Abstract
Rapid genomic testing in critically ill neonatal and paediatric patients has transformed the paradigm of rare disease diagnosis, delivering results in real time to inform patient management. More than 20 studies totalling over 1500 patients from diverse healthcare settings worldwide have now been published, forming a compelling evidence base for healthcare system implementation. We review the reported diagnostic and clinical outcomes, as well as broader evaluations of family and professional experiences, cost effectiveness, implementation challenges and bioethical issues arising from rapid testing. As rapid genomic testing transitions from the research to the healthcare setting to become a ‘standard of care’ test, there is a need to develop effective service delivery models to support scalability at both the laboratory and clinical level and promote equity of access, prompt test initiation, integrated multidisciplinary input and holistic family support. Harnessing the high level of professional engagement with rapid genomic testing programmes will continue to drive innovation and adoption, while close integration with emerging precision medicine approaches will be necessary to deliver on the promise of reduced infant and child mortality.
Similar content being viewed by others
Introduction
Twenty years after the completion of the Human Genome project, genomic sequencing can now deliver life-saving rare disease diagnoses in just 13 hours [1]. Technological advances have driven an explosion in rare disease gene discovery research [2], and substantial government investments have accelerated the implementation of genomic testing in healthcare worldwide [3]. To truly impact on healthcare outcomes for patients and families, however, it is imperative that results are delivered in real time to influence clinical decisions. This is particularly true for infants and children with rare disease who are critically unwell, and this patient group has become the focus of efforts to develop rapid genomic testing pathways. Since the first ‘proof-of-principle’ demonstration of a 50-hour turnaround time to genomic result nearly a decade ago [4], >20 studies have now been published describing experience with rapid genomic testing in over 1500 critically ill neonatal and paediatric patients worldwide [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26] (Table 1). Here, we review the current evidence for diagnostic and clinical utility, as well as broader evaluation of family and professional perspectives and make recommendations for healthcare system implementation.
What is rapid genomic testing?
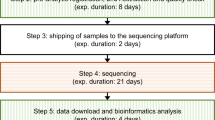
Conventional diagnostic genomic testing in rare disease typically returns results in 1–6 months from sample receipt. Given the relatively high cost of sequencing, laboratory workflows are generally designed to achieve economies of scale by processing groups of samples together (batching). Batching can occur at multiple steps including sample preparation, sequencing, bioinformatic processing, analysis and reporting. Considerable shortening of laboratory processing times (in the range of 2–3 weeks to report) is possible by simply prioritising samples within established workflows [11]. However, achieving very rapid turnaround times such as <5 days generally requires additional changes, which increase cost. Depending on laboratory testing volumes, it may require the use of separate lower capacity sequencers or using sequencers at below their sample capacity. Specialised bioinformatics pipelines may accelerate data processing, and analysis and reporting may need to occur outside of usual laboratory hours. Concomitant sequencing of parental samples (trio sequencing) enables faster reporting of definitive results by reducing the number of variants considered during analysis and establishing variant inheritance (such as de novo status in dominant disorders, or confirming variants are in trans for recessive disorders). Linkage with electronic medical records to facilitate extraction of phenotypic information, and the use of artificial intelligence in analysis and reporting hold promise to further shorten test times [27]. Variable requirements for confirming diagnostic findings using orthogonal methods such as Sanger sequencing may lead some laboratories to issue ‘preliminary’ reports, by e-mail or verbally, with final reports following weeks later.
While targeted panels, exomes and genomes have all been used in rapid genomic testing programmes, genome sequencing (GS) may be the preferred modality for achieving faster results due to shorter sample preparation times and the ability to comprehensively assess multiple variant types, including copy number variants, short tandem repeats, and mitochondrial genome variants, in a single test [26]. These advantages, however, need to be balanced against increased computational load and increased overall cost. Some key points for laboratory services to consider in deciding which testing modality to employ are presented in Table 2.
