Abstract
Background
Pre-eclampsia can lead to maternal and neonatal complications and is a common cause of maternal mortality worldwide. This review has examined the effect of micronutrient supplementation interventions in women identified as having a greater risk of developing pre-eclampsia.
Methods
A systematic review was performed using the PRISMA guidelines. The electronic databases MEDLINE, EMBASE and the Cochrane Central Register of Controlled trials were searched for relevant literature and eligible studies identified according to a pre-specified criteria. A meta-analysis of randomised controlled trials (RCTs) was conducted to examine the effect of micronutrient supplementation on pre-eclampsia in high-risk women.
Results
Twenty RCTs were identified and supplementation included vitamin C and E (n = 7), calcium (n = 5), vitamin D (n = 3), folic acid (n = 2), magnesium (n = 1) and multiple micronutrients (n = 2). Sample size and recruitment time point varied across studies and a variety of predictive factors were used to identify participants, with a previous history of pre-eclampsia being the most common. No studies utilised a validated prediction model. There was a reduction in pre-eclampsia with calcium (risk difference, −0.15 (−0.27, −0.03, I2 = 83.4%)), and vitamin D (risk difference, −0.09 (−0.17, −0.02, I2 = 0.0%)) supplementation.
Conclusion
Our findings show a lower rate of pre-eclampsia with calcium and vitamin D, however, conclusions were limited by small sample sizes, methodological variability and heterogeneity between studies. Further higher quality, large-scale RCTs of calcium and vitamin D are warranted. Exploration of interventions at different time points before and during pregnancy as well as those which utilise prediction modelling methodology, would provide greater insight into the efficacy of micronutrient supplementation intervention in the prevention of pre-eclampsia in high-risk women.
Similar content being viewed by others
Introduction
Pre-eclampsia is a hypertensive disorder of pregnancy associated with a high risk of maternal, fetal and neonatal morbidity [1]. Pre-eclampsia has been defined as high blood pressure after 20 weeks’ gestation associated with one or more of the following: proteinuria, multisystemic maternal organ dysfunction or placental dysfunction [2, 3]. Pre-eclampsia affects around 2–8% of pregnancies globally, with approximately 10–15% of direct maternal deaths being attributed to pre-eclampsia and eclampsia [4].
The pathophysiology of pre-eclampsia is not fully understood and this disorder presents as a clinical syndrome with a wide spectrum [5]. Early onset pre-eclampsia is generally considered as a defect in placentation whilst late onset pre-eclampsia is more often attributed to a range of interacting factors including normal placental senescence and a genetic predisposition to cardiovascular and metabolic disease [5]. Poor placental function has repeatedly been associated with oxidative stress [6].
Several systematic reviews have assessed the effects of single and multiple micronutrients on the risk of developing pre-eclampsia. High dose calcium supplementation has been shown to be effective in reducing pre-eclampsia, particularly in women with low dietary calcium intake, but with limited evidence on the effects of low dose supplementation [7]. A recent umbrella review [8] reported that vitamin D supplementation reduced pre-eclampsia, while reporting limited or no effect of iron, folic acid supplementation or of the antioxidants, vitamin C and/or E. Despite magnesium being utilised in the treatment of pre-eclampsia and eclampsia, previous reviews have not been able to establish an effect of magnesium supplementation [9]. Similarly, many reviews [10, 11] report no established effect of zinc supplementation. There is increasing interest in the role of multiple micronutrient supplements (MMS) and their potential benefit for pregnant women, particularly in low-income countries where more than one micronutrient deficiency may co-exist. A meta-analysis of 28 RCTs [12] reported that despite evidence from observational cohort studies reporting a reduction in the risk of pre-eclampsia following MMS, there was a lack of effect from RCTs.
Interventions may be better targeted to women with more specific risk for adverse pregnancy outcomes. Several studies have used prediction modelling to identify those women more likely to develop pre-eclampsia [13,14,15]. An externally validated model from The Avon Longitudinal Study of Parents and Children cohort used routinely collected data to predict pre-eclampsia risk in a 12,996 women. The study combined maternal early pregnancy characteristics (including initial mean arterial pressure [MAP]) with repeatedly measured MAP collected from 20–36 weeks’ gestation. The authors found that blood pressure recorded from 28 weeks’ gestation improved the model’s identification of women who would go on to develop pre-eclampsia with an area under the curve of 0.81 and 0.83 in the validated cohort [13]. Other cohorts have frequently combined clinical risk factors with biomarkers and imaging techniques such as uterine artery Doppler ultrasound recorded in the first trimester [16]. Multivariable prediction models such as this have often demonstrated better performance with predicting early-onset pre-eclampsia [17]. The application of predictive modelling in the context of preventative micronutrient interventions in pre-eclampsia may offer insight into effectiveness of predictive factors and algorithms in clinical practice.
To the best of our knowledge, there has been no review of studies which have utilised prediction tools to stratify interventions intended to reduce pre-eclampsia. There is a need to evaluate interventions that utilise micronutrient supplementation in women who have been identified as high-risk for pre-eclampsia. Moreover, there is a paucity of data on the effects of pre-pregnancy micronutrient interventions on the development of pre-eclampsia. As healthcare aims to move towards primary prevention, it is important to assess supplementation interventions prior to pregnancy on the development of pre-eclampsia. Finally, few reviews report the effect of micronutrient supplementation in women with differing severity of pre-eclampsia (mild, severe and superimposed) who have been identified as high risk, which could allow more tailored and personalised preventative approaches in the future.
