Abstract
Human research ethics committees (HRECs) are evaluating increasing quantities of genomic research applications with complex ethical considerations. Genomic confidence is reportedly low amongst many non-genetics-experts; however, no studies have evaluated genomic confidence levels in HREC members specifically. This study used online surveys to explore genomic confidence levels, predictors of confidence, and genomics resource needs of members from 185 HRECs across Australia. Surveys were fully or partially completed by 145 members. All reported having postgraduate 94 (86%) and/or bachelor 15 (14%) degrees. Participants consisted mainly of researchers (n = 45, 33%) and lay members (n = 41, 30%), affiliated with either public health services (n = 73, 51%) or public universities (n = 31, 22%). Over half had served their HREC \(\ge\)3 years. Fifty (44%) reviewed genomic studies \(\le\)3 times annually. Seventy (60%) had undertaken some form of genomic education. While most (94/103, 91%) had high genomic literacy based on familiarity with genomic terms, average genomic confidence scores (GCS) were moderate (5.7/10, n = 119). Simple linear regression showed that GCS was positively associated with years of HREC service, frequency of reviewing genomic applications, undertaking self-reported genomic education, and familiarity with genomic terms (p < 0.05 for all). Conversely, lay members and/or those relying on others when reviewing genomic studies had lower GCSs (p < 0.05 for both). Most members (n = 83, 76%) agreed further resources would be valuable when reviewing genomic research applications, and online courses and printed materials were preferred. In conclusion, even well-educated HREC members familiar with genomic terms lack genomic confidence, which could be enhanced with additional genomic education and/or resources.
Similar content being viewed by others
Background
With the growing integration of genomics into clinical medicine [1], the scale, scope and complexity of genomic research is inevitably increasing [2]. There are many ethical considerations associated with genomic research, including the nature of consent [3,4,5,6], procedures around the disclosure/non-disclosure of results [7,8,9,10,11], sample ownership and data storage/sharing [12,13,14,15], the shared nature of genetic information [16], and genetic discrimination with insurance policies [17,18,19]. Genomic research, therefore, requires robust ethical review. However, comfort and confidence levels of human research ethics committee (HREC) members who review and approve genomic studies is unknown.
Within Australia and internationally, members of the public and non-genetic clinicians have reported low levels of awareness and confidence when accessing genomic services. Specifically, members of the public have reported low awareness of genomic services [17], low confidence comprehending genetic/genomic results [20], and hesitation toward genomic testing due to the possibility of discrimination in certain insurance policies [17, 18]. Similarly, both primary care physicians and oncologists have reported perceived low levels of knowledge, awareness and understanding of genetics and genomics [21,22,23,24], and/or low confidence when ordering and interpreting genetic/genomic tests [18, 25,26,27,28].
In Australia, HRECs oversee and review the design and conduct of human research studies [29], adhering to national criteria [30] for ethical review principles, procedures, policies and guidelines. Certified HRECs are those that have been assessed and deemed compliant by the National Health and Medical Research Council (NHMRC) [30]. In Australia, HRECs consist of a minimum of eight members, captured by one of six categories: chair, at least two “lay” people (one male and one female), a person with knowledge of, and current experience in, the professional care, counselling or treatment of people, one pastoral carer, one lawyer, and at least two people with research experience relevant to the research proposals being evaluated [29].
No studies have explored overall comfort and confidence levels of HREC members when reviewing genomic research applications. However, a North American study reported low confidence levels of ethics committee/review board members when assessing the risks and benefits of disclosing incidental findings from genomic studies [31, 32]. In Australia, a case report highlighted that multiple ethics committees reviewing the same genomics application had divergent assessments, which suggests a lack of clear guidance when reviewing these applications [33]. Recently, revisions were made to the NHMRC national statement [30], expanding the chapter on genetic and genomic research studies. However, it is unclear whether further resources and guidelines are necessary to support HRECs in reviewing these studies.