Most diagnostic laboratories are capable of processing occasional or small numbers of samples within urgent timeframes. However, the reliable, consistent delivery of rapid genomic test results at scale requires the establishment of robust laboratory workflows, and additional investment in infrastructure and workforce capacity [23]. Business continuity plans must seek to minimise delays caused by equipment breakdowns, plastics and reagent supply issues, computing delays or failures, and staff shortages. Highly complex cases, for example those with variants identified in genes with new or limited aetiological evidence, benefit from multi-disciplinary input prior to reporting and may require follow up studies to fully determine the clinical significance of results. Clinical presentations can evolve quickly, requiring effective communication between clinicians and the laboratory to optimise data interpretation. Rapid turnaround times of around two weeks are typical of most studies reported to date, with only two reporting routinely achieving ultra-rapid times of <5 calendar days [23, 24].
Diagnostic and clinical utility
Over 20 studies have now reported clinical outcomes of rapid genomic testing in cohorts ranging in size from 10 to 213 patients [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. The vast majority of these studies have been observational, with rapid genomic testing applied to selected groups of patients, without a comparator against which to evaluate outcomes. However, benchmarking studies capturing diagnostic outcomes prior to the implementation of genomic testing indicate that around 21–26% of NICU patients referred for genetic consultation receive a confirmed diagnosis, though rarely during hospital admission [28, 29]. Two studies evaluating the diagnostic outcomes of rapid genomic testing have adopted a randomised controlled trial approach to study design [10, 25]. The NSIGHT-1 trial randomised infants to receive rapid GS or standard genetic tests. It was terminated early due to loss of equipoise, with 73% of controls receiving genomic tests and 15% undergoing compassionate cross-over to rapid GS [10]. The NICUSeq Study Group randomised infants to receive GS results 15 days (early) or 60 days (delayed) after enrolment [25]. At 60 days, twice as many infants in the early group vs delayed group received a change in clinical management (P = 0.009). At 90 days the number of infants with a change in clinical management was similar in both groups (25% overall).
Studies have typically originated at single academic paediatric centres, although six studies have described experience with expansion of testing to larger multi-site networks [11, 16, 22,23,24,25]. Most studies have included patients from both neonatal and paediatric intensive care units, but a substantial number have restricted testing to infants. Only two studies have reported experience with testing a small number of adults [14, 16]. Study patient selection criteria generally emphasise high acuity coupled with high pre-test probability of a monogenic disorder and anticipated clinical utility. Team approaches to patient selection are commonly employed [23, 30] and are recognised to increase diagnostic yield. Professional guidance on patient selection is limited [31], and an example framework developed by the Australian Genomics Acute Care study [23, 32] to guide clinicians is presented in Fig. 1.
The framework provides examples of common clinical presentations suggestive of syndromic, metabolic, neurological and other rare disorders and the probability of identifying an underlying genetic condition, to help guide test initiation (developed by the Australian Genomics Acute Care study, modified with permission from Baynam [32] etc…).
Diagnostic yields have varied between 21% and 73%, and the overall yield across all studies is 37% (567/1533). The NSIGHT-2 trial aimed to evaluate the performance of exome and genome and of singleton and trio-based analysis in this setting and did not demonstrate any differences in diagnostic yield between these approaches, despite the superior analytical performance of GS [26]. The diagnostic yield in adults is 40%; however, the total number reported to date is very small (N = 10 across two studies) and at present it is uncertain whether the results achieved in paediatric cohorts can be extrapolated to adults.
