosslinking. The dispersion of nonionic boronate ester crosslinks in a hydrophobic polymer probably contributes to this remarkable combination of properties. Xia et al [53] reported another type of catechol polymer with a spontaneous healing ability under seawater. A lipophilic hyperbranched polyurethane (HBPU) with carboxyl and catechol end groups was prepared and dynamically crosslinked by catechol–Fe3+ coordination. In artificial seawater with pH = 8.3, the tris-coordination complex was the main component [54], and the dynamics between coordination–dissociation were maintained. As a result, the material was saturated with a water absorption of ~20 wt% in seawater and behaved as an elastomer (σfv = 2.5 MPa, εfv = 2900%) with a healing ability (R(σfv) = 87%). The same group also reported autonomous healing polymers in acidic water [55] and water at pH values of 7 and 9 [56] with similar HBPU-based structures.
Chemical control-assisted healing using aqueous solutions
Some dynamic bonds are susceptible to aqueous solutions, including saline, acids and bases, which trigger activation and chemical equilibrium shifts. Chemical control of dynamic bonds has been demonstrated to be valuable in self-healing. Ahn et al. [57] reported acid-triggered healing of poly(meth)acrylates with triethylsilyl-protected catechol groups in their side chain. Immersion of these polymers in acidic water (pH = 3) led to deprotection and activation of the catechol groups on the surface. When the surfaces of this material were in contact, the catechol groups on the surfaces initiated healing by forming interfacial hydrogen bonds and, subsequently, other attractive interactions. The rigid methacrylate polymer (Ev = 350 MPa) recovered a fracture stress σfh of ∼4 MPa (from σfv = 6 MPa) after an acidic water treatment, whereas the samples did not show a healing ability in neutral or basic water.
Reisch et al [58] demonstrated that a polyelectrolyte complex of a polyanion, Poly-, and polycation, Poly+, had a healing ability with the assistance of NaCl solutions. The complex of poly(acrylic acid) and poly(allylamine hydrochloride) quantitatively recovered its strength (σfh ≈ σfv = 3.2 MPa) by immersion in a 2.5 M NaCl solution and subsequent conditioning in a 0.15 M NaCl solution. The healing mechanism was proposed (eq. 1).
As the salt concentration increases, the equilibrium of eq. 1 shifts to the right, which would lead to a higher chain mobility and effective interdiffusion of the chain molecules across the broken surfaces to facilitate reinteractions between the polyelectrolytes. A similar mechanism was demonstrated to work in another tough hydrogel with a tensile strength of 3.7 MPa and toughness of 14.8 MJ m−3, although the healing efficiency of the toughness R(T) was 66% [59]. Zheng et al. [60] developed an acid-triggered healing gel of poly(acrylamide-co-acrylic acid). In the presence of Fe3+, the polymer formed a gel at a weakly acidic pH (pH = 4.45) due to the formation of a stable tris-carboxyl−Fe3+ complex. With the decreasing pH, the carboxyl groups were protonated, and the stability of the complex decreased. Eventually, the gel became a sol at pH ≤ 1. This reversible sol−gel transition facilitated healing of the gel. When a drop of 1 M HCl solution was first applied to the cut sample to soften and activate the fracture surfaces and was followed by swelling in a 0.1 M FeCl3 solution for recrosslinking and pure water for mechanical enhancement, the gel recovered a σfh of 4.5 MPa and εfh of 480% (initial values: σfv = 6.2 MPa, εfv = 520%).