This study used online surveys to evaluate Australian HREC members’ comfort and confidence in evaluating genomic studies. The term “genomic” is used, in this paper and survey, to encompass both genetic and genomic subject matter. Specifically, we aimed to investigate factors influencing confidence levels in reviewing genomic research, identify currently used resources and the need for further resources, and understand preferences for resource types to support the review of genomic research applications.
Methods
Ethics approval for this study was obtained through the University of Queensland HREC (UQ #2019002416) and ratified by the University of Technology Sydney HREC (ETH19-C0005).
Participants and recruitment
The members of 196 HRECs (fifty-one of which were certified) [29] in Australia were invited to participate. Contact details of HREC administrators were obtained from the NHMRC Research Quality and Priorities group [30]. Email invitations to participate in an anonymous online survey were sent to all HREC administrators for forwarding to their members. Administrators were asked to notify the researcher (RP) when they had emailed their members. A follow up email was sent to each HREC administrator after two weeks, if no response had been received.
Individuals interested in participating accessed the survey via email link. Surveys were hosted by the University of Queensland (UQ) using the Checkbox survey tool®. A brief participant information statement was included at the start informing participants of the study rationale, ethics approval information, and how data would be stored and processed (See Supplementary Data). Consent was considered implied if members opted to complete the survey.
Data collection
The survey comprised of 26 items (See Survey in Supplementary Data), most of which were custom, due to a lack of validated relevant tools. Four multiple choice and one open field item captured membership category, HREC affiliation, and experience reviewing general and genomic specific studies. Eight items asked participants to rate (/10) their perceived confidence levels reviewing genomic research. Four additional items asked participants about different types of resources (expert consultation, NHMRC National Statement, internet for ethics and internet for science), whether they were currently used when assessing genomic studies (yes/no), and if so, how useful they were (/10). An open field was provided for further comments. Two items asked participants to rate (/10) how heavily they relied on other HREC members when reviewing research in general, and genomic research specifically. One (yes/no) item assessed the perceived need for further resources to support HREC members reviewing genomic studies, which resource types would be most useful (select all that apply), which resources types would be least useful (select all that apply), and provided open fields for participants to add comments. One multiple-choice item captured participants’ highest level of educational attainment, and three additional (yes/no) items captured genomic specific education through attendance of courses/lectures and their utility (/10). Genetic/genomic literacy was assessed using GeneLiFT, a previously validated tool to assess familiarity with genomic terms via word recognition [34]. Finally, an open field item was available for participants to add any further comments at the end of the survey.
Data analysis
Data were exported from Checkbox in a text-delimited format, and imported into Microsoft Excel and IBM SPSS Statistics [35] for analysis. The GeneLiFT tool assessed genomic literacy by calculating participants ability to identify genetic specific words (2 points), general medical words (1 point), and non-words (−1 point) as real terms. The GeneLiFT tool uses 51 terms; 15 genetic specific, 16 general medical, and 20 non-word terms (possible scores ranging from −20 to 46). Due to human error, two genetic specific terms and one non-word, were not included in the survey tool, therefore possible scores ranged from −19 to 42.
Individual dimensions of genomic confidence with genomic science, ethics, participant risk and consent were consolidated to create an overall genomic confidence score (GCS) (/10) with genomic research review. Simple linear regression was used to identify associations between GCS and other variables, p values < 0.05 were considered significant. Student’s t-tests were used to identify any significant differences between ratings of reliance on other HREC members when reviewing general vs genomic research, ratings of usefulness for genomic education modalities, and between dimensions of confidence when reviewing genomic studies.
Descriptive analyses of resources currently accessed by HREC members summarised their perceived “usefulness”, adequacy (whether further resources were needed), and which resource types were preferred. Content analysis of open field responses identified illustrative quotes which explained participants’ preferences. Researcher CW categorised text responses about preferences for educational resources into codes which were discussed and consolidated by researcher AML. Codes were subsequently sorted into categories and overarching themes.