No consensus exists on how clinical utility of genomic testing should be measured [33, 34], hampering robust evaluation and comparison between studies. Rapid genomic testing studies have typically collected information on changes in management following result from referring clinicians (perceived utility). While perceived utility is an important factor in driving adoption, as an outcome measure it may be prone to bias. Changes in management have nevertheless been reported in 20–100% of diagnosed patients, depending on how clinical utility was defined. Studies have consistently emphasised the benefits of a definitive diagnosis, including the avoidance of unnecessary investigations, interventions, and surgical procedures, and for a minority of patients, enabling access to precision treatments, particularly for neurological and metabolic disorders. Many have also highlighted the role that a definitive diagnosis of life-limiting conditions plays in decisions to redirect care towards palliation, reducing unnecessary suffering. Three studies have also reported clinical utility from uninformative results, particularly where this has contributed towards an alternative, non-genetic diagnosis being made, with attendant avoidance of investigative procedures such as tissue biopsies [17, 23, 24]. All studies reported so far have only captured short-term impact on clinical outcomes, although it can be expected that longer-term benefits such as restoration of reproductive confidence in the parents will be similar to that reported in other rare disease infant cohorts [35, 36].
Cost effectiveness
The measurement of cost effectiveness of genomic testing in rare disease remains another fraught area, with very few studies incorporating measures such as quality of life years (QALYs) [35, 36], which are typically considered ‘gold standard’ by health technology assessments. In the critical care setting, it has been postulated that the relatively higher cost of rapid genomic testing will be outweighed by reductions in length of stay in intensive care, typically another high-cost intervention (AU$5000 per day in Australia and approximately £2000 per day in the UK). Four studies have reported substantial cost savings from rapid genomic testing in the order of US$500,000 to US$1400,000 per 100 patients tested [9, 11, 24]. These have typically focused the analysis on the small number of patients where substantial changes in management follow a rapid result and have used expert opinion on investigations, interventions and days in hospital avoided or matched historical controls where possible. One study used a Delphi method to increase the robustness of estimates provided by experts [9]. In addition, two case reports have provided further examples using real hospital cost data [37, 38]. In the first case report, a diagnosis of a syndromic disorder prompted same-day redirection of care to palliation in an infant with congenital diaphragmatic hernia. The diagnosis was made 250 days into the hospital admission, genomic testing having been initiated on day 236, after a complicated clinical course involving multiple surgical procedures, including cardiac surgeries requiring extracorporeal membrane oxygenation (ECMO) support, gastrostomy placement, Nissen fundoplication, and tracheostomy. The cumulative costs of the patient’s admission were in excess of US$1.8 M. Had a referral for rapid testing been made on admission, with result available in 14 days, and decision to palliate six days later, the total costs of the hospital admission would have been US$115,000, a cost saving of US$1.7M [38]. The second case report compared the cost of admission between two siblings with the same condition, investigated seven years apart, with a cost saving of AU$108,828 in hospital costs demonstrated [37].
The importance of considering both health and non-health outcomes when informing funding decisions in healthcare is recognised [39], and discrete choice experiments (DCEs) are increasingly used to provide estimates of personal utility. A DCE specifically aimed at eliciting preferences with regards to rapid and ultra-rapid testing in critically ill children with rare disease demonstrated strong preference for faster turnaround times, with members of the Australian public willing to pay an additional AU$9510 (US$6657) for rapid and AU$11,000 (US$7700) for ultra-rapid genomic testing relative to standard diagnostic care [40].
Ethical issues
Genomic testing in children, and especially newborns, raises important ethical issues related to informed consent, incidental findings, data sharing and privacy, disability rights and discrimination [41, 42]. All of these are pertinent to rapid testing in critically ill children; however, three analyses have examined issues specifically raised by the rapidity of testing within the ICU setting [43,44,45]. These have centred on the impact of the perceived urgency of testing combined with the vulnerable state of parents heightening the existing challenges of informed consent [43]. The prompt return of results, frequently identifying disabling or life-limiting conditions, raises questions about impact of on parent–child bonding, particularly in newborns, and how results may impact decisions about treatment, and especially treatment limitation [43,44,45]. And finally, in light of the relatively high cost of rapid testing, the issue of distributive justice has been examined, including concerns about diverting resources from non-urgent testing [43] or inequitable access [44].