Boronic acid derivatives, such as boroxine, trigonal boronic ester, and tetrahedral boronate ester bonds, are fascinating dynamic bonds due to their kinetics, which are easily controllable by their pKa values, as well as conditional factors including the temperature, humidity, pH, and addition of Lewis bases [61, 62]. In refs. 52 and 56 mentioned above, boronate ester bonds were used as stable dynamic bonds in seawater to develop in situ healing polymers. On the other hand, Cash et al. [63] and Lai et al. [64] developed healing polymers triggered by hydrolysis (hydration/dehydration). Cash et al. [63] incorporated trigonal boronic ester bonds into a hydrophobic network polymer. This polymer was healable by placing it in a chamber with 85% relative humidity after dabbing it with water. During this process, the trigonal boronic ester bonds exposed on the surfaces were hydrolyzed and facilitated the network rearrangement. After the drying step to shift the equilibrium to the ester bond formation again, the sample recovered σfh ∼ 4 MPa and εfh ∼ 50% with a good healing efficiency. Lai et al [64] prepared a poly(dimethylsiloxane) (PDMS) crosslinked by aryl-boroxine and analyzed its healing ability. Even though the wetted surfaces of this polymer healed only partially at room temperature, a full recovery of the mechanical properties (Eh ≈ Ev = 182 MPa, σfh ≈ σfv = 9 MPa) was achieved at a healing temperature of 70 °C along with a water treatment due to its high Tg (65 °C).
Concluding remark
In this review, we first surveyed successful attempts to combine mechanical robustness and an in situ autonomous healing ability in a polymer. Many of these successes were achieved through smart molecular design of the environment around the dynamic bonds and the careful selection of the dynamic bonds. The polymer chain structure around the dynamic bonds determines the chain mobility and kinetics of the dynamic bond and, as a result, plays a crucial role in the healing process. In the latter part of this review, the studies on healing assisted by ubiquitous stimuli were summarized. Such a healing process is a suboptimal choice for practical healing materials, even though it is difficult to achieve at the present time. The properties of the polymer chains, including thermal properties (Tm, Tg, crystallization kinetics), hydrophobicity/hydrophilicity and so on, are critical to realize this type of healing. In addition to an extension of these previous studies attempting to create stiffer, stronger and/or tougher self-healing polymers, the remaining challenges include the development of healing materials triggered by unprecedented ubiquitous stimuli such as visible light, moisture or low temperature. To this end, we believe that the total design of the polymer structure, not only the dynamic bond, will contribute to the development of novel strategies for mechanically robust healing polymers that are applicable to a wide range of polymer materials.
References
Bergman DS, Wudl F, Mendable polymers. J Mater Chem. 2008;18:41–62.
Chen X, Dam MA, Ono K, Mal A, Shen H, Nutt SR. A thermally re-mendable cross-linked polymeric material. Science. 2002;295:1698–702.
Canadell J, Goossens H, Klumperman B. Self-healing materials based on disulfide links. Macromolecules. 2011;44:2536–41.
Ying H, Zhang Y, Cheng J. Dynamic urea bond for the design of reversible and self-healing polymers. Nat Commun. 2014;5:3218.
Zheng P, McCarthy TJ. A surprise from 1954: Siloxane equilibration is a simple, robust, and obvious polymer self-healing mechanism. J Am Chem Soc. 2012;134:2024–7.
Deng G, Tang C, Li F, Jiang H, Chen Y. Covalent cross-linked polymer gels with reversible sol−gel transition and self-healing properties. Macromolecules. 2010;43:1191–4.
Capelot M, Montarnal D, Tournilhac F, Leibler L. Metal-catalyzed transesterification for healing and assembling of thermosets. J Am Chem Soc. 2012;134:7664–7.
Lu Y-X, Guan Z. Olefin metathesis for effective polymer healing via dynamic exchange of strong carbon–carbon double bonds. J Am Chem Soc. 2012;134:14226–31.
García JM, Jones GO, Virwani K, McCloskey BD, Boday DJ, Huurne GM. Recyclable, strong thermosets and organogels via paraformaldehyde condensation with diamines. Science. 2014;344:732–5.
Yuan C, Rong MZ, Zhang MQ, Zhang ZP, Yuan YC. Self-healing of polymers via synchronous covalent bond fission/radical recombination. Chem Mater. 2011;23:5076–81.