The overall aim of this review was to assess RCTs of micronutrient supplementation (single and multiple micronutrients) either pre-pregnancy and/or during pregnancy to prevent pre-eclampsia in women identified as high risk. An additional aim included examining the effect of intervention on different severities of pre-eclampsia in higher-risk women.
Methods
This systematic review was registered in the PROSPERO database (CRD42021240941) and conducted according to the PRISMA guidelines [18].
Inclusion and exclusion criteria
The PICOS (population, intervention, comparison, outcomes and study design) framework described in Table 1 was used to develop the inclusion and exclusion criteria. Studies were eligible if they met the following criteria: (1) RCTs evaluating single or multiple micronutrient supplementation before and/or during pregnancy compared with a control arm (no supplementation, placebo, dose difference or alternative micronutrient supplementation intervention) with a primary or secondary outcome of any classification of pre-eclampsia; (2) reproductive aged women between 18 and 50 years who intended to become pregnant or were pregnant at any gestation; (3) women identified as high risk of developing pre-eclampsia using a defined eligibility criteria at study entry. Studies meeting the following criteria were excluded: (1) observational and non-randomised studies; (2) abstracts, reviews, letters, comments and editorials; (3) women with existing pre-eclampsia; (4) women aged less than 18 years or more than 50 years; (5) studies not published in English.
Primary and secondary outcomes
The primary outcome of this review was the development of pre-eclampsia of any classification including mild, severe and superimposed pre-eclampsia, defined by any diagnostic criteria ranging from the use of systolic (SBP) and diastolic blood pressures (DBP), urinary protein measurements and other relevant clinical indicators such as liver enzymes and platelet count. Trials that reported definitions for severe pre-eclampsia generally defined this with the same diagnostic criteria, however with higher thresholds for blood pressure and urinary protein measurement. The secondary outcomes included gestational hypertension, eclampsia, diastolic and systolic blood pressure, HELLP syndrome, premature rupture of membranes, placental abruption, preterm birth, low birthweight, birth weight centile, small for gestational age (SGA), caesarean section, miscarriage, Apgar scores and maternal death, of which 7 of these secondary outcomes have been identified as part of the recommended core outcome set for pre-eclampsia for future studies [19]. Data were extracted on secondary outcomes from studies when available.
Literature search and study selection
The electronic databases MEDLINE, EMBASE and the Cochrane Central Register of Controlled trials were searched by two reviewers (SG, DDALS) on 14th April 2021. Search strategies are shown in Supplementary Information 1. Results of the search strategy were imported into EndNote for removal of duplicates and the remaining articles were imported into Rayyan for title and abstract screening [20]. If eligibility could not be determined by the title and abstract, full-text articles were screened. Any disagreement was resolved by a third reviewer (ACF). Additional studies were examined for eligibility through hand searching of reference lists.
Data extraction
Two reviewers (SG, DDALS) carried out the data extraction in duplicate and any disagreements were resolved by discussion or by consultation with another author to achieve a consensus opinion (ACF and KVD). A data extraction template was developed which included: title, authors, publication data, trial periods, study design, country of study, aim of study, sample size, characteristics of participants, inclusion and exclusion criteria, period of intervention (preconception and/or pregnancy), type and dose of intervention and clinical outcomes.
Data synthesis
The interventions and outcomes were assessed for suitability for data pooling to perform a meta-analysis. The analysis focused on assessing the effect of micronutrient interventions (single or multiple micronutrients) on the development of pre-eclampsia, the primary outcome, in high-risk populations. Micronutrient interventions included calcium, vitamin D, vitamin C and E, folic acid, magnesium and MMS in women defined as high risk of developing any classification of pre-eclampsia including severe pre-eclampsia, as previously defined. Summaries of exposure effect for each intervention were provided using a risk difference, calculated using Stata, version 16. The risk differences were calculated using a random effects model and the I2 statistic was used to assess heterogeneity amongst studies, with a threshold of >50% indicating significant heterogeneity. When meta-analysis was not possible due to too few studies, a narrative synthesis was performed.
Risk of bias (quality) assessment
The Cochrane Risk of Bias tool for randomised trials (RoB 2) [21] was used to assess the quality of each study included. The domains assessed include randomisation selection (selection bias), allocation concealment (selection bias), follow-up of participant from recruitment to termination of study and dropout (attrition bias) and other potential sources of bias. Disagreement between reviewers was resolved by discussion, with the overall risk of each study being deemed as either “low risk of bias,” “some concerns” or “high risk of bias.”
Results
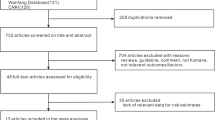
The electronic database search resulted in 8922 articles. Following removal of duplicates, a total of 7237 articles were screened for eligibility using titles and abstracts. Full-text screening was conducted on 168 articles. Major reasons for exclusion included: publication type (e.g. commentary articles or protocols of clinical trials), ineligible outcome (e.g. studies that did not include pre-eclampsia in the results), ineligible population (e.g. low risk women, adolescents), studies which were not published in English, ineligible study design (e.g. review and observational studies), incorrect type of intervention (e.g. pharmacological intervention) and retraction of trials. Twenty articles [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] met the inclusion criteria and were included in this review. The study identification and selection process are summarised in Fig. 1.