Family experiences
The ability of parents to provide informed consent for genomic testing and generally process complex information while their child is critically unwell is key area of not just ethical but also practical concern [46, 47], and investigating parental experiences forms an important part of evaluating rapid testing programmes. Post-test surveys conducted as part of two large rapid testing studies [23, 26], one in Australia [48] and the other in the US [49] elicited response rates of 57% (55 families) and 54% (114 families), respectively. Overall, a high proportion of parents perceived testing as being useful. There was little or no decisional regret, over 90% reported receiving enough information at consent, and many reported greater empowerment [48, 49]. Another Canadian study compared responses from 20 families enroled in a NICU-based rapid testing cohort against responses from 44 families from an outpatient-based genomic testing cohort, where the average age of affected children was 10 years. Parents whose infants underwent rapid testing were significantly more likely to select ‘diagnosis’ as the primary motivation for testing and indicated ‘feeling overwhelmed’ as their main concern [50]. Qualitative explorations through interviews provide more nuanced understanding of parental experiences, highlighting further the clinical and psychosocial benefits of alleviating diagnostic uncertainty, though also the challenges for informed decision-making and increased vulnerability during intensive care unit admissions [51,52,53].
Views of health professionals
The views of intensive care physicians and genetics professionals, in particular genetic counsellors, are also key in evaluating and learning from existing research programmes to inform future service design. A mixed-methods study conducted at 13 Australian hospitals and three laboratories prior to implementing a multi-site rapid genomic testing programme, revealed high levels of support for rapid genomic testing in NICU/PICU, and a strong preference for a clinical genetics-led service delivery model [54]. This preference was echoed in interviews with UK health professionals involved in a single-site rapid genomic testing programme [51], specifically in relation to support with patient selection, pre-test counselling and result interpretation. Semi-structured interviews at a single US centre similarly highlighted the need for decision support for intensive care clinicians with clinicians expressing moral distress at the prospect of making high-stakes, irrevocable decisions involving genomic results, with only a partial understanding of them [55]. Despite these concerns, clinicians caring for paediatric patients enroled in rapid genomic testing programmes have consistently reported high perceived clinical utility [23, 51, 56], both for diagnostic and uninformative results [23, 56], and low perceived harms [56].
Genetic counselling experiences and challenges specifically related to rapid genomic testing in critically ill children have been explored in more detail as part of professional reflections [30, 47] or interviews [57]. The importance of pre-test counselling for facilitating informed consent for genomic testing is well recognised, particularly in exploring with families the possibility of variants of uncertain significance, unexpected findings, and data privacy and storage considerations [58]. Genetic counsellors are well placed to assist families through the process of genomic testing and with adjusting to results [59], although traditionally have had little involvement in paediatric critical care settings [30, 57]. Early experiences reported by genetic counsellors working as part of multi-disciplinary teams delivering rapid genomic testing highlight some key challenges: the highly medicalised nature of the environment, with reduced opportunities for privacy; the unpredictable and urgent nature of referrals with lack of preparation time; the vulnerable psychological state of parents contributing to reduced ability to process complex information; and logistical issues associated with test coordination [30, 47, 57]. However, parallels have also been drawn to other established areas of genetic counselling practice involving clinical urgency such as prenatal and cancer settings, transferrable models such as ‘crisis intervention counselling’ and the importance of flexibility and maintaining a family-centred focus [47, 57]. The value of comprehensive post-test counselling cannot be underestimated, not only in helping families adjust to the results, which frequently reveal extremely rare and/or life-limiting conditions, but also in facilitating informed decision-making in future pregnancies and supporting broader family communication [30, 47].