Imato K, Takahara A, Otsuka H. Self-healing of a cross-linked polymer with dynamic covalent linkages at mild temperature and evaluation at macroscopic and molecular levels. Macromolecules. 2015;48:5632–9.
Cordier P, Tournilhac F, Soulié-Ziakovic C, Leibler L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature. 2008;451:977–80.
Haraguchi K, Uyama K, Tanimoto H. Self-healing in nanocomposite hydrogels. Macromol Rapid Commun. 2011;32:1253–8.
Burnworth M, Tang L, Kumpfer JR, Duncan AJ, Beyer FL, Fiore GL. Optically healable supramolecular polymers. Nature. 2011;472:334–7.
Nakahata M, Takashima Y, Yamaguchi H, Harada A. Redox-responsive self-healing materials formed from host–guest polymers. Nat Commun. 2011;2:511.
Zhang M, Xu D, Yan X, Chen J, Dong S, Zheng B. Self-healing supramolecular gels formed by crown ether based host–guest interactions. Angew Chem Int Ed. 2012;51:7011–5.
Burattini S, Colquhoun MH, Fox DJ, Friedmann D, Greenland WB, Harris FPJ. A self-repairing, supramolecular polymer system: healability as a consequence of donor–acceptor π–π stacking interactions. Chem Commun. 2009;0:6717–9.
Huynh T-P, Sonar P, Haick H. Advanced materials for use in soft self-healing devices. Adv Mater. 2017;29:1604973–86.
Thakur VK, Kessler MR. Self-healing polymer nanocomposite materials: a review. Polymer. 2015;69:369–83.
Urdl K, Kandelbauer A, Kern W, Müller U, Thebault M, Zikulnig-Rusch E. Self-healing of densely crosslinked thermoset polymers—a critical review. Prog Org Coat. 2017;104:232–49.
Das A, Sallat A, Böhme F, Suckow M, Basu D, Wießner S Ionic modification turns commercial rubber into a self-healing material. ACS Appl Mater Interfaces. 2015;7:20623–30.
Yoshie N, Saito S, Oya N. A thermally-stable self-mending polymer networked by Diels–Alder cycloaddition. Polymer. 2011;52:6074–9.
Wu J, Cai L-H, Weitz DA, Tough self-healing elastomers by molecular enforced integration of covalent and reversible networks. Adv Mater. 2017;29:1702616–23.
Chen Y, Kushner AM, Williams GA, Guan Z. Multiphase design of autonomic self-healing thermoplastic elastomers. Nat Chem. 2012;4:467–72.
Chen Y, Guan Z. Multivalent hydrogen bonding block copolymers self-assemble into strong and tough self-healing materials. Chem Commun. 2014;50:10868–70.
Kim S-M, Jeon H, Shin S-H, Park S-A, Jegal J, Hwang SY. Superior toughness and fast self-healing at room temperature engineered by transparent elastomers. Adv Mater. 2018;30:1705145–52.
Aguirresarobe RH, Martin L, Fernandez-Berridi MJ, Irusta L. Autonomic healable waterborne organic-inorganic polyurethane hybrids based on aromatic disulfide moieties. Express Polym Lett. 2017;11:266–77.
He X, Zhang C, Wang M, Zhang Y, Liu L, Yang W. An electrically and mechanically autonomic self-healing hybrid hydrogel with tough and thermoplastic properties. ACS Appl Mater Interfaces. 2017;9:11134–43.
Aida T, Meijer EW, Stupp SI. Functional supramolecular polymers. Science. 2012;335:813–7.
Bose RK, Hohlbein N, Garcia SJ, Schmidt AM, Zwaag S, Connecting supramolecular bond lifetime and network mobility for scratch healing in poly(butyl acrylate) ionomers containing sodium, zinc and cobalt. Phys Chem Chem Phys. 2014;17:1697–704.
Susa A, Bose RK, Grande AM, van der Zwaag S, Garcia SJ. Effect of the dianhydride/branched diamine ratio on the architecture and room temperature healing behavior of polyetherimides. ACS Appl Mater Interfaces. 2016;8:34068–79.