Characteristics of included studies
This review included RCTs published between 1991 to 2021. A summary of these RCTs is shown in Table 2. The characteristics of studies including women identified as high-risk for pre-eclampsia are shown in Table 3. Six studies [23, 24, 29, 30, 32, 38] were conducted in Iran, three studies [31, 33, 39] in the United States, two studies [25, 40] in the United Kingdom, two studies [26, 34] in Brazil, one study in each of Colombia [27], India [28], Mexico [35] and China [36]. Three studies were multicentre and were conducted in South Africa, Zimbabwe and Argentina [22]; India, Peru, South America and Vietnam [37]; Argentina, Australia, Canada, Jamaica and the United Kingdom [41]. Fifteen studies were carried out in low- and middle-income countries [22,23,24, 26,27,28,29,30, 32, 34,35,36,37,38, 41]. The lowest sample size was 50 [28] and the largest sample size 2464 [41]. The studies aimed to assess the efficacy of either the supplementation of calcium, vitamin D, vitamin C in combination with vitamin E, magnesium, folic acid or MMS in reducing the incidence of pre-eclampsia in women classified at high-risk of pre-eclampsia at study entry. Of the 20 studies, two targeted women before pregnancy [18, 32]. The earliest time a pregnancy intervention was introduced was at 8 weeks’ gestation [41] with most of the studies continuing the micronutrient supplementation until delivery.
Predictive factors of pre-eclampsia
The studies used a variety of strategies to identify women at risk of developing pre-eclampsia. The number of variables used ranged from one to 15 and included a range of factors from maternal history to clinical biomarkers (Table 4). Thirteen studies [22, 24,25,26, 31, 34,35,36,37,38,39,40,41] considered a history of pre-eclampsia in a prior pregnancy, with two [35, 38] of these also including family history of pre-eclampsia. Seven studies [26, 31, 37,38,39,40,41] required participants to have at least one risk factor of pre-eclampsia such as chronic hypertension, type 1 or 2 diabetes mellitus, multiple gestation, or history of preterm birth. Five studies [25, 29, 32, 37, 40] used uterine artery doppler waveforms to select participants, either in isolation or within a combination of other maternal characteristics. Three studies [23, 27, 30] included a positive rollover test, while only one study [33] utilised a positive angiotensin sensitivity test. This test was performed in women at 24–28 weeks’ gestation, by infusing increasing doses of angiotensin II every 5 min until the normal cut-off value (12 ng/kg/min) was reached or the DBP increased to 20 mmHg above the baseline before reaching this cut-off value (the effective pressor dose). If a participant’s effective pressor dose was achieved with a rate of less than 12 ng/kg/min, they were deemed to have a positive angiotensin sensitivity test and were then randomised. Three studies [25, 27, 30] required participants to have a combination of predictive factors, for example, both an abnormal two-stage uterine-artery doppler analysis and a previous history of pre-eclampsia [25]. None reported using a validated prediction model to identify women at high-risk of developing pre-eclampsia.
Micronutrient interventions
The type of intervention varied between studies (Table 4). The majority evaluated the effect of single micronutrients including calcium [22, 23, 27, 30, 33], combined vitamin C and E [25, 28, 34, 35, 37, 39, 40], vitamin D [24, 29, 32], folic acid [36, 41] and magnesium [26]. Two studies evaluated the effect of MMS which included multimineral-vitamin D supplements (calcium, magnesium, zinc and vitamin D) compared to vitamins C and E [38] and phytonutrient supplementation [31].
Seventeen studies compared the micronutrient supplementation intervention to a placebo, whilst one study compared the intervention to standard clinic protocol [28]. One study compared the effects of different dosages of micronutrient supplementation [36] another study made comparisons between MMS and vitamin C and E supplementation [38].
Calcium supplementation
Three calcium interventions used a high dose between 1500 to 2000 mg daily [23, 30, 33]. Two studies [22, 27] used a lower dose of 500 and 600 mg calcium, respectively. Hofmeyr et al. started 500 mg of calcium supplementation before pregnancy until 20 weeks’ gestation whilst Herrera et al. used a 600 mg calcium supplement with 50 mg of linoleic acid between 28 to 32 weeks’ gestation until delivery [22, 27]. Similarly, Babadizavandy et al. utilised 2000 mg of calcium supplementation between 28 to 32 weeks’ gestation until delivery [23]. Both Niromanesh et al. and Sanchez-Ramos et al. also used 2000 mg of daily calcium supplementation. However, the period of the intervention was unclear [30, 33]. Of the 2 trials that reported data on compliance, Sanchez-Ramos et al. had a compliance rate of 91.1% while Hofmeyr et al. trial reported that approximately 50% of participants took at least 80% of their expected tablets. Random effects meta-analysis of five studies showed a lower rate of pre-eclampsia with calcium supplementation (risk difference = −0.15, 95% CI = −0.27 to −0.03), with significant heterogeneity among the studies (I2 = 83.41%, Fig. 2), however, there was no reduction in severe pre-eclampsia (Fig. 3).
Vitamin D supplementation interventions
All three studies used a dose of 50,000 IU of vitamin D every 2 weeks, with 2 using this intervention in isolation [24, 29] and the third in combination with 1000 mg of calcium [32], with a reported compliance rate of 90–100% across all three trials. All 3 studies were conducted in the pregnancy period until 32 to 36 weeks’ gestation. There was a lower risk of pre-eclampsia with vitamin D supplementation in comparison to no supplementation (risk difference = −0.09, 95% CI = −0.17 to −0.02, I2 = 0.00%, Fig. 2), however there was no effect on severe pre-eclampsia (Fig. 3).
