Abstract
Capturing CO2 from various sources, such as postcombustion exhaust gases and the atmosphere, is essential for a sustainable human society. Effective CO2 separation materials such as adsorbents and membranes are of utmost importance in efficient CO2 capture. This short review is focused on CO2 separation materials consisting of hydrogel particles. The first chapter introduces stimuli-responsive micro- and nanogel particles that reversibly absorb CO2 in response to thermal stimuli. The development of temperature-responsive hydrogel films comprising gel particles for reversible CO2 capture is introduced. The importance of choosing amines with optimal pKa values for efficient CO2 capture from various sources is explained in detail. The assembly of CO2 separation membranes consisting of amine-containing hydrogel particles is introduced in the final chapter. The paper highlights the promise of separation materials consisting of hydrogel particles for efficient CO2 capture from postcombustion gases and air and the prospects for further advances in this area.
Similar content being viewed by others
Introduction
The development of efficient CO2 capture processes is crucial for rebalancing the global carbon cycle [1,2,3]. Chemical absorption processes using aqueous amine solutions to absorb CO2 have been the standard industrial processes for CO2 capture. However, high temperatures and large amounts of energy are needed to regenerate the solutions that absorb CO2, which limits the use of this process for carbon capture from sources with low concentrations of CO2, such as postcombustion gases (~10 vol% CO2) and air (400 ppm CO2), for economic reasons. Thus, the development of materials and processes that do not require high temperatures and large energy input is of great importance.
Several porous solid sorbents, such as activated carbon [4], metal-organic frameworks (MOFs) [4, 5], zeolites [4, 5], and physically or chemically supported amine polymers [4,5,6,7,8,9] that can be regenerated under relatively mild conditions, have been proposed to address the aforementioned problems. These sorbents show large absorption/adsorption capacities of up to ~3 mmol CO2 g−1 [4,5,6,7,8,9] due to the large surface areas of the pores in the sorbents. However, most porous sorbents cannot be used without drying the gases before CO2 capture because water adsorption competes with CO2 and/or capillary condensed water to prevent gas diffusion in the pores [4,5,6,7,8,9]. Thus, the development of solid sorbents that work in wet conditions and can be regenerated under mild conditions is of great interest.
The membrane separation process has been developed as an alternative approach for CO2 capture [10,11,12]. Ideal membranes showing highly selective permeation of CO2 enable CO2 capture without heat. To realize the ideal membrane, ultrathin membranes without defects must be prepared that allow rapid sorption, diffusion, and desorption of CO2 and minimal sorption of competing gases [10]. Defect-free thin membranes consisting of graphene oxide [13], metal–organic frameworks [14], polymers [15,16,17], zeolites [18], organic/inorganic interpenetrating networks [19], and liquid water [20] have been reported. However, further improvements in their CO2 permeabilities and selectivities are needed [21].
This short review is focused on the development of hydrogel particles for CO2 capture. Hydrogels are aqueous materials solidified by polymer networks. Diffusion of the solutes in the gels is much faster than it is in other solids and comparable to those of liquids [22,23,24], so it is attractive for CO2 absorption and CO2 separation membranes [25]. The chemical properties of gels, such as the equilibrium and kinetics for CO2 absorption, can be tuned by introducing appropriate functional groups into the hydrogels and designing the structures of the polymers [26,27,28]. The physical properties of gels, such as the viscoelasticity, flexibility, adaptability, deformability, and diffusivity, can also be tailored by selecting the properties and structures of the polymers [26,27,28].
Temperature-responsive hydrogels with various functions have been designed with poly-N-isopropyl acrylamide (pNIPAm) as the network polymer. pNIPAm is highly hydrated at low temperatures, but above the lower critical solution temperature (LCST), the nonpolar side chains dehydrate and aggregate, which causes precipitation of the polymer [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44]. Hydrogels composed of pNIPAm swell at temperatures below the LCST but shrink due to dehydration when the temperature is raised above the LCST. Various stimulus-responsive gels have been proposed that use these volume phase transitions (VPTs). In volume phase transitions of bulk gels, dense polymer layers, named the skin layers, formed on the surface of the gel during VPT restricts mass transfer of water from inside to outside the gels. Thus, the VPT of the entire gel takes a long time [45]. On the other hand, hydrogel particles with particle sizes ranging from tens of nanometers to micrometers have very large surface areas relative to the volume of each gel, so they show rapid VPTs [46].