Yanagisawa Y, Nan Y, Okuro K, Aida T. Mechanically robust, readily repairable polymers via tailored noncovalent cross-linking. Science. 2018;359:72–6.
Yoshie N, Watanabe M, Araki H, Ishida K. Thermo-responsive mending of polymers crosslinked by thermally reversible covalent bond: Polymers from bisfuranic terminated poly (ethylene adipate) and tris-maleimide. Polym Degrad Stab. 2010;95:826–9.
Rodriguez ED, Luo X, Mather PT. Linear/network poly(ε-caprolactone) blends exhibiting Shape Memory Assisted Self-Healing (SMASH). ACS Appl Mater Interfaces. 2011;3:152–61.
Du P, Liu X, Zheng Z, Wang X, Joncheray T, Zhang Y. Synthesis and characterization of linear self-healing polyurethane based on thermally reversible Diels–Alder reaction. RSC Adv. 2013;3:15475–82.
Liu J, Liu J, Wang S, Huang J, Wu S, Tang Z. An advanced elastomer with an unprecedented combination of excellent mechanical properties and high self-healing capability. J Mater Chem A. 2017;5:25660–71.
Huang L, Yi N, Wu Y, Zhang Y, Zhang Q, Huang Y. Multichannel and repeatable self-healing of mechanical enhanced graphene-thermoplastic polyurethane composites. Adv Mater. 2013;25:2224–8.
Yang Y, Pei Z, Zhang X, Tao L, Wei Y, Ji Y. Carbon nanotube–vitrimer composite for facile and efficient photo-welding of epoxy. Chem Sci. 2014;5:3486–92.
Zhang H, Zhao Y. Polymers with dual light-triggered functions of shape memory and healing using gold nanoparticles. ACS Appl Mater Interfaces. 2013;5:13069–75.
Kohlmeyer RR, Lor M, Chen J. Remote, local, and chemical programming of healable multishape memory polymer nanocomposites. Nano Lett. 2012;12:2757–62.
Michal BT, Jaye CA, Spencer EJ, Rowan SJ. Inherently photohealable and thermal shape-memory polydisulfide networks. ACS Macro Lett. 2013;2:694–9.
Hong G, Zhang H, Lin Y, Chen Y, Xu Y, Weng W. Mechanoresponsive healable metallosupramolecular polymers. Macromolecules. 2013;46:8649–56.
Peterson AM, Jensen RE, Palmese GR. Room-temperature healing of a thermosetting polymer using the diels−alder reaction. ACS Appl Mater Interfaces. 2010;2:1141–9.
Zeng C, Seino H, Ren J, Hatanaka K, Yoshie N. Bio-based furan polymers with self-healing ability. Macromolecules. 2013;46:1794–802.
Zeng C, Seino H, Ren J, Hatanaka K, Yoshie N. Self-healing biobased furan polymers cross-linked with various bis-maleimides. Polymer. 2013;54:5351–7.
Phadke A, Zhang C, Arman B, Hsu C-C, Mashelkar RA, Lele AK. Rapid self-healing hydrogels. Proc Natl Acad Sci USA. 2012;109:4383–8.
Taylor DL. & in het Panhuis, M. Self-healing hydrogels. Adv Mater. 2016;28:9060–93.
Xing X, Li L, Wang T, Ding Y, Liu G, Zhang G. A self-healing polymeric material: from gel to plastic. J Mater Chem A. 2014;2:11049–53.
Kakuta T, Takashima Y, Sano T, Nakamura T, Kobayashi Y, Yamaguchi H. Adhesion between semihard polymer materials containing cyclodextrin and adamantane based on host–guest interactions. Macromolecules. 2015;48:732–8.
Zhang D-L, Ju X, Li L-H, Kang Y, Gong X-L, Li B-J. An efficient multiple healing conductive composite via host–guest inclusion. Chem Commun. 2015;51:6377–80.