Implementation barriers and enablers
It takes an average of 17 years to implement innovations into healthcare [60], and the approach to implementation not only determines whether an evidence-based intervention is adopted, but also whether it delivers the anticipated benefits. The paucity of theoretically informed implementation in genomics has already been identified as a factor hindering the uptake and long-term viability of clinical genomic testing [61]. Three studies have used implementation science frameworks to systematically identify barriers and enablers to rapid genomic testing in the paediatric setting, and to identify interventions to facilitate uptake [11, 62, 63]. The first two studies used the Consolidated Framework for Implementation Research [64] iteratively, firstly to understand barriers and enablers during an initial study across two Australian hospitals in 2016–17 [11], which then informed an implementation strategy to scale up rapid testing to a national network in 2018–19 [23]. The implementation strategy emphasised communication and feedback, standardised processes, co-ordination, distributed leadership and the importance of fostering collective learning [23]. Key professionals involved in the delivery of the national programme were subsequently interviewed to inform future ‘mainstream’ implementation [62]. Findings illustrate a shift in priorities as implementation progresses, with an initial focus on the ‘relative advantage’ of rapid testing over traditional investigations, and on resolving process issues. Trust in consistent delivery, as well as feedback on outcomes were important in engagement, and appropriate resourcing for both the clinical and laboratory components were highlighted as important for long-term sustainability [62]. The third study [63] examined professional perspectives as part of a multi-site implementation in the US [24], using the NASSS (non-adoption, abandonment, scale-up, spread, and sustainability) technology adoption framework [65]. The study highlighted the role of local champions, and of addressing the educational needs of intensivists in relation to genomic testing. While rapid genomic testing disrupts established workflows and professional roles, both intensivist-led and genetics-led models successfully delivered the service, and professionals held largely positive views about implementing rapid genomic testing, but were concerned about cost, funding models and equity of access [63].
Rapid genomic testing for critically ill children: ready to become standard of care
A large body of evidence and a wealth of practical experience have now been generated through research studies, particularly in North America, Europe and Australia, to inform policy and practice. The clear diagnostic and clinical utility, and cost-effectiveness of rapid testing in critically ill children with rare disease make a compelling case for healthcare system funding. Indeed, the first national healthcare system-funded implementation of rapid genomic sequencing for acutely unwell children commenced in England on 1st October 2019 under the auspices of the new NHS Genomic Medicine Service. During the first year 361 children underwent testing, with a diagnostic yield of 38% (141/361). The molecular diagnosis influenced management in 94% (133/141).
As other healthcare systems embark on implementation, it is important to consider that effective service delivery encompasses supporting not just the laboratory component of testing but also the clinical pathways that promote equity of access, prompt test initiation, multidisciplinary input in result interpretation and holistic family support. The existing high level of professional and service-level engagement provides an opportunity for rapid testing programmes to serve as exemplars for best practice in clinical genomics, including promoting bioinformatics innovation, integration of multi-omic approaches and data sharing to improve diagnostic outcomes. Beyond delivering answers for families, rapid genomic testing programmes need to integrate with emerging precision medicine approaches, if they are to live up to the promise of reducing neonatal and paediatric mortality.
References
Owen MJ, Niemi AK, Dimmock DP, Speziale M, Nespeca M, Chau KK, et al. Rapid sequencing-based diagnosis of thiamine metabolism dysfunction syndrome. N. Engl J Med. 2021;384:2159–61.
Bamshad MJ, Nickerson DA, Chong JX. Mendelian gene discovery: fast and furious with no end in sight. Am J Hum Genet. 2019;105:448–55.
Stark Z, Dolman L, Manolio TA, Ozenberger B, Hill SL, Caulfied MJ, et al. Integrating genomics into healthcare: a global responsibility. Am J Hum Genet. 2019;104:13–20.
Saunders CJ, Miller NA, Soden SE, Dinwiddie DL, Noll A, Alnadi NA, et al. Rapid whole-genome sequencing for genetic disease diagnosis in neonatal intensive care units. Sci Transl Med. 2012;4:154ra135.
Soden SE, Saunders CJ, Willig LK, Farrow EG, Smith LD, Petrikin JE, et al. Effectiveness of exome and genome sequencing guided by acuity of illness for diagnosis of neurodevelopmental disorders. Sci Transl Med. 2014;6:265ra168.
Willig LK, Petrikin JE, Smith LD, Saunders CJ, Thiffault I, Miller NA, et al. Whole-genome sequencing for identification of Mendelian disorders in critically ill infants: a retrospective analysis of diagnostic and clinical findings. Lancet Resp Med. 2015;3:377–87.