Results
Participants
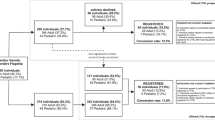
Invitations were sent to HREC committees in February 2020. Contact details could not be obtained for five committee co-ordinators and six declined to circulate the survey, leaving the assumed number of HRECs surveyed to be 185/196 (94%). Seven hundred and fifty respondents accessed the online survey and 141/750 fully or partially completed it (19% completion rate). The mode of recruitment, through HREC administrators, did not allow for the collection of individual data on reasons for non-attempt and non-completion.
Participant characteristics
The characteristics of the participants can be seen in Table 1. The majority (93/109; 85.3%) had a postgraduate education, some (15/109; 13.8%) had undergraduate/bachelor degrees, and one (1/109, 0.9%) had trade/technical/vocational training. Most were members of HRECs affiliated with a public hospital/health services (72/141, 51.1%) or public university/educational institutions (31/141, 22.0%). Forty five of 137 (32.8%) were researchers, 40/137 (29.2%) lay members, 17/137 (12.4%) nurses or allied health workers, 12/137 (8.8%) chairs, 12/137 (8.8%) who worked in pastoral care, and 11/137 (8.0%) were lawyers. Eighty-eight of 141 (62.4%) reported serving \(\ge\)3 years on their HREC while 53/141 (37.6%) had served \(\le\!\!2\) years.
Participants’ HRECs reviewed genomic studies variably: 32/112 (28.6%) every 2–3 months, 28/112 (25.0%) 2–3 times per year, and 22/112 (19.6%) once per year. The average estimated number of genomic research applications reviewed by HRECs annually was fifteen (n = 81; CI 7.1, 25.3). Most members (n = 45, 56%) reported reviewing \( < \)10 genomic studies annually. Participants were comparably reliant on other HREC members when reviewing genomic research specifically (7.0/10; CI = 6.4, 7.5; n = 106) as compared to reviewing all research in general (6.6/10; CI = 6.2, 7.1; n = 109). This was confirmed by Student’s t-test (P = 0.18) (Table 1).
Seventy three participants (73/116, 62.9%) reported having undertaken some or multiple forms of genomic specific education. This was most commonly through lectures about the ethical considerations of genomic research (n = 60/116; 51.7%) with a “usefulness” rating of 7.4/10 (CI = 6.9, 7.9). Some participants (n = 25/116; 21.6%) had completed an award unit of study in genomics and ranked it as moderately useful 6.8/10 (CI = 5.6, 7.9). Short courses on genomics were the least common form of genomic education among participants (n = 9/115; 7.8%) with a “usefulness” rating of 6.6/10 (CI = 4.5, 8.8). Of note, 46/116 (39.7%) did not report attending/receiving any form of genomic education.
Familiarity with genetic/genomic terms was evaluated for 103/141 (73.0%) respondents using the GeneLiFT tool [36]. On average, participants scored 28.0/42 (CI 25.7, 30.4) distinguishing between non-words and real terms related to genomics and medical research in general. Of note, 81 participants (78.6%) were highly familiar with genetic/genomic terms (score ≥ 21), while 22 (21.4%) lacked familiarity (score < 21).
Genomic confidence
Average scores for each dimension measuring confidence in reviewing genomic research are summarised in Table 2. Participants reported the highest levels of confidence when evaluating the ethical considerations of genomic studies (6.4/10; CI 6.0, 6.9), followed by understanding the content to be included in consent forms for genomic research studies (6.1/10; CI 5.6, 6.6). Confidence was lowest in recognising genomics studies that could result in incidental findings (5.4/10; CI 4.9, 5.9), and when reviewing the science of genomic studies (5.2/10; CI 4.7, 5.6). The cumulative average revealed an overall genomic confidence score (GCS) of 5.7/10.
Student’s t-tests comparing confidence levels between individual dimensions of genomic confidence are displayed in Table 2. Participants were significantly more confident evaluating the ethics as compared to the science of genomic studies (p < 0.001), the type of sample used (p = 0.002), the types of results (p = 0.003), implications for family members (p = 0.006), and incidental findings (p = 0.001). Members were also more confident in evaluating the type of research as compared to the science of genomics (p = 0.005) and incidental findings (p = 0.025), and the consent content as compared to the science of genomics (p = 0.003), the sample type (p = 0.036), the result type (p = 0.043) and incidental findings (p = 0.016).