CO2 absorbents that reversibly absorb CO2 in response to small temperature changes have been developed to take advantage of the quick temperature-responsive changes of nano- to micrometer-sized hydrogel particles [26, 27, 47, 48]. The flexibilities and shape adaptabilities of the gel particles enabled the formation of temperature-responsive hydrogel films on the surfaces of various substrates for reversible CO2 capture [26, 49, 50]. It has also been reported that polymerization as well as the combinations and populations of functional monomers in particles can be engineered to optimize adsorption for efficient CO2 capture from various CO2 sources [26, 27, 51]. The deformabilities of the particles enabled assembly of defect-free nanometer-thick CO2 separation membranes consisting of the particles. The development of each material is reviewed in this manuscript (Fig. 1) [21].
Stimuli-responsive micro- and nanogel particles that reversibly adsorb CO2 in response to thermal stimuli
Aqueous amine solutions (such as ethanol amine) have been tested for CO2 capture from point sources such as fossil fuel power plants [52, 53]. The amine solutions absorbed CO2 at low temperatures (~40 °C) via an exothermic reaction and desorbed the CO2 upon heating (>120 °C) [54,55,56,57]. Although this amine process, called a chemical absorption process, exhibits a high capacity for CO2 capture, a high energy consumption equivalent to 20–40% of that for a typical power plant output prevented sustainable use of this process [55,56,57,58,59,60]. Furthermore, amines are easily oxidized and volatilized during high-temperature regeneration and need must recovered to avoid air pollution [61]. Thus, nonvolatile sorbents that desorb CO2 at low temperatures (<100 °C) must be developed [54, 62].
In the bloodstreams of living animals, a protein called hemoglobin plays a key role in efficient CO2 transport in an ambient environment. The dynamic and reversible shifts of the pKa values of Brønsted acids and bases in hemoglobin are crucial for energy-efficient CO2 transport (Bohr effect) [63, 64]. In the lungs, the pKa of the ammonium and imidazolium ions in/on hemoglobin decrease due to drastic conformational changes of the polypeptides caused by oxygen binding to heme. The pKa shift triggers the release of protons into the blood, lowers the pH of the blood, and drives efficient CO2 release from the lungs without heating the system. When hemoglobin reaches a capillary blood vessel where the concentration of oxygen is low, dissociation of oxygen from the heme triggers a change in the conformation back to the original structure. Subsequently, the pKa values of the ammonium and imidazolium ions in/on the hemoglobin increase back to their original values. The high pKa of the ammonium ions on the hemoglobin enables efficient CO2 capture from the tissues around the capillaries in the form of ammonium and imidazolium bicarbonates.
Motivated by the process of reversible ion capture instigated by the structural transitions in proteins, the research conducted by Shinkai and his team is of significance [65, 66]. It has also been reported that temperature-responsive pNIPAm hydrogel films, nanoparticles (GPs), and fibers comprising functional groups reversibly captured targets such as ionic dyes [67, 68], drugs [40, 69], peptides [33], proteins [70, 71], nucleotides [30], and cells [72] during heating and cooling cycles. The ability of pNIPAm GPs to capture target ions [37], peptides [33], and proteins [35] can be enhanced by polymerizing the GPs in the presence of target molecules that serve as templates. During molecular/ion imprinting polymerization, the three-dimensional structure of the target molecule is imprinted into the polymer matrix to generate complementary binding sites within the network after extraction [37, 73, 74]. The imprinted GPs showed greater affinity shifts in response to phase transitions than the nonimprinted GPs [26].
Recently, it was reported that the pKa values of Brønsted acids and bases incorporated in pNIPAm shifted reversibly in response to phase transitions of the polymers, which were induced by cooling-heating cycles [30, 33, 70,71,72, 75]. pNIPAm-based GPs that show large and reversible pKa shifts can be prepared by the “proton imprinting” [37] and “microenvironment imprinting” [31] strategies. When acidic monomers such as acrylic acids are protonated during the polymerization process, the protonated acids are incorporated into the hydrophobic domains of the growing dehydrated-pNIPAm GPs and are subsequently stabilized with cross-linkers. As a result, acids with high pKas were imprinted in the GPs. When the degree of cross-linking was not too high, the structures of the high-pKa acidic sites were reversibly denatured by temperature-induced conformational changes of the polymer chains, resulting in large, reversible pKa shifts. The greatest pKa variation range observed in proton-imprinted GPs was significantly larger than that of the nonimprinted particles (the particles polymerized with deprotonated acrylic acids) and enzymes that transport protons in the blood [64]. The pKa shift enabled reversible capture and release of the target ions [31, 37, 47, 76, 77].