Vitamin C and E supplementation interventions
All 7 studies [25, 28, 34, 35, 37, 39, 40] used vitamin C and E together in combination with 6 studies using a dose of 1000 mg vitamin C and 400 IU vitamin E [25, 28, 34, 37, 39, 40]. The remaining study used a dose of 500 mg vitamin C and 400 IU vitamin E with or without L-arginine [35]. The majority of studies started the intervention in the second trimester of pregnancy, ranging from 14 to 24 weeks’ gestation, whilst Spinnato et al. initiated the intervention at the end of the first trimester [34]. Five trials reported a moderate to high level of compliance [25, 34, 35, 37, 40].
Random effects meta-analysis showed that the risk difference for pre-eclampsia was 4% for vitamin C and E, (risk difference = −0.04, 95% CI = −0.09 to 0.00) however, there was significant heterogeneity between the studies (I2 = 65.76%, Fig. 2). There was no effect on the rate of severe pre-eclampsia. Poston et al. reported no significant effect of vitamin C and E supplementation in the prevention of pre-eclampsia, however, infants born with a low birthweight were higher in the intervention group (I = 387 vs. C = 335, p = 0.023, RR = 1.15, 95% CI = 1.02–1.30).
Folic acid supplementation interventions
One study evaluated the effect of high dose folic acid (4 mg daily) compared to low dose folic acid (0.4 mg daily) from before pregnancy until delivery [36], whilst one study assessed the effect of the daily supplementation of 4 mg of folic acid from 8 to 16 weeks’ gestation until delivery [41]. Zheng et al. assessed homocysteine plasma levels and reported that levels were significantly lower in the high dose group which had a compliance rate of 74.1% compared to 73% in the low dose group. Among the high dose group, the incidence of pre-eclampsia was reduced when compliance was greater than 80% compared to 50%. Overall, there was no effect of folic acid supplementation on pre-eclampsia (Fig. 2) or severe pre-eclampsia (Fig. 3).
Magnesium supplementation interventions
De Araújo et al. assessed the effect of 300 mg of daily magnesium supplementation compared to a placebo from 12 to 20 weeks’ gestation until delivery and showed no significant impact on pre-eclampsia or severe pre-eclampsia [26]. Although adherence was defined in this trial, compliance rates were not reported.
MMS interventions
The two studies [31, 38] that used multiple micronutrients differed according to composition. Azami et al. compared three groups of pregnant women who received a daily ferrous sulphate tablet with a multimineral-vitamin D tablet consisting of 800 mg calcium, 9 mg zinc and 400 IU vitamin D (Group A), a daily ferrous sulphate tablet with vitamin C and vitamin E (Group B) or a daily ferrous sulphate tablet alone (Group C) [38], while Parrish et al. assessed the effect of a combination of phytonutrients including 7.5 mg of beta-carotene, 234 mg of Vitamin C, 30 mg of vitamin E, 420 mg of folate and 60 mg of calcium, taken twice daily until delivery, compared to placebo [31]. There were no data reported on compliance.
Pooled estimate of the 2 studies showed that MMS was not associated with a reduction in overall pre-eclampsia (Fig. 2). Parrish et al. also showed no reduction in the rate of severe pre-eclampsia [31].
Diagnostic criteria for pre-eclampsia
The studies diagnosed and classified pre-eclampsia using primarily SBP and DBP, urinary protein measurements and other clinical indicators such as liver enzymes and platelet count (Table 5).
Quality of included studies
The quality of the studies included in this review is shown in Supplementary Information 2. In total, 12 trials [22, 24, 25, 27, 29, 32,33,34,35, 37, 40, 41] were assessed as ‘low risk of bias,’ seven trials [23, 28, 30, 31, 36, 38, 39] as “high risk of bias” and one [26] was classified as “some concerns.” The main source of bias across the studies was either lack of information available on compliance and adherence in the study or non-adherence to the micronutrient supplementation intervention.
Discussion
This review aimed to evaluate the effect of micronutrient supplementation interventions on the development of pre-eclampsia in women identified as high risk. Our findings showed a lower rate of pre-eclampsia with calcium and vitamin D supplementation. There was no effect of micronutrient supplementation on severe pre-eclampsia. The review was limited by studies not adequately powered to detect a difference in pre-eclampsia and heterogeneity between studies was high.
Calcium supplementation has been previously reported to reduce the risk of pre-eclampsia [7] with the greatest effects observed in women with low calcium diets, however doses of calcium varied significantly across the trials, ranging from 500 mg to 2000 mg. The mechanism is largely unknown; however it is suggested that calcium lowers blood pressure [42] and may also reduce activation of the vascular endothelium [43]. Our findings support the use of calcium, in women with low dietary intake, to reduce pre-eclampsia in high-risk women, however, the studies had a small sample size and were under powered for the outcome [22]. The variance amongst these trials, ranging from different diagnostic criteria to the differences in sample size, highlights the need for more well-powered and larger-scale trials to establish when and how calcium supplementation can be the most beneficial to women identified as high risk of developing pre-eclampsia.