Predictors of genomic confidence score
Simple linear regression identified independent predictors of GCS (Table 1): being a category B “lay” member (p = 0.001); heavier reliance on other HREC members when reviewing genomic studies (p < 0.001), a greater number of years served on the HREC by the member (p = 0.03), HRECs’ frequency reviewing genomic studies (p < 0.001), the annual estimated number of genomic studies reviewed by the HREC (p = 0.02), familiarity with genomic terms (p = 0.01) and genomic education. Specifically, genomic education award courses (p < 0.001), short course (p < 0.001), or lectures on ethical considerations of genomic research (p = 0.001) were positively associated with GCS.
HREC member preferences for educational resources
Resources currently used by HREC members and their “usefulness” ratings are summarised in Table 3. The most frequently used resource was the NHMRC National Statement (90/114, 78.9%) with a “usefulness” rating of 7.0/10 (CI 6.6, 7.4), followed by the internet for information on the science of genomics (61/112, 54.5%) with a rating of 7.0/10 (CI 6.6, 7.4), the internet for information on the ethics of genomic research (n = 41, 36.9%) with a rating of 7.2/10, (CI 6.6, 7.7), and seeking guidance from an expert (30/115, 26.1%) rated as 8.2/10 (CI 7.5, 8.9). Most frequently cited internet resources for understanding the science of genomics, were scientific literature, databases and journals (54/112, 48.2%), and public educational sites (n = 39/112, 34.8%). Videos and “other” resources were the least accessed resources (5/112, 4.5% and 1/112, 0.9% respectively). Similarly, the most frequently accessed internet resources for ethics of genomics were scientific literature, databases and journals (32/111, 28.8%). Educational sites for the lay public (1/111, 0.9%), videos (3/111, 2.7%) and other resources (3/111, 2.7%) were not frequently accessed. Supplementary Table 1 presents specific websites and non-internet resources that participants found helpful when reviewing genomic studies.
The majority (n = 83, 75.5%) agreed on the need for further resources to support HREC members reviewing genomic studies (Table 3). Resource preferences and open field quotes are summarised in Table 3. The most preferred resource types were online courses (56/85, 65.9%), printed materials (55/85, 64.7%), and face to face courses (45/85, 52.9%). Responses in open fields revealed that online courses are easily accessible, easy to refer back to, and up to date. Printed materials were selected because of their ease of access, ability to reference quickly, and because they allowed for annotation/mark-up. Participants selected a hotline as the least helpful resource type (n = 42, 46.7%), followed by face-to-face courses (n = 13, 14.4%), printed materials (n = 9, 10.0%), and online courses (n = 8, 8.9%). Face-to-face courses were not viewed as ideal due to time, scheduling, and economic constraints.
There were a limited number of responses to the “additional comments” open field section (n = 13). Some respondents (n = 2) expressed their support for this research as well as their agreement regarding HREC committees’ needs for further education around genomic research, detailed their interest in the area of genomic research (n = 1), outlined topics they would personally like to learn more about (n = 2), and pointed to the need for lay language in genomic research ethics applications (n = 3).
Discussion
Participants were highly educated, and the majority were affiliated with public health services and universities. Two thirds of participants reported receiving some form of genomic education and the majority had high familiarity with genomic terms. Despite this, confidence in evaluating genomic research applications remained low/moderate. Factors positively associated the GCS included number of years served on the HREC committee, familiarity with genomic terms, the frequency and number genomic studies reviewed, and having undertaken genomic education. Lay members and/or those who reported higher reliance on colleagues when reviewing genomic studies, had lower genomic confidence levels. Participants agreed on the need for further resources to support the review of genomic studies and expressed preferences for online courses and/or printed materials.