Taking advantage of the reversible pKa shifts of the pNIPAm GPs, materials that reversibly absorb and desorb CO2 via phase transitions induced by small temperature changes were synthesized (Fig. 2) [47, 76, 77]. Amine-containing monomers, such as N-[3-(dimethylamino)propyl]methacrylamide (DMAPM), were incorporated into the GPs as functional units to capture CO2 at 30 °C, which is below the volume phase transition temperature (VPTT). Most amines present in the swollen GPs undergo protonation and create ion pairs with the absorbed bicarbonate ions. However, at 75 °C, above the VPTT, shrinkage of the GPs lowers the pKas of the protonated amines (ammonium ions), thereby resulting in almost complete desorption of CO2. The amines in the GPs undergoing VPTs reversibly absorb more CO2 than the DMAPM monomer and polymer without VPTs. This indicates the importance of polymer temperature-responsive phase transitions in determining the degree of absorption.
Development of temperature-responsive hydrogel films comprising microgel particles for reversible CO2 capture
The amount of CO2 reversibly absorbed by amines in the GPs during a small temperature change was greater than those of traditional CO2 absorbents such as ethanolamine [47]. However, the absorption capacity per weight of the absorbent solution was very small because the concentration of GPs in the solution (~0.1 wt%) was much lower than those of traditional amines (20–30 wt%) [47]. As a result, the energy consumed by the GP solution in separating CO2 was significantly larger than those of amine solutions, despite the use of a low temperature for the regeneration process. To improve the energy efficiency, the amount of water had to be dramatically reduced.
Hydrogel films consisting of temperature-responsive GPs (GP films) were developed to prepare energy-efficient CO2 absorbents with high absorption capacities per weight [26, 50]. The films were prepared by simply casting a solution of the temperature-responsive GPs onto the surface of a solid substrate. The films combined the best of the high stoichiometric absorption efficiencies of the GP solutions with the large absorption capacities per weight of the solid sorbents. Furthermore, it was revealed that the GP films responded much faster to external stimuli than monolithic hydrogel films (Fig. 3) [26, 50].
Fabrication methods for energy-efficient temperature-responsive GP films with high absorption capacities and reversible CO2 absorption-desorption characteristics in response to temperature [50]
Tuning the pK as of amines in the particles to design thermally responsive particle films that reversibly absorb large amounts of CO2 from postcombustion gases
The capacity for reversible absorption by a GP film can be enhanced with the use of more DMAPM. However, GPs with larger amounts of DMAPM do not always release CO2 completely during heating [26]. In the fabrication of thermally responsive GP films capable of capturing and liberating substantial quantities of CO2 within a confined temperature range (30–75 °C), meticulous optimization of the pKa values for the ammonium ions in the GPs at both the CO2 absorption (30 °C) and release (75 °C) temperatures is required [26]. At 30 °C, a sufficiently high pKa value of the ammonium ion is needed to drive the reaction between the amine and CO2 to form ammonium bicarbonate for efficient CO2 capture. Conversely, a sufficiently low pKa is needed to protonate bicarbonate at 75 °C for efficient release of the CO2.
Yue et al. reported that the pKa values of GPs containing DMAPM were readily adjusted to the desired level via four methods [26]. These were: (1) Controlling the VPTT of the GPs above and close to the absorbing temperature (30 °C) so the phase transition induced a large pKa shift over a wide temperature range above the VPTT. The VPTT is increased by incorporating larger amounts of amines in the particles but decreased by replacing the NIPAm with more hydrophobic monomers such as N-tert-butylacrylamide (TBAm). (2) Controlling the sizes of the GPs, since smaller GPs show higher pKa values because the proportion of amines incorporated in the polar microenvironment on the exterior of the gel particles increases for smaller GPs. (3) Regulating the swelling ratio, which impacts the extent of the pKa fluctuations and, in turn, directly affects the pKa of the GPs. The particles with larger amounts of cross-linker showed less swelling during the cooling process, which limited the pKa increase for the of ammonium ions in the GPs. (4) Controlling the imprinted microenvironments of amines in the GPs. Amines in the GPs that were synthesized in acidic environments showed relatively high pKa values because the amines were protonated and thus incorporated into the hydrophilic microenvironment. On the other hand, amines in the GPs synthesized in a basic environment showed relatively high pKas because the amines were protonated and thus incorporated into the hydrophilic microenvironment.
The optimal GPs comprising the designed proportions of 55 mol% DMAPM, 43 mol% TBAm, and 2% N,N’-methylenebis(acrylamide) (BIS), showed a high capture capacity (68 mL CO2/g dry GPs, 3.0 mmol CO2/g dry GPs) for simulated postcombustion gases (10% CO2, water-saturated gas), although the regeneration temperature was as low as 75 °C. The GP film comprising these GPs is expected to become a new CO2 absorbent used to separate CO2 from large point sources such as fire power plants with low energy costs.