The vitamin D supplementation trials showed a slight reduction in pre-eclampsia, however, from studies with a small sample size. Previous reviews have reported on the lack of consistent evidence of benefit of vitamin D supplementation and its role in the prevention of pre-eclampsia, adding that inconsistencies in reporting the timing and duration of the intervention have not yet been adequately addressed [44]. Our findings in high-risk women support this, with some studies lacking clarity in reporting the intervention components [24] and time of gestation during the intervention, highlighting the need for further studies in higher risk women and better reporting in clinical trials.
There was a slightly lower rate of pre-eclampsia with antioxidants (vitamin C and E), however the confidence interval included zero and adverse outcomes such as low birthweight [40] were higher in the intervention arm. Previous reviews have reported no benefit of vitamin C and E supplementation on pre-eclampsia in ‘all-risk’ women, however there might be a protective effect in low- and middle-income countries [8]. This review provides some evidence that vitamin C and E supplementation may reduce pre-eclampsia in higher-risk women by 4%, including in low- and middle-income countries [35], however secondary outcomes were unfavourable, including a higher instance of SGA infants in the intervention arm. Oxidative stress is known to play a pivotal role in the manifestation of conditions such as pre-eclampsia [45], and alternative methods of reversing oxidative stress or poor antioxidant status may be worth investigating further in higher risk women.
This review found no evidence of benefit of folic acid supplementation on the development of pre-eclampsia. A recent 2018 review of observational studies [46] reported that folic acid was associated with lower risk of developing pre-eclampsia in pregnancy, perhaps being most effective in combination with multivitamins. However, RCTs evaluating the effect of folic acid on the development of pre-eclampsia are scarce, with conflicting data about the optimal dosages and whether it is best in isolation or combination with other micronutrients [46] and suggests a need for further research in women identified as high risk. Appropriately designed RCTs which encompass both dosage comparison of folic acid and stratify participants by high vs low risk are needed to elucidate the role of folic acid in the prevention of pre-eclampsia.
A limited number of studies addressed the impact of magnesium. Additionally, few studies assessed effects of MMS supplements with considerable variation in the combination of micronutrients used in each trial. A 2019 review reported improvements in preterm birth, low birth weight and SGA when supplemented with MMS together with iron and folic acid [47], however the effect of MMS on pre-eclampsia is still unclear. Higher-quality studies are needed to evaluate the potential benefits of MMS on the prevention of pre-eclampsia with a focus on finding the most effective combination of micronutrients. We did not retrieve any studies assessing the efficacy of zinc in this review.
Specific classifications of pre-eclampsia were reported in 11 studies [22, 25, 28, 31, 33, 34, 36, 37, 39,40,41], including mild, severe and superimposed pre-eclampsia. There was no effect of micronutrient supplementation interventions on severe pre-eclampsia. Tailoring micronutrients and their dosages to predicted severity of pre-eclampsia may be more beneficial than more generalised preventative approaches, particularly in this stratified population as opposed to all-risk women. With a lack of reviews evaluating the effect of micronutrient supplementation interventions on different classifications of pre-eclampsia, future studies on pre-eclampsia and its various severities, along with more exploration of the associated predictive factors, could inform primary prevention strategies to improve maternal healthcare.
The studies in this review used a wide variety of predictive factors at study entry. Of the eight studies [24, 25, 27, 30, 33, 35, 36, 38] with positive findings, five [24, 25, 35, 36, 38] used a history of pre-eclampsia to identify high-risk women whilst two studies used a positive roll-over test in addition to another predictive factor [27, 30], with the remaining study using a positive angiotensin sensitivity test [33]. A history of pre-eclampsia was the most common factor used to select participants at study entry, with very few studies using a combination of more than one risk factor. Predictive factors have shown to be most effective through a combination of using maternal characteristics, biomarkers and other variables such as the uterine artery doppler [17]. For example, a new first-trimester screening algorithm combining MAP, uterine artery doppler and circulating levels of placental growth factor (PIGF) to predict preterm pre-eclampsia, has a true positive rate of 82%, almost double the rate of detection via the UK National Institute for Health and Care Excellence (NICE) guidelines which uses clinical factors alone [48]. Many trials in this review may have benefited from using prediction models, which utilise a combination of specific variables and may offer higher predictive ability [48]. With no study in this review using a validated prediction model to identify high-risk women and a lack of externally validated prediction algorithms, future studies assessing the effect of micronutrient supplementation interventions in women at high risk of developing pre-eclampsia could use validated prediction models to select participants, with more trials also exploring alternative combinations of predictive factors that accurately determine women who are at high risk of developing pre-eclampsia.
The trials included in this review also varied in other aspects of study design and reporting of findings. In addition to differences in the identification of high-risk women, there was variability in the eligibility criteria, with one trial including women with other co-morbidities, for example, kidney disease [38]. Furthermore, some trials reported the diagnostic criteria of severe pre-eclampsia, but several did not [31, 33, 36, 39, 41] while other trials did not report a diagnostic criterion for any classification of pre-eclampsia [29, 31, 32, 39]. There was a lack of reporting on statistical methodology, in particular the inclusion of confounding variables in the analysis. Several trials did not clearly state whether confounding variables had been adjusted for. In those that did, the most common confounding factors adjusted for were maternal age and BMI. Compliance across the trials ranged from moderate to high, with the majority of studies using tablet count while Zheng et al. used plasma homocysteine levels as a method of demonstrating the potential confounding effect of compliance on the outcome of pre-eclampsia. Data for compliance was not reported in several trials [23, 26,27,28, 30, 31, 38, 39] while there was also variation across trials in the frequency, dosing and timing of supplementation, while the gestational age at the time of intervention was often not reported.