The high level of education in this group combined with the fact that the majority of participants had undertaken some form of genomic specific education, may explain the high familiarity with genomic terms. Overall educational attainment [37] and genomic education specifically [18], have previously been shown to be positively associated with genomic literacy/knowledge. However, one study showed that even highly educated individuals may be less familiar with genomics as compared to healthcare in general [17]. Approximately, two thirds of participants indicated that they had undertaken some form of genomic education, which is an unexpectedly high proportion, considering findings within Australia have shown that when continuing genomic education sessions are offered to healthcare providers, attendance is limited [38]. Similarly, awareness and understanding of genetic services among health consumers is low [17]. Furthermore, the literature reports that, internationally, non-genetic physicians and members of the general public reported receiving limited genomic education [36].
Participants reported low/moderate confidence levels in their ability to review genomic research applications, despite having demonstrated familiarity with genetic/genomic terms, indicating one component of high genomic literacy, and the fact that a substantial portion had undertaken some type of genomics education. Lack of confidence about genomic information despite adequate knowledge has been previously reported in a United States study where non-genetics physicians with relatively high perceived medical knowledge about genetics had less confidence in their understanding of the benefits risks and limitations of genetic testing [39]. We found that GCS was positively influenced by having undertaken genomic education, familiarity with genomic terms, years of experience on the HREC committee, and experience reviewing genomic studies. Previous studies have shown that genomic education [18, 38, 40], and genomic literacy [24, 38] are both positively associated with genomic confidence. Education levels have also been previously associated with improved genomic confidence and self-efficacy among health consumers [20], however, given the homogenous nature of this cohort, where the majority had a postgraduate qualification, it is not possible to explore associations between education level and GCS.
Of note, HREC members in this study reported feeling most confident evaluating ethical considerations in general, but least confident reviewing the science of genomics research. According to the Australian Statement on Ethical Conduct in Human Research, it is the role of the HREC to assess whether the benefits of the study outweigh the risks, and whether these risks have been adequately communicated to participants [30]. However, HRECs are not expected to be experts in the science of genomics. It is, therefore, the responsibility of the researcher to clearly communicate the study design and methods in lay terms to enable review and adequate consideration of ethical issues by HRECs [30].
This is the first study to report that years of HREC membership and experience reviewing genomic research studies, positively influenced overall genomic confidence. One qualitative study showed that healthcare providers believed experience working with genomics increased genomic confidence levels [38]. In Australia, a study showed that the frequency with which cancer physicians ordered genetic testing was associated with increased genomic confidence levels, however, it was unclear whether genomic confidence was a cause or effect of test ordering behaviour [26]. Similarly, a study in the United States reported genetic test ordering to be a key predictor of genomic confidence in family physicians [18]. Of note, the findings from our study imply that it is the regular practice of reviewing genomic information which improves confidence of HREC members, and not vice versa. However, almost half of participants reported that their HRECs reviewed genomic studies infrequently (three or less times per year). Unsurprisingly, members of non-health/university related affiliations, non-experts in genetics, and those who reported relying more heavily upon other HREC members when evaluating genomic studies had lower GCSs. This is reflective of previous studies from Australia and internationally showing that the lay public have low perceived knowledge of genomics and genomic testing [17, 20, 39].
We found that ethics committee members access a variety of appropriate resources when reviewing genomic studies, most frequently, the NHMRC national statement and scientific literature, which all received similar helpfulness ratings (moderately/very helpful). Nonetheless, they expressed a need for further support resources. The literature echoes calls for additional resources to support consumers and healthcare professionals when utilising genomic information [27, 38, 41]. However, this is the first study to showcase this need for HREC members specifically. Participants from this study preferred resources in the form of online courses and printed materials. Open-field responses related to this topic suggested that online resources were preferred to face to face courses and hotlines, primarily due to accessibility, particularly with regards to working around time constraints and scheduling. Preference for online learning in Australia [17] and internationally [42] have been expressed previously. Of note, a recent study of Australian health consumers reported that they were less inclined to access printed materials [17], which contradicts this study’s findings. This may reflect the different needs and motivations of the health consumers in contrast with HREC members who are actively using online resources daily.