Importance of optimizing the pK a values of amines in the particles for efficient CO2 capture from various CO2 sources
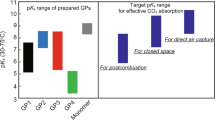
Honda et al. reported the design principles for temperature-responsive hydrogels used with various CO2 concentrations [27]. Computational predictions and experimental observations indicated that the pKas of the ammonium ions in the hydrogel particles must be tuned based on the concentration of CO2. It was shown that a pKa of approximately 8 was sufficient to absorb relatively concentrated CO2 (~10%), such as that in postcombustion exhaust gas, whereas a pKa of 10 or more was needed to absorb dilute CO2 efficiently, such as that in the atmosphere (400 ppm).
Tuning the structures, combinations, and populations of functional groups in the hydrogels
Hydrogels with various functional groups have been proposed for use as CO2 absorbents. Early studies reported the effects of the polymerization conditions and the comonomer compositions on CO2 absorption by hydrogel particles using DMAPM, a commercially available tertiary amine-containing monomer [47]. NIPAm and TBAm were used as comonomers to achieve ideal VPTs [26]. A small amount of BIS, a water-soluble cross-linker, was added to adjust the degree of swelling of the gel particles [26].
Rieger et al. proposed a microgel-based CO2 absorbent in which acrylamide with 2,2,6,6-tetramethyl-piperidine, which is a cyclic secondary amine, was copolymerized with NIPAm and BIS [78].The particles showed a VPTT with a large shift in pKa from 8.6 (at room temperature) to 6.7 (at 85 °C) with increased temperature. An aqueous solution of the gel particles reversibly and quickly released CO2. Rieger et al. also proposed a temperature-responsive CO2 absorbent consisting of polyethylenimine functionalized with a hydrophobic functional group [79]. Polyethylenimine that was partially acylated with butyric anhydride (b-PEI) showed an LCST together with a reversible pH shift in water. The solution showed improved CO2 release associated with the LCST behavior of the polymer.
Yue et al. proposed a temperature-responsive hydrogel-based CO2 absorbent composed of polyvinylamine, which is a typical primary polyamine [48]. Microgels with polyvinyl amine (PVAm) were synthesized by copolymerizing N-vinylformamide with pNIPAm and BIS followed by hydrolysis of formamide with strong acids. Temperature-responsive gel particles with high colloidal stabilities and a large amounts of primary amine (3.2 mmol/g) were synthesized by increasing the amount of BIS introduced along with the increased content of the vinylamine.
The design principle for a temperature-responsive hydrogel film consisting of linear polymers was reported by Yue et al. [51]. Reversible CO2 capture by hydrogel films consisting of acylated PVAm copolymers, as well as blended films with the homopolymer of PVAm and 100% acylated PVAm, were compared in detail. The findings indicated that simply by combining with temperature-responsive polymers, the efficiency of reversible CO2 capture by the polyamine films was enhanced. While the homopolymer films prepared through blending demonstrated marginally lower capacities for reversible CO2 absorption compared to their partially acylated copolymer counterparts, the strategy of polymer blending remains appealing due to the ease of creating high-performance films merely by combining readily available and/or inexpensive materials.
Assembly of CO2 separation membranes consisting of amine-containing hydrogel particles
CO2-separation membranes are of great interest for energy-efficient CO2 capture because they enable complete CO2 separation without heat consumption [10, 11, 80]. To achieve ideal membranes, materials that allow fast CO2 absorption, diffusion, and release should be developed. Hydrogels are attractive options for separating CO2 due to their high solute diffusivities, which are comparable to those of liquids [22,23,24,25].
Taniguchi and colleagues found that hydrated amine-containing membranes made of poly(vinyl alcohol) (PVA) and 3-(1-piperazinyl)-1,2-propanediol (PzPD) exhibited highly selective permeation of CO2 against hydrogen [81]. Amine-containing PVA membranes with thicknesses of 3–10 µm were fabricated by casting an aqueous solution of PVA and the amine on a flat surface followed by drying. The high concentration and diffusivity of the bicarbonate ions in the membrane enabled selective CO2 permeation.
Hoshino et al. proposed that ultrathin defect-free hydrogel membranes (hydrogel nanomembranes) for CO2 separation could be prepared by coating an aqueous suspension of amine-containing colloidal hydrogel particles (GPs) onto a porous support [21]. Given that the pores are hydrophilic and the pore dimensions are smaller than the microgel hydrodynamic diameter, planar, coarse, and microstructured porous membranes can be used as substrates. The deformable characteristics of the GPs facilitated autonomous assembly of defect-free CO2 separation layers with thicknesses of 30 to 50 nm, which originated from deformed discoidal entities approximately 15 nm thick. (Fig. 4)
Illustration of ultrathin, defect-free hydrogel membranes prepared by spray coating of GPs for CO2 separation [21]
Dried films with tertiary amine groups did not exhibit significant CO2 permeance. However, when the membranes were adequately hydrated to form a hydrogel, the CO2 permeance dramatically increased, indicating that free water in the membranes enabled quick bicarbonate ion diffusion. Selective CO2 permeance (850 GPU, αCO2/N2 = 25) against simulated postcombustion gases (10% CO2, water-saturated gas) was observed for the hydrogel nanomembranes contained in amine (DMAPM)-containing gel particles. In addition, acid-containing microgel membranes, when doped with amines, displayed exceptional CO2 selectivity against simulated postcombustion gases (1010 GPU, αCO2/N2 = 216) as well as in direct air capture (1270 GPU, αCO2/N2 = 2380). The versatile route of fabricating hydrogel nanomembranes via autonomous assembly of deformable GPs will enable large-scale manufacturing of high-performance separation membranes and low-cost carbon capture from postcombustion gases and air.