Li J, Ejima H, Yoshie N. Seawater-assisted self-healing of catechol polymers via hydrogen bonding and coordination interactions. ACS Appl Mater Interfaces. 2016;8:19047–53.
Kim C, Ejima H, Yoshie N. Non-swellable self-healing polymer with long-term stability under seawater. RSC Adv. 2017;7:19288–95.
Xia NN, Xiong XM, Wang J, Rong MZ, Zhang MQ. A seawater triggered dynamic coordinate bond and its application for underwater self-healing and reclaiming of lipophilic polymer. Chem Sci. 2016;7:2736–42.
Holten-Andersen N, Harrington MJ, Birkedal H, Lee BP, Messersmith PB, Lee KYC. pH-induced metal-ligand cross-links inspired by mussel yield self-healing polymer networks with near-covalent elastic moduli. Proc Natl Acad Sci USA. 2011;108:2651–5.
Xia NN, Xiong XM, Rong MZ, Zhang MQ, Kong F. Self-healing of polymer in acidic water toward strength restoration through the synergistic effect of hydrophilic and hydrophobic interactions. ACS Appl Mater Interfaces. 2017;9:37300–9.
Nan Xia N, Zhi Rong M, Qiu Zhang M. Stabilization of catechol–boronic ester bonds for underwater self-healing and recycling of lipophilic bulk polymer in wider pH range. J Mater Chem A. 2016;4:14122–31.
Ahn BK, Lee DW, Israelachvili JN, Waite JH. Surface-initiated self-healing of polymers in aqueous media. Nat Mater. 2014;13:867–72.
Reisch A, Roger E, Phoeung T, Antheaume C, Orthlieb C, Boulmedais F. On the benefits of rubbing salt in the cut: self-healing of saloplastic PAA/PAH compact polyelectrolyte complexes. Adv Mater. 2014;26:2547–51.
Luo F, Sun TL, Nakajima T, Kurokawa T, Zhao Y, Sato K. Oppositely charged polyelectrolytes form tough, self-healing, and rebuildable hydrogels. Adv Mater. 2015;27:2722–7.
Zheng SY, Ding H, Qian J, Yin J, Wu ZL, Song Y. Metal-coordination complexes mediated physical hydrogels with high toughness, stick–slip tearing behavior, and good processability. Macromolecules. 2016;49:9637–46.
Wu X, Li Z, Chen X-X, Fossey JS, James TD, Jiang Y-B. Selective sensing of saccharides using simple boronic acids and their aggregates. Chem Soc Rev. 2013;42:8032.
Brooks WLA, Sumerlin BS. Synthesis and applications of boronic acid-containing polymers: from materials to medicine. Chem Rev. 2016;116:1375–97.
Cash JJ, Kubo T, Bapat AP, Sumerlin BS. Room-temperature self-healing polymers based on dynamic-covalent boronic esters. Macromolecules. 2015;48:2098–106.
Lai J-C, Mei J-F, Jia X-Y, Li C-H, You X-Z, Bao Z. A stiff and healable polymer based on dynamic-covalent boroxine bonds. Adv Mater. 2016;28:8277–82.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kim, C., Yoshie, N. Polymers healed autonomously and with the assistance of ubiquitous stimuli: how can we combine mechanical strength and a healing ability in polymers?. Polym J 50, 919–929 (2018). https://doi.org/10.1038/s41428-018-0079-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-018-0079-x
This article is cited by
-
Development of heat-responsive adhesive materials that are stable during use and quickly deteriorate during dismantling
Polymer Journal (2024)
-
3D-printed self-healing hydrogels via Digital Light Processing
Nature Communications (2021)
-
Biobased and mechanically stiff lignosulfonate/cationic-polyelectrolyte/sugar complexes with coexisting ionic and covalent crosslinks
Polymer Journal (2021)
-
A novel kind of room temperature self-healing poly(urethane-urea) with robust mechanical strength based on aromatic disulfide
Journal of Polymer Research (2021)