Meng L, Pammi M, Saronwala A, Magoulas P, Ghazi AR, Vetrini F, et al. Use of exome sequencing for infants in intensive care units: ascertainment of severe single-gene disorders and effect on medical management. JAMA Pediatr. 2017;171:e173438.
van Diemen CC, Kerstjens-Frederikse WS, Bergman KA, de Koning TJ, Sikkema-Raddatz B, van der Velde JK, et al. Rapid targeted genomics in critically Ill Newborns. Pediatrics. 2017;140:e20162854.
Farnaes L, Hildreth A, Sweeney NM, Clark MM, Chowdhury S, Nahas S, et al. Rapid whole-genome sequencing decreases infant morbidity and cost of hospitalization. NPJ Genom Med. 2018;3:10.
Petrikin JE, Cakici JA, Clark MM, Willig LK, Sweeney NM, Farrow EG, et al. The NSIGHT1-randomized controlled trial: rapid whole-genome sequencing for accelerated etiologic diagnosis in critically ill infants. NPJ Genom Med. 2018;3:6.
Stark Z, Lunke S, Brett GR, Tan NB, Stapleton R, Kumble S, et al. Meeting the challenges of implementing rapid genomic testing in acute pediatric care. Genet Med. 2018;20:1554–63.
Mestek-Boukhibar L, Clement E, Jones WD, Drury S, Ocaka L, Gagunashvili A, et al. Rapid Paediatric Sequencing (RaPS): comprehensive real-life workflow for rapid diagnosis of critically ill children. J Med Genet. 2018;55:721–8.
French CE, Delon I, Dolling H, Sanchis-Juan A, Shamardina O, Mégy K, et al. Whole genome sequencing reveals that genetic conditions are frequent in intensively ill children. Intensive Care Med. 2019;45:627–36.
Powis Z, Farwell Hagman KD, Blanco K, Au M, Graham JM, Singh K, et al. When moments matter: finding answers with rapid exome sequencing. Mol Genet Genom Med. 2020;8:e1027.
Carey AS, Schacht JP, Umandap C, Fasel D, Weng C, Cappell J, et al. Rapid exome sequencing in PICU patients with new-onset metabolic or neurological disorders. Pediatr Res. 2020;88:761–8.
Kamolvisit W, Phowthongkum P, Boonsimma P, Kuptanon C, Rojnueangnit K, Wattanasirichaigoon D, et al. Rapid exome sequencing as the first-tier investigation for diagnosis of acutely and severely ill children and adults in Thailand. Clin Genet. 2021;100:100–5.
Freed AS, Clowes Candadai SV, Sikes MC, Thies J, Byers HM, Dines JN, et al. The impact of rapid exome sequencing on medical management of critically Ill children. J Pediatr. 2020; online ahead of print.
Elliott AM, du Souich C, Lehman A, Guella I, Evans DM, Candido T, et al. RAPIDOMICS: rapid genome-wide sequencing in a neonatal intensive care unit-successes and challenges. Eur J Pediatr. 2019;178:1207–18.
Sanford EF, Clark MM, Farnaes L, Williams MR, Perry JC, Ingulli EG, et al. Rapid whole genome sequencing has clinical utility in children in the PICU. Pediatr Crit Care Med. 2019;20:1007–20.
Śmigiel R, Biela M, Szmyd K, Błoch M, Szmida E, Skiba P, et al. Rapid whole-exome sequencing as a diagnostic tool in a neonatal/pediatric intensive care unit. J Clin Med. 2020;9:2220.
Gubbels CS, VanNoy GE, Madden JA, Copenheaver D, Yang S, Wojcik MH, et al. Prospective, phenotype-driven selection of critically ill neonates for rapid exome sequencing is associated with high diagnostic yield. Genet Med. 2020;22:736–44.
Chung CCY, Leung GKC, Mak CCY, Fung JLF, Lee M, Pei SLC, et al. Rapid whole-exome sequencing facilitates precision medicine in paediatric rare disease patients and reduces healthcare costs. Lancet Reg Health-W. 2020;1:100001.