Prospective
In this paper, we briefly reviewed the recent developments in CO2 separation materials consisting of hydrogel particles. By taking advantage of temperature-responsive shifts in the pKa values of ammonium ions in the amine-containing micro- and nanogel particles induced by quick and reversible volume phase transitions of the particles, CO2 absorbents that reversibly absorb CO2 in response to small temperature stimuli have been developed. The flexibilities and shape adaptabilities of the gel particles enabled the formation of temperature-responsive hydrogel films on the surfaces of various substrates for reversible CO2 capture. The polymerization conditions as well as the combinations and feed ratios of the functional monomers in the particles were optimized for efficient CO2 capture from various sources. The deformability of the gel particles allowed the assembly of defect-free nanometer-thick CO2 separation membranes consisting of amine-containing hydrogel particles. Although durability tests and the development of separation processes are needed, the excellent mass production capability of hydrogel particles in addition to their high functionalities will provide low-cost CO2 separation from postcombustion gases and air.
References
Goeppert A, Czaun M, Prakash GKS, Olah GA. Air as the renewable carbon source of the future: an overview of CO2 capture from the atmosphere. Energy Environ Sci. 2012;5:7833.
Sanz-Perez ES, Murdock CR, Didas SA, Jones CW. Direct capture of CO2 from Ambient Air. Chem Rev. 2016;116:11840.
Bui M, Adjiman CS, Bardow A, Anthony EJ, Boston A, Fennell SBPS. et al. Carbon capture and storage (CCS): the way forward. Energy Environ Sci. 2018;11:1062
Choi S, Drese JH, Jones CW. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem. 2009;2:796–854.
Lee KB, Beaver MG, Caram HS, Sircar S. Reversible chemisorbents for carbon dioxide and their potential applications. Ind Eng Chem Res. 2008;47:8048–62.
Choi S, Gray ML, Jones CW. Amine-tethered solid adsorbents coupling high adsorption capacity and regenerability for CO2 capture from ambient air. ChemSusChem. 2011;4:628–35.
Harlick PJE, Sayari A. Applications of pore-expanded mesoporous silica. 5. Triamine grafted material with exceptional CO2 dynamic and equilibrium adsorption performance. Ind Eng Chem Res. 2007;46:446–58.
Serna-Guerrero R, Da’na E, Sayari A. New insights into the interactions of CO2 with amine-functionalized silica. Ind Eng Chem Res. 2008;47:9406–12.
Goeppert A, Czaun M, May RB, Prakash GKS, Olah GA, Narayanan SR. Carbon dioxide capture from the air using a polyamine based regenerable solid adsorbent. J Am Chem Soc. 2011;133:20164–7.
Park HB, Kamcev J, Robeson LM, Elimelech M, Freeman BD. Maximizing the right stuff: the trade-off between membrane permeability and selectivity. Science (1979). 2017;356:eaab0530.
Merkel TC, Lin H, Wei X, Baker R. Power plant post-combustion carbon dioxide capture: an opportunity for membranes. J Membr Sci. 2010;359:126.
Du N, Park HB, Dal-Cin MM, Guiver MD. Advances in high permeability polymeric membrane materials for CO2 separation. Energy Environ Sci. 2012;5:7306.
Kim HW, Yoon HW, Yoon S-M, Yoo BM, Ahn BK, Cho YH, et al. Selective gas transport through few-layered graphene and graphene oxide membranes. Science (1979). 2013;342:91.
Peng Y, Li Y, Ban Y, Jin H, Jiao W, Liu X, et al. Metal-organic framework nanosheets as building blocks for molecular sieving membranes. Science (1979). 2014;346:1356.
Jimenez-Solomon MF, Song Q, Jelfs KE, Munoz-Ibanez M, Livingston AG. Polymer nanofilms with enhanced microporosity by interfacial polymerization. Nat Mater. 2016;15:760.
Chen Y, Ho WSW. High-molecular-weight polyvinylamine/piperazine glycinate membranes for CO2 capture from flue gas. J Membr Sci. 2016;514:376.