Two trials [18, 32] commenced in the preconception period. Calcium is known to have a benefit in reducing the risk of pre-eclampsia from 20 weeks’ gestation onwards, however our findings suggest that calcium supplementation before this point may not necessarily be effective as is shown by Hofmeyr et al. [22]. On the other hand, Zheng et al. reported that supplementation of folic acid from preconception to delivery may effectively reduce risk of severe pre-eclampsia [36]. Micronutrient supplementation interventions may also be effective for different durations and at different time points, which could be investigated in future trials to further elucidate the effect of these micronutrients. However, caution is imperative as our findings show that some micronutrient interventions, notably vitamin C and E, are not entirely benign.
Strengths and limitations
This systematic review has several strengths. This study addressed whether micronutrient interventions are effective in reducing the development of pre-eclampsia in women identified as high risk for the condition. Previous reviews have focused on an unselected approach, often with no risk stratification. The identification of women with an increased risk of developing pre-eclampsia might enable targeted intervention in women most likely to benefit. This work complements previous findings that calcium and vitamin D are beneficial in reducing pre-eclampsia including in those identified as high risk, whilst highlighting the need for more large-scale well-powered studies with improved and more consistent reporting of interventions and findings. A comprehensive search strategy was used to screen for studies that targeted interventions at higher risk populations by using a pre-specified eligibility criteria. The findings from this review are important to inform the design of future RCTs to improve the data quality and clarify the effects of micronutrient supplementation, particularly in women at high risk for pre-eclampsia and the effect on different classifications of pre-eclampsia.
This review has limitations. It only included studies published in English which may have introduced publication bias. The high risk of bias in several studies in this review highlights the low-quality evidence in this research area and supports the need for more robust future trials. Additionally, many trials with significant results were not adequately powered to detect a difference in pre-eclampsia between treatment groups or may have overestimated the effect of the given intervention as a result of a small sample size. The analysis was limited in scope as pooling of data were not possible for all micronutrient interventions, in addition to a lack of adjustment for potential confounders. Furthermore, there was high methodological variability between studies particularly with calcium, vitamin D and vitamin C and E trials, limiting the consistency of data across these studies. One reason for this may be because of differences in the interventions themselves, as Herrera et al. did not investigate calcium alone but in combination with linoleic acid, whilst Samimi et al. investigated vitamin D given with calcium. Additionally, the gestational age at which interventions were administered varied significantly amongst trials. Finally, the risk factors used to screen for women at high risk of pre-eclampsia differed significantly between trials.
Recommendations for further research and practice
Our review highlighted the lack of studies of interventions targeting higher risk women in the preconception period. Only two studies [22, 36] in this review initiated the intervention in the preconception period. With symptoms of pre-eclampsia beginning from 20 weeks’ gestation, preconception and early pregnancy interventions require further exploration as they may provide greater insight into how to improve maternal and neonatal outcomes. We have reported a lower rate of pre-eclampsia with calcium and vitamin D, however, conclusions were limited by small sample sizes, methodological variability and heterogeneity between studies. Future studies of these two micronutrients are warranted, however they must be larger-scale and well-powered to allow more thorough and reliable conclusions to be drawn. Additionally, several studies [24, 25, 30, 38, 39] in this review did not clearly state the gestation of the participants at the beginning and end of the given micronutrient supplementation intervention, as well as failing to report on adherence and compliance. Better reporting of trials is required in future studies to ascertain the relationship between the effectiveness of the given micronutrient supplementation intervention and the timing with which it is administered. Further studies using externally validated prediction models that have demonstrated higher predictive performance such as models by Poon et al. [49] and Odibo et al. [50] with a detection rate of 91.7% and 80.0% respectively for pre-eclampsia requiring early delivery and use a variety of predictive factors such as chronic hypertension and PAPP-A may additionally clarify the effects of these micronutrients. Finally, future research is needed to determine the effect of micronutrient supplementation interventions on different classifications of pre-eclampsia, from mild to superimposed pre-eclampsia, to progress towards a more personalised and tailored approach in the primary prevention of pre-eclampsia.
Conclusion
This study showed a small effect of calcium and vitamin D in the prevention of pre-eclampsia in women who were identified as higher risk of developing the condition. The review was limited by the inclusion of studies with a small sample size. Significant heterogeneity between studies as well as methodological variability was evident. Further higher quality, large-scale RCTs of calcium and vitamin D, the use of prediction modelling, and particularly at different points of time before and during pregnancy, may be beneficial to assess the efficacy of micronutrient supplementation intervention in the prevention of pre-eclampsia in high-risk women.
References
World Health Organization. WHO recommendations for Prevention and treatment of pre-eclampsia and eclampsia [Internet]. 2011 [cited 2021 Sep 2]. Available from: http://apps.who.int/iris/bitstream/handle/10665/44703/9789241548335_eng.pdf
NICE. Hypertension in pregnancy: diagnosis and management NICE guideline [Internet]. 2019. Available from: www.nice.org.uk/guidance/ng133
Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet. 2016;387:999–1011.
Rawlins B, Plotkin M, Rakotovao JP, Getachew A, Vaz M, Ricca J, et al. Screening and management of pre-eclampsia and eclampsia in antenatal and labor and delivery services: Findings from cross-sectional observation studies in six sub-Saharan African countries. BMC Pregnancy Childbirth. 2018;18:1–11.