Australian Genomics Health Alliance Acute Care F, Lunke S, Eggers S, Wilson M, Patel C, Barnett CP, et al. Feasibility of ultra-rapid exome sequencing in critically Ill infants and children with suspected monogenic conditions in the Australian Public Health Care System. JAMA. 2020;323:2503–11.
Dimmock D, Caylor S, Waldman B, Benson W, Ashburner C, Carmichael JL, et al. Project Baby Bear: Rapid precision care incorporating rWGS in 5 California children’s hospitals demonstrates improved clinical outcomes and reduced costs of care. Am J Hum Genet. 2021;108:1231–8.
Krantz ID, Medne L, Weatherly JM, Wild KT, Biswas S, Devkota B, et al. Effect of whole-genome sequencing on the clinical management of acutely Ill infants with suspected genetic disease: a randomized clinical trial. JAMA Pediatr. 2021, online ahead of print.
Kingsmore SF, Cakici JA, Clark MM, Gaughran M, Feddock M, Batalov S, et al. A randomized, controlled trial of the analytic and diagnostic performance of Singleton and Trio, rapid genome and exome sequencing in Ill Infants. Am J Hum Genet. 2019;105:719–33.
Clark MM, Hildreth A, Batalov S, Ding Y, Chowdhury S, Watkins K, et al. Diagnosis of genetic diseases in seriously ill children by rapid whole-genome sequencing and automated phenotyping and interpretation. Sci Transl Med. 2019;11:eeat6177.
Malam F, Hartley T, Gillespie MK, Armour CM, Bariciak E, Graham GE, et al. Benchmarking outcomes in the Neonatal Intensive Care Unit: Cytogenetic and molecular diagnostic rates in a retrospective cohort. Am J Med Genet Part A. 2017;173:1839–47.
Tan NB, Tan TY, Martyn MM, Savarirayan R, Amor DJ, Moody A, et al. Diagnostic and service impact of genomic testing technologies in a neonatal intensive care unit. J Paediatr Child Health. 2019;55:1309–14.
Clowes Candadai SV, Sikes MC, Thies JM, Freed AS, Bennett JT. Rapid clinical exome sequencing in a pediatric ICU: Genetic counselor impacts and challenges. J Genet Couns. 2019;28:283–91.
Borghesi A, Mencarelli MA, Memo L, Ferrero GB, Bartuli A, Genuardi M, et al. Intersociety policy statement on the use of whole-exome sequencing in the critically ill newborn infant. Ital J Pediatr. 2017;43:100.
Baynam G. A diagnostic odyssey—red flags in the red sand. Medicus. 2015;55:4043.
Hayeems RZ, Luca S, Assamad D, Bhatt A, Ungar WJ. Utility of genetic testing from the perspective of parents/caregivers: a scoping review. Children (Basel) 2021;8:259.
Hayeems RZ, Dimmock D, Bick D, Belmont JW, Green RC, Lanpher B, et al. Clinical utility of genomic sequencing: a measurement toolkit. NPJ Genom Med. 2020;5:56.
Schofield D, Rynehart L, Shresthra R, White SM, Stark Z. Long-term economic impacts of exome sequencing for suspected monogenic disorders: diagnosis, management, and reproductive outcomes. Genet Med. 2019;21:2586–93.
Stark Z, Schofield D, Martyn M, Rynehart L, Shrestha R, Alam K, et al. Does genomic sequencing early in the diagnostic trajectory make a difference? A follow-up study of clinical outcomes and cost-effectiveness. Genet Med. 2018;21:173–80.
Akesson LS, Bournazos A, Fennell A, Krzesinski EI, Tan K, Springer A, et al. Rapid exome sequencing and adjunct RNA studies confirm the pathogenicity of a novel homozygous ASNS splicing variant in a critically ill neonate. Hum Mutat. 2020;41:1884–91.