Qiao Z, Zhao S, Sheng M, Wang J, Wang S, Wang Z, et al. Metal-induced ordered microporous polymers for fabricating large-area gas separation membranes. Nat Mater. 2018;18:163.
Jeon MY, Kim D, Kumar P, Lee PS, Rangnekar N, Bai P, et al. Ultra-selective high-flux membranes from directly synthesized zeolite nanosheets. Nature. 2017;543:690.
Vendamme R, Onoue S-Y, Nakao A, Kunitake T. Robust free-standing nanomembranes of organic/inorganic interpenetrating networks. Nat Mater. 2006;5:494.
Fu Y, Jiang Y-B, Dunphy D, Xiong H, Coker E, Chou SS, et al. Ultra-thin enzymatic liquid membrane for CO2 separation and capture. Nat Commun. 2018;9:990.
Hoshino Y, Gyobu T, Imamura K, Hamasaki A, Honda R, Horii R, et al. Assembly of defect-free microgel nanomembranes for CO2 separation. ACS Appl Mater Interfaces. 2021;13:30030–8.
Sagle AC, Ju H, Freeman BD, Sharma MM. PEG-based hydrogel membrane coatings. Polym (Guildf). 2009;50:756.
Ju H, Sagle AC, Freeman BD, Mardel JI, Hill AJ. Characterization of sodium chloride and water transport in crosslinked poly(ethylene oxide) hydrogels. J Membr Sci. 2010;358:131.
Wu Y, Joseph S, Aluru NR. Effect of cross-linking on the diffusion of water, ions, and small molecules in hydrogels. J Phys Chem B. 2009;113:3512.
Liu Y, Yu S, Wu H, Li Y, Wang S, Tian Z, et al. High permeability hydrogel membranes of chitosan/poly ether-block-amide blends for CO2 separation. J Membr Sci. 2014;469:198.
Yue M, Hoshino Y, Miura Y. Design rationale of thermally responsive microgel particle films that reversibly absorb large amounts of CO2: fine tuning the pKa of ammonium ions in the particles. Chem Sci. 2015;6:6112.
Honda R, Hamasaki A, Miura Y, Hoshino Y. Thermoresponsive CO2 absorbent for various CO2 concentrations: tuning the pKa of ammonium ions for effective carbon capture. Polym J. 2021;53:157.
Zhang X, Chen L, Lim KH, Gonuguntla S, Lim KW, Pranantyo D, et al. The pathway to intelligence: using stimuli-responsive materials as building blocks for constructing smart and functional systems. Adv Mater. 2019;31:1804540.
Lynch I, de Gregorio P, Dawson KA. Simultaneous release of hydrophobic and cationic solutes from thin-film “plum-pudding” gels: a multifunctional platform for surface drug delivery? J Phys Chem B. 2005;109:6257–61.
Nagase K, Kobayashi J, Kikuchi A, Akiyama Y, Kanazawa H, Okano T. Preparation of thermoresponsive cationic copolymer brush surfaces and application of the surface to separation of biomolecules. Biomacromolecules. 2008;9:1340–7.
Hoshino Y, Kodama T, Okahata Y, Shea KJ. Peptide imprinted polymer nanoparticles: a plastic antibody. J Am Chem Soc. 2008;130:15242–3.
Hoare T, Pelton R. Impact of microgel morphology on functionalized microgel−drug interactions. Langmuir. 2008;24:1005–12.
Hoshino Y, Haberaecker WWIII, Kodama T, Zeng Z, Okahata Y, Shea KJ. Affinity purification of multifunctional polymer nanoparticles. J Am Chem Soc. 2010;132:13648–50.
Rao TP, Kala R, Daniel S. Metal ion-imprinted polymers—novel materials for selective recognition of inorganics. Anal Chim Acta. 2006;578:105–16.
Yoshimatsu K, Lesel BK, Yonamine Y, Beierle JM, Hoshino Y, Shea KJ. Temperature-responsive “catch and release” of proteins by using multifunctional polymer-based nanoparticles. Angew Chem Int Ed. 2012;51:2405–8.
Hoshino Y, Miyoshi T, Nakamoto M, Miura Y. Wide-range pKa tuning of proton imprinted nanoparticles for reversible protonation of target molecules via thermal stimuli. J Mater Chem B. 2017;5:9204–10.
Hoshino Y, Ohashi RC, Miura Y. Rational design of synthetic nanoparticles with a large reversible shift of acid dissociation constants: proton imprinting in stimuli responsive nanogel particles. Adv Mater. 2014;26:3718–23.