Burton GJ, Redman CW, Roberts JM, Moffett A Pre-eclampsia: pathophysiology and clinical implications. Vol. 366, The BMJ. BMJ Publishing Group; 2019.
Aouache R, Biquard L, Vaiman D, Miralles F. Oxidative stress in preeclampsia and placental diseases. Int J Mol Sci. 2018;19:1496.
Hofmeyr GJ, Lawrie TA, Atallah ÁN, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. 2018;2018:1–123.
Kinshella MLW, Omar S, Scherbinsky K, Vidler M, Magee LA, von Dadelszen P, et al. Effects of maternal nutritional supplements and dietary interventions on placental complications: an umbrella review, meta-analysis and evidence map. Nutrients. MDPI AG. 2021;13:1–29.
Makrides M, Crosby DD, Shepherd E, Crowther CA. Magnesium supplementation in pregnancy. Cochrane Database Syst Rev. 2014;2019:1–57.
Wilson R, Grieger J, Bianco-Miotto T, Roberts C. Association between maternal zinc status, dietary zinc intake and pregnancy complications: a systematic review. Nutrients. 2016;8:1–28.
Oh C, Keats E, Bhutta Z. Vitamin and mineral supplementation during pregnancy on maternal, birth, child health and development outcomes in low- and middle-income countries: a systematic review and meta-analysis. Nutrients. 2020;12:1–30.
Fu ZM, Ma ZZ, Liu GJ, Wang LL, Guo Y. Vitamins supplementation affects the onset of preeclampsia. J Formos Med Assoc. 2018;117:6–13.
Macdonald-Wallis C, Silverwood RJ, de Stavola BL, Inskip H, Cooper C, Godfrey KM, et al. Antenatal blood pressure for prediction of pre-eclampsia, preterm birth, and small for gestational age babies: development and validation in two general population cohorts. BMJ. 2015;351:h5948–48.
Kenny LC, Black MA, Poston L, Taylor R, Myers JE, Baker PN, et al. Early pregnancy prediction of preeclampsia in nulliparous women, combining clinical risk and biomarkers. Hypertension. 2014;64:644–52.
Kleinrouweler CE, Cheong-See FM, Collins GS, Kwee A, Thangaratinam S, Khan KS, et al. Prognostic models in obstetrics: available, but far from applicable. Am J Obstet Gynecol. 2016;214:79–90.e36.
Onwudiwe N, Yu CKH, Poon LCY, Spiliopoulos I, Nicolaides KH. Prediction of pre-eclampsia by a combination of maternal history, uterine artery Doppler and mean arterial pressure. Ultrasound Obstet Gynecol. 2008;32:877–83.
Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. The Lancet. Elsevier B.V. 2021;398:341–54.
Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:1–6.
Duffy J, Cairns A, Richards‐Doran D, ’t Hooft J, Gale C, Brown M, et al. A core outcome set for pre‐eclampsia research: an international consensus development study. BJOG: Int J Obstet Gynaecol. 2020;127:1516–26.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. The BMJ. 2019;366:1–8.
Hofmeyr GJ, Betrán AP, Singata-Madliki M, Cormick G, Munjanja SP, Fawcus S, et al. Prepregnancy and early pregnancy calcium supplementation among women at high risk of pre-eclampsia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2019;393:330–9.
Baba Dizavandy E, Seyyedi Alavi G, Cordi M. The effect of calcium supplementation in the prevention of hypertensive disorder of pregnancy in nulliparous women. Med J Islamic Repub Iran. 1998;12:11–4.
Behjat Sasan S, Zandvakili F, Soufizadeh N, Baybordi E. The effects of vitamin D supplement on prevention of recurrence of preeclampsia in pregnant women with a History of preeclampsia. Obstet Gynecol Intern. 2017;2017:1–5.
Chappell LC, Seed PT, Briley AL, Kelly FJ, Lee R, Hunt BJ, et al. Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomised trial. Lancet. 1999;354:810–6.
de Araújo CAL, Ray JG, Figueiroa JN, Alves JG. BRAzil magnesium (BRAMAG) trial: a double-masked randomized clinical trial of oral magnesium supplementation in pregnancy. BMC Pregnancy Childbirth. 2020;20:1–7.
Herrera MJA, Arevalo-Herrera M, Herrera S. Prevention of preeclampsia by linoleic acid and calcium supplementation: a randomized controlled trial. Obstet Gynecol. 1998;91:585–90.
Kalpdev A, Saha SC, Dhawan V. Vitamin C and E supplementation does not reduce the risk of superimposed PE in pregnancy. Hypertens Pregnancy. 2011;30:447–56.
Karamali M, Beihaghi E, Mohammadi A, Asemi Z. Effects of high-dose vitamin D supplementation on metabolic status and pregnancy outcomes in pregnant women at risk for pre-eclampsia. Hormone Metab Res. 2015;47:867–72.
Niromanesh S, Laghaii S, Mosavi-Jarrahi A. Supplementary calcium in prevention of pre-eclampsia. Int J Gynecol Obstet. 2001;74:17–21.
Parrish MR, Martin JN, Lamarca BB, Ellis B, Parrish SA, Owens MY, et al. Randomized, placebo controlled, double blind trial evaluating early pregnancy phytonutrient supplementation in the prevention of preeclampsia. J Perinatol. 2013;33:593–9.