Limitations
Limitations of this study include low participation levels from ethics committee members, possibly in part, due to the study design that did not allow for the researcher to remind individual participants directly. Lack of feedback from HREC administrators meant that there was uncertainty regarding the number of committees to whom the survey had been circulated. Self-selection of participants may have resulted in an ascertainment bias whereby individuals who are particularly interested in this topic elected to participate. This combined with the low response rate limits the generalisability of these results. The question capturing the estimated number of genomics applications reviewed by participants’ HRECs annually could have been more clearly worded. As it stands it is unclear whether this number pertains to full-review of new applications only or also includes amendments. The GeneLiFT tool also has limitations in that it assesses only one component or pillar of genetic literacy, familiarity with vocabulary, which aligns with awareness of genetics [43]. This does not imply that people with high genetic literacy, based on the GeneLiFT tool, have high levels of understanding or principles knowledge, as these are usually individually assessed. Due to human error, two genomic specific terms and one non-word were omitted from the GeneLiFT tool, potentially affecting its accuracy.
Conclusions
Our findings show low/moderate genomic confidence levels in highly educated HREC members with high familiarity with genomic terms. Genomic education, familiarity with genomic terms, and the frequency with which HRECs reviewed genomic studies all positively influenced genomic confidence. This study demonstrates that even well-educated HREC members, where many have undertaken genomics education modules, expressed a need for further support resources when evaluating genomic research applications, and preferred both online-courses and printed materials due to their ease of access. Encouragingly, even short-term genomic education interventions appear to positively affect confidence in reviewing genomic information.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Khan R, Mittelman D. Consumer genomics will change your life, whether you get tested or not. Genome Biol. 2018;19:4–7.
Martinez-martin N, Magnus D, Martinez-martin N, Magnus D. Privacy and ethical challenges in next-generation sequencing Privacy and ethical challenges in next-generation sequencing. Expert Rev Precis Med Drug Dev. 2019;4:95–104.
Barazzetti G, Cavalli S, Benaroyo L, Kaufmann A. Still rather hazy at present: citizens’ and physicians’ views on returning results from Biobank research using broad consent. Genet Test Mol Biomark. 2017;21:159–65.
Budin-Ljøsne I, Teare HJA, Kaye J, Beck S, Bentzen HB, Caenazzo L, et al. Dynamic consent: a potential solution to some of the challenges of modern biomedical research. BMC Med Ethics. 2017;18:1–10.
Mascalzoni D, Hicks A, Pramstaller P, Wjst M. Informed consent in the genomics era. PLoS Med. 2008;5:1302–5.
Johnson SB, Slade I, Giubilini A, Graham M. Rethinking the ethical principles of genomic medicine services. Eur J Hum Genet. 2020;28:147–54.
Kaye J, Boddington P, De Vries J, Hawkins N, Melham K. Ethical implications of use of whole genome methods in medical research. Eur J Hum Genet. 2010;18:398–403.
Eckstein L, Garrett JR, Berkman BE. A framework for analyzing the ethics of disclosing genetic research findings. 2014;42:190–207.
Bombard Y, Brothers KB, Fitzgerald-Butt S, Garrison NA, Jamal L, James CA, et al. The responsibility to recontact research participants after reinterpretation of genetic and genomic research results. Am J Hum Genet. 2019;104:578–95.
Tibben A, Dondorp W, Cornelis C, Knoers N, Brilstra E, van Summeren M, et al. Parents, their children, whole exome sequencing and unsolicited findings: growing towards the child’s future autonomy. Eur J Hum Genet. 2021;29:911–9.
Hart MR, Biesecker BB, Blout CL, Christensen KD, Amendola LM, Bergstrom KL, et al. Secondary findings from clinical genomic sequencing: prevalence, patient perspectives, family history assessment, and health-care costs from a multisite study. Genet Med. 2019;21:1100–10.
Kaye J, Terry SF, Juengst E, Coy S, Harris JR, Chalmers D, et al. Including all voices international datasharing governance. Hum Genom. 2018;12:18–23.
Arbour L, Cook D. DNA on loan: Issues to consider when carrying out genetic research with aboriginal families and communities. Commun Genet. 2006;9:153–60.