Honda R, Gyobu T, Shimahara H, Miura Y, Hoshino Y. Electrostatic interactions between acid-/base-containing polymer nanoparticles and proteins: impact of polymerization pH. ACS Appl Bio Mater. 2020;3:3827–34.
Paril A, Alb AM, Giz AT, Çatalgil-Giz H. Effect of medium pH on the reactivity ratios in acrylamide acrylic acid copolymerization. J Appl Polym Sci. 2007;103:968–74.
Serpe MJ, Yarmey KA, Nolan CM, Lyon LA. Doxorubicin uptake and release from microgel thin films. Biomacromolecules. 2005;6:408–13.
Koide H, Saito K, Yoshimatsu K, Chou B, Hoshino Y, Yonezawa S, et al. Cooling-induced, localized release of cytotoxic peptides from engineered polymer nanoparticles in living mice for cancer therapy. J Controll Release. 2023;355:745–59.
Hoshino Y, Jibiki T, Nakamoto M, Miura Y. Reversible pKa modulation of carboxylic acids in temperature-responsive nanoparticles through imprinted electrostatic interactions. ACS Appl Mater Interfaces. 2018;10:31096–105.
Guo B, Miura Y, Hoshino Y. Rational design of thermocells driven by the volume phase transition of hydrogel nanoparticles. ACS Appl Mater Interfaces. 2021;13:32184–92.
Guo B, Hoshino Y, Gao F, Hayashi K, Miura Y, Kimizuka N, et al. Thermocells driven by phase transition of hydrogel nanoparticles. J Am Chem Soc. 2020;142:17318–22.
Kaneko Y, Yoshida R, Sakai K, Sakurai Y, Okano T. Temperature-responsive shrinking kinetics of poly (N-isopropylacrylamide) copolymer gels with hydrophilic and hydrophobic comonomers. J Memb Sci. 1995;101:13–22.
Wang J, Gan D, Lyon LA, El-Sayed MA. Temperature-Jump investigations of the kinetics of hydrogel nanoparticle volume phase transitions. J Am Chem Soc. 2001;123:11284–9.
Hoshino Y, Imamura K, Yue M, Inoue G, Miura Y. Reversible absorption of CO2 triggered by phase transition of amine-containing micro- and nanogel particles. J Am Chem Soc. 2012;134:18177–80.
Yue M, Imai K, Miura Y, Hoshino Y. Design and preparation of thermo-responsive vinylamine-containing micro-gel particles for reversible absorption of carbon dioxide. Polym J. 2017;49:601–6.
Gao J, Liu Y, Terayama Y, Katafuchi K, Hoshino Y, Inoue G. Polyamine nanogel particles spray-coated on carbon paper for efficient CO2 capture in a milli-channel reactor. Chem Eng J. 2020;401:126059.
Yue M, Hoshino Y, Ohshiro Y, Imamura K, Miura Y. Temperature-responsive microgel films as reversible carbon dioxide absorbents in wet environment. Angew Chem Int Ed. 2014;53:2654–7.
Yue M, Imai K, Yamashita C, Miura Y, Hoshino Y. Effects of hydrophobic modifications and phase transitions of polyvinylamine hydrogel films on reversible CO2 capture behavior: comparison between copolymer films and blend films for temperature-responsive CO2 absorption. Macromol Chem Phys. 2017;218:1600570.
Olah GA, Goeppert A, Prakash GKS. Beyond Oil and Gas: The Methanol Economy. Angew Chem Int Ed. 2005;44:2636–39.
Olah GA, Goeppert A, Prakash GKS. Chemical recycling of carbon dioxide to methanol and dimethyl ether: from greenhouse gas to renewable, environmentally carbon neutral fuels and synthetic hydrocarbons. J Org Chem. 2009;74:487–98.
Rochelle GT. Amine scrubbing for CO2 capture. Science (1979). 2009;325:1652–4.
Fout T, Murphy JT. DOE/NETL’s Carbon Capture R&D Program for Existing Coal-Fired Power Plants. 2009.
Aaron D, Tsouris C. Separation of CO2 from flue gas: a review. Sep Sci Technol. 2005;40:321–48.
Davis JD. Thermal degradation of aqueous amines used for carbon dioxide capture. University of Texas, 2009.
Huang HY, Yang RT, Chinn D, Munson CL. Amine-grafted MCM-48 and silica xerogel as superior sorbents for acidic gas removal from natural gas. Ind Eng Chem Res. 2003;42:2427–33.
Park Y, Shin D, Jang YN, Park A-HA. CO2 capture capacity and swelling measurements of liquid-like nanoparticle organic hybrid materials via attenuated total reflectance fourier transform infrared spectroscopy. J Chem Eng Data. 2012;57:40–5.
D’Alessandro DM, Smit B, Long JR. Carbon dioxide capture: prospects for new materials. Angew Chem Int Ed. 2010;49:6058–82.