Samimi M, Kashi M, Foroozanfard F, Karamali M, Bahmani F, Asemi Z, et al. The effects of vitamin D plus calcium supplementation on metabolic profiles, biomarkers of inflammation, oxidative stress and pregnancy outcomes in pregnant women at risk for pre-eclampsia. J Hum Nutri Diet. 2016;29:505–15.
Sanchez-Ramos L, Briones DK, Kaunitz AM, Delvalle GO, Gaudier FL, Walker CD. Prevention of pregnancy-induced hypertension by calcium supplementation in angiotensin II-sensitive patients. Obstet Gynecol [Internet]. 1994;84:349–53. https://pubmed.ncbi.nlm.nih.gov/8058229/
Spinnato JA, Freire S, Pinto E, Silva JL, Cunha Rudge MV, Martins-Costa S, Koch MA, et al. Antioxidant therapy to prevent preeclampsia. Obstet Gynecol. 2007;110:1311–8.
Vadillo-Ortega F, Perichart-Perera O, Espino S, Avila-Vergara MA, Ibarra I, Ahued R, et al. Effect of supplementation during pregnancy with L-arginine and antioxidant vitamins in medical food on pre-eclampsia in high risk population: randomised controlled trial. BMJ. 2011;342:1–8.
Zheng L, Huang J, Kong H, Wang F, Su Y, Xin H. The effect of folic acid throughout pregnancy among pregnant women at high risk of pre-eclampsia: a randomized clinical trial. Pregnancy Hypertens. 2020;19:253–8.
Villar J, Purwar M, Merialdi M, Zavaleta N, thi Nhu Ngoc N, Anthony J, et al. World Health Organisation multicentre randomised trial of supplementation with vitamins C and E among pregnant women at high risk for pre-eclampsia in populations of low nutritional status from developing countries. BJOG: Int J Obstet Gynaecol. 2009;116:780–8.
Azami M, Azadi T, Sc M, Farhang S, Rahmati S, Pourtaghi K. The effects of multi mineral-vitamin D and vitamins (C+E) supplementation in the prevention of preeclampsia: an RCT. Int J Reprod BioMed. 2017;15:273–8.
Beazley D, Ahokas R, Livingston J, Griggs M, Sibai BM. Vitamin C and E supplementation in women at high risk for preeclampsia: a double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2005;192:520–1.
Poston L, Briley A, Seed P, Kelly F, Shennan A. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367:1145–54.
Wen SW, White RR, Rybak N, Gaudet LM, Robson S, Hague W, et al. Effect of high dose folic acid supplementation in pregnancy on pre-eclampsia (FACT): double blind, phase III, randomised controlled, international, multicentre trial. BMJ. 2018;362:1–8.
Hatton DC, Yue Q, McCarron DA. Mechanisms of calcium’s effects on blood pressure. Semin Nephrol. 1995;15:593–602.
DeSousa J, Tong M, Wei J, Chamley L, Stone P, Chen Q. The anti-inflammatory effect of calcium for preventing endothelial cell activation in preeclampsia. J Hum Hypertens. 2016;30:303–8.
Purswani JM, Gala P, Dwarkanath P, Larkin HM, Kurpad A, Mehta S. The role of vitamin D in pre-eclampsia: a systematic review. BMC Pregnancy Childbirth. 2017;17:1–15.
Aouache R, Biquard L, Vaiman D, Miralles F. Oxidative stress in preeclampsia and placental diseases. Int J Mol Sci. 2018;19:1–29.
Liu C, Liu C, Wang Q, Zhang Z. Supplementation of folic acid in pregnancy and the risk of preeclampsia and gestational hypertension: a meta-analysis. Archives Gynecol Obstet. 2018;298:697–704.
Keats EC, Haider BA, Tam E, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev. 2019;3:1–128.
Tan MY, Wright D, Syngelaki A, Akolekar R, Cicero S, Janga D, et al. Comparison of diagnostic accuracy of early screening for pre-eclampsia by NICE guidelines and a method combining maternal factors and biomarkers: results of SPREE. Ultrasound Obstet Gynecol. 2018;51:743–50.
Poon LCY, Stratieva V, Piras S, Piri S, Nicolaides KH. Hypertensive disorders in pregnancy: combined screening by uterine artery Doppler, blood pressure and serum PAPP-A at 11–13 weeks. Prenatal Diagnosis. 2010;30:216–23.
Odibo AO, Zhong Y, Goetzinger KR, Odibo L, Bick JL, Bower CR, et al. First-trimester placental protein 13, PAPP-A, uterine artery Doppler and maternal characteristics in the prediction of pre-eclampsia. Placenta. 2011;32:598–602.
Funding
ACF and LP are supported by Tommy’s charity. KVD is supported by the Medical Research Council (MR/V005839/1).
Author information
Authors and Affiliations
Contributions
The research question and study design were formulated by ACF. Titles, abstracts and full text articles were independently screened by SG and DDALS. OQ contributed to data extraction and provided feedback on the systematic review process. Data extraction and analysis was carried out by SG, DDALS and KVD. The writing of the article was completed by SG, DDALS, KVD, LP, LM, SLW, JRF and ACF. ACF had overall responsibility for the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gunabalasingam, S., De Almeida Lima Slizys, D., Quotah, O. et al. Micronutrient supplementation interventions in preconception and pregnant women at increased risk of developing pre-eclampsia: a systematic review and meta-analysis. Eur J Clin Nutr 77, 710–730 (2023). https://doi.org/10.1038/s41430-022-01232-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-022-01232-0