Kowal E, Orphan DNA. Indigenous samples, ethical biovalue and postcolonial science. Soc Stud Sci. 2013;43:577–97.
Vidgen ME, Kaladharan S, Malacova E, Hurst C, Waddell N. Sharing genomic data from clinical testing with researchers: public survey of expectations of clinical genomic data management in Queensland, Australia. BMC Med Ethics. 2020;21:1–11.
Liao SM. Is there a duty to share genetic information? J Med Ethics. 2009;35:306–9.
Wallingford CK, Cutler K, Istiko SN, Fowles LF, Lamb R, Bean J, et al. Queensland consumers’ awareness and understanding of clinical genetics services. Front Genet. 2020;11:1–8.
Haga SB, Carrig MM, O’Daniel JM, Orlando LA, Killeya-Jones LA, Ginsburg GS, et al. Genomic risk profiling: attitudes and use in personal and clinical care of primary care physicians. J Gen Intern Med. 2011;26:834–40.
Tiller J, Keogh L, Wake S, Delatycki M, Otlowski M, Lacaze P. Genetics, insurance and professional practice: survey of the Australasian clinical genetics workforce. Front Public Heal. 2018;6:1–7.
Carere DA, Kraft P, Kaphingst KA, Roberts JS, Green RC. Consumers report lower confidence in their genetics knowledge following direct-to-consumer personal genomic testing. Genet Med. 2016;18:65–72.
Hamilton JG, Abdiwahab E, Edwards HM, Fang M, et al. Primary care providers’ cancer genetic testing-related knowledge, attitudes and communication behaviors: a systematic review and research agenda. J Gen Intern Med. 2017;32:315–24.
Chow-White P, Ha D, Laskin J. Knowledge, attitudes, and values among physicians working with clinical genomics: a survey of medical oncologists. Hum Resour Health. 2017;15:1–9.
Papaioannou K, Kampourakis K. Health-care professionals’ awareness and understanding of genomics. In Applied genomics and public health. Cambridge, Massachusetts, Academic Press 2020 (pp. 225–242).
Gingras I, Sonnenblick A, De Azambuja E, Paesmans M, Delaloge S, Aftimos P, et al. The current use and attitudes towards tumor genome sequencing in breast cancer. Sci Rep. 2016;6:1–8.
Blazer KR, Nehoray B, Solomon I, Niell-swiller M, Culver JO, Uman GC, et al. Next-generation testing for cancer risk: perceptions, experiences, and needs among early adopters in community healthcare settings. Genet Test Mol Biomarkers. 2015;19:657–65.
Demeshko A, Pennisi DJ, Narayan S, Gray SW, Brown MA, McInerney-Leo AM. Factors influencing cancer genetic somatic mutation test ordering by cancer physician. J Transl Med. 2020;18:413.
Harding B, Webber C, Rühland L, Dalgarno N, Armour C, Birtwhistle R, et al. Bridging the gap in genetics: a progressive model for primary to specialist care. BMC Med Educ. 2019;19:1–10.
Johnson LM, Valdez JM, Quinn EA, Sykes AD, McGee RB, Nuccio R, et al. Integrating next-generation sequencing into pediatric oncology practice: an assessment of physician confidence and understanding of clinical genomics. Cancer. 2017;123:2352–9.
National Health and Medical Research Council. Report on the Activity of Human Research Ethics Committees and Certified Institutions for the period: 1 January 2019 to 31 December 2019. National Health and Medical Research Council: 2020. Available from https://www.nhmrc.gov.au/about-us/resources/activity-human-research-ethics-committees-and-certified-institutions.
National Health and Medical Research Council. List of Human Research Ethics Committees registered with NHMRC. National Health and Medical Research Council: 2018. Available from: https://www.nhmrc.gov.au/sites/default/files/documents/attachments/registered-hrecs.pdf.
Gliwa C, Yurkiewicz IR, Lehmann LS, Hull SC, Jones N, Berkman BE. Institutional review board perspectives on obligations to disclose genetic incidental findings to research participants. Genet Med. 2016;18:705–11.