Choi S, Drese JH, Eisenberger PM, Jones CW. Application of amine-tethered solid sorbents for direct CO2 capture from the ambient air. Environ Sci Technol. 2011;45:2420–7.
Carbon Dioxide Capture from Existing Coal Fired Power Stations. 2007.
Pedersen PL. Biochemistry, 2nd edit., edited by Donald Voet and Judith G. Voet. New York: Wiley, 1995, 1392 pages, $86.95. Proteins: Structure, Function, and Genetics 1995;23:613.
Kilmartin JV, Breen JJ, Roberts GCK, Ho C. Direct measurement of the pK values of an alkaline Bohr group in human hemoglobin. Proc Natl Acad Sci. 1973;70:1246–9.
Shinmori H, Takeuchi M, Shinkai S. Spectroscopic detection of diols and sugars by a colour change in boronic acid-appended spirobenzopyrans. J Chem Soc, Perkin Transactions 2. 1996;1. https://doi.org/10.1039/p29960000001.
Shinkai S, Ogawa T, Nakaji T, Kusano Y, Nanabe O. Photocontrolled extraction ability of azobenzene-bridged azacrown ether. Tetrahedron Lett. 1979;20:4569–72.
Lynch I, Dawson KA. Release of model compounds from “plum-pudding”-type gels composed of microgel particles randomly dispersed in a gel matrix. J Phys Chem B. 2004;108:10893–8.
Tanaka T, Wang C, Pande V, Grosberg AY, English A, Masamune S, et al. Polymer gels that can recognize and recover molecules. Faraday Discuss. 1995;101:201.
Kavanagh CA, Rochev YA, Gallagher WM, Dawson KA, Keenan AK. Local drug delivery in restenosis injury: thermoresponsive co-polymers as potential drug delivery systems. Pharm Ther. 2004;102:1–15.
Yonamine Y, Hoshino Y, Shea KJ. ELISA-mimic screen for synthetic polymer nanoparticles with high affinity to target proteins. Biomacromolecules. 2012;13:2952–7.
Lee S-H, Hoshino Y, Randall A, Zeng Z, Baldi P, Doong R, et al. Engineered synthetic polymer nanoparticles as IgG affinity ligands. J Am Chem Soc. 2012;134:15765–72.
Akiyama Y, Kikuchi A, Yamato M, Okano T. Ultrathin Poly(N -isopropylacrylamide) grafted layer on polystyrene surfaces for cell adhesion/detachment control. Langmuir. 2004;20:5506–11.
Wulff G. Enzyme-like catalysis by molecularly imprinted polymers. Chem Rev. 2002;102:1–28.
Mosbach K, Ramström O. The emerging technique of molecular imprinting and its future impact on biotechnology. Nat Biotechnol. 1996;14:163–70.
Feil H, Bae YH, Feijen J, Kim SW. Mutual influence of pH and temperature on the swelling of ionizable and thermosensitive hydrogels. Macromolecules. 1992;25:5528–30.
Nayak S, Debord SB, Lyon LA. Investigations into the deswelling dynamics and thermodynamics of thermoresponsive microgel composite films. Langmuir. 2003;19:7374–9.
Lynch I, Dawson KA. Synthesis and characterization of an extremely versatile structural motif called the “Plum-Pudding” gel. J Phys Chem B. 2003;107:9629–37.
Werz PDL, Kainz J, Rieger B. Thermo- and pH-responsive nanogel particles bearing secondary amine functionalities for reversible carbon dioxide capture and release. Macromolecules. 2015;48:6433–9.
Kainz J, Werz PDL, Troll C, Rieger B. Temperature and CO2 responsive polyethylenimine for highly efficient carbon dioxide release. RSC Adv. 2015;5:9556–60.
Du N, Park HB, Dal-Cin MM, Guiver MD. Advances in high permeability polymeric membrane materials for CO2 separations. Energy Environ Sci. 2012;5:7306–22.
Taniguchi I, Kinugasa K, Toyoda M, Minezaki K, Tanaka H, Mitsuhara K. Piperazine-immobilized polymeric membranes for CO2 capture: mechanism of preferential CO2 permeation. Polym J. 2021;53:129–36.
Acknowledgements
This research was supported by JST ALCA, Japan (Grant No. JPMJAL1403), the Japan Aerospace Exploration Agency (JAXA) open innovation hub center, the MEXT Program: Data Creation and Utilization-Type Material Research and Development Project Grant Number JPMXP1122714694, JST Grant Number JPMJPF2114, and JCCL, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hoshino, Y., Aki, S. Hydrogel particles for CO2 capture. Polym J 56, 463–471 (2024). https://doi.org/10.1038/s41428-023-00850-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-023-00850-0