Dressler LG, Smolek S, Ponsaran R, Markey JM, Starks H, Gerson N, et al. IRB perspectives on the return of individual results from genomic research. Genet Med. 2012;14:215–22.
De Smit E, Kearns L, Clarke L, Dick J, Hill C, Hewitt A. Heterogeneity of human research ethics committees and research governance offices across Australia: an observational study. Australas Med J. 2016;9:33–9.
Milo Rasouly H, Cuneo N, Marasa M, DeMaria N, Chatterjee D, Thompson JJ, et al. GeneLiFT: a novel test to facilitate rapid screening of genetic literacy in a diverse population undergoing genetic testing. J Genet Couns. 2020;30:742–54.
IBM Corp. Released 2020. IBM SPSS Statistics for Macintosh, Version 27.0. Armonk, NY; IBM Corp.
Calzone KA, Kirk M, Tonkin E, Badzek L, Benjamin C, Middleton A. The global landscape of nursing and genomics. J Nurs Scholarsh. 2018;50:249–56.
Wright CF, FitzPatrick DR, Firth HV. Paediatric genomics: diagnosing rare disease in children. Nat Rev Genet. 2018;19:253–68.
Mcclaren BJ, Crellin E, Janinski M, Nisselle AE. Preparing medical specialists for genomic medicine: continuing education should include opportunities for experiential learning. Front Genet. 2020;11:1–11.
Haga SB, Barry WT, Mills R, Ginsburg GS, Svetkey L, Sullivan J, et al. Public knowledge of and attitudes toward genetics and genetic testing. Genet Test Mol Biomark. 2013;17:327–35.
Whitley KV, Tueller JA, Weber KS. Genomics education in the era of personal genomics: academic, professional, and public considerations. Int J Mol Sci. 2020;21:768.
Savard J, Hickerton C, Tytherleigh R, Terrill B, Turbitt E, Newson AJ, et al. Australians’ views and experience of personal genomic testing: survey findings from the Genioz study. Eur J Hum Genet. 2019;27:711–20.
Rubanovich CK, Cheung C, Mandel J, Bloss CS. Physician preparedness for big genomic data: a review of genomic medicine education initiatives in the United States. Hum Mol Genet. 2018;27:R250–8.
Abrams LR, McBride CM, Hooker GW, Cappella JN, Koehly LM. The many facets of genetic literacy: assessing the scalability of multiple measures for broad use in survey research. PLoS One. 2015;10:1–11.
Acknowledgements
We thank the NHMRC Research Quality and Priorities group for providing contact details for the HREC co-ordinators. We would also like to thank the HREC members who graciously agreed to participate.
Funding
AML is funded by a National Health and Medical Research Council (NHMRC) Early Career Fellowship (ID 1158111). RMW was supported by an ARC Discovery Project (ID 180100269). The University of Queensland Diamantina Institute is located in The Translational Research Institute, supported by a grant from the Australian Government. CW is supported by an Australian Government Research Training Program Scholarship.
Author information
Authors and Affiliations
Contributions
RP, CJ and AML were responsible for study design, and data collection. RP, AML and CW were responsible for data analysis, interpreting results, and writing the manuscript. JB, SBC, LE, RM, BT, and CJ gave input on questionnaire design and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
LE has recently received funds from Praxis Australia to develop training materials for HRECs. The other authors declare no competing interests.
Ethics approval
Ethics approval for this study was obtained through the University of Queensland Human Research Ethics Committee (HREC) (UQ #2019002416) and ratified by the University of Technology Sydney HREC (ETH19-C0005).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pysar, R., Wallingford, C.K., Boyle, J. et al. Australian human research ethics committee members’ confidence in reviewing genomic research applications. Eur J Hum Genet 29, 1811–1818 (2021). https://doi.org/10.1038/s41431-021-00951-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-021-00951-5
This article is cited by
-
Genomics elucidates both common and rare disease aetiology
European Journal of Human Genetics (2021)