Sweeney NM, Nahas SA, Chowdhury S, Campo MD, Jones MC, Dimmock DP, et al. The case for early use of rapid whole-genome sequencing in management of critically ill infants: late diagnosis of Coffin-Siris syndrome in an infant with left congenital diaphragmatic hernia, congenital heart disease, and recurrent infections. Cold Spring Harb Mol Case Stud. 2018;4:a002469.
Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–103.
Goranitis I, Best S, Christodoulou J, Boughtwood T, Stark Z. Preferences and values for rapid genomic testing in critically ill infants and children: a discrete choice experiment. Eur J Hum Genet. 2021;29:1645–53.
Newson AJ. Whole genome sequencing in children: ethics, choice and deliberation. J Med Ethics. 2017;43:540–2.
Lantos JD. Ethical and psychosocial issues in whole genome sequencing (WGS) for newborns. Pediatrics. 2019;143:S1–5.
Gyngell C, Newson AJ, Wilkinson D, Stark Z, Savulescu J. Rapid challenges: ethics and genomic neonatal intensive care. Pediatrics. 2019;143:S14–21.
Wilkinson DJ, Barnett C, Savulescu J, Newson AJ. Genomic intensive care: should we perform genome testing in critically ill newborns? Arch Dis Child Fetal Neonatal Ed. 2016;101:F94–8.
Deem MJ. Whole-genome sequencing and disability in the NICU: exploring practical and ethical challenges. Pediatrics. 2016;137:S47–55.
Diamonstein CJ. Factors complicating the informed consent process for whole exome sequencing in neonatal and pediatic intensive care units. J Genet Couns. 2019;28:256–62.
Ayres S, Gallacher L, Stark Z, Brett GR. Genetic counseling in pediatric acute care: reflections on ultra-rapid genomic diagnoses in neonates. J Genet Couns. 2019;28:273–82.
Brett GR, Martyn M, Lynch F, de Silva MG, Ayres S, Gallacher L, et al. Parental experiences of ultrarapid genomic testing for their critically unwell infants and children. Genet Med. 2020;22:1976–85.
Cakici JA, Dimmock DP, Caylor SA, Gaughran M, Clarke C, Triplett C, et al. A prospective study of parental perceptions of rapid whole-genome and -exome sequencing among seriously Ill infants. Am J Hum Genet. 2020;107:953–62.
Smith EE, du Souich C, Dragojlovic N, Study C, Study R, Elliott AM. Genetic counseling considerations with rapid genome-wide sequencing in a neonatal intensive care unit. J Genet Couns. 2019;28:263–72.
Hill M, Hammond J, Lewis C, Mellis R, Clement E, Chitty LS. Delivering genome sequencing for rapid genetic diagnosis in critically ill children: parent and professional views, experiences and challenges. Eur J Hum Genet. 2020;28:1529–40.
Aldridge CE, Osiovich H, Hal Siden H, Study R, Gen CS, et al. Rapid genome-wide sequencing in a neonatal intensive care unit: a retrospective qualitative exploration of parental experiences. J Genet Couns. 2021;30:616–29.
Lynch F, Nisselle A, Stark Z, Gaff CL, McClaren B: Parents’ experiences of decision making for rapid genomic sequencing in intensive care. Eur J Hum Genet. 2021; online ahead of print.
Stark Z, Nisselle A, McClaren B, Lynch F, Best S, Long JC, et al. Attitudes of Australian health professionals towards rapid genomic testing in neonatal and paediatric intensive care. Eur J Hum Genet. 2019;27:1493–501.
Char DS, Lee SS, Magnus D, Cho M. Anticipating uncertainty and irrevocable decisions: provider perspectives on implementing whole-genome sequencing in critically ill children with heart disease. Genet Med. 2018;20:1455–61.
Dimmock DP, Clark MM, Gaughran M, Cakici JA, Caylor SA, Clarke C, et al. An RCT of rapid genomic sequencing among seriously Ill infants results in high clinical utility, changes in management, and low perceived harm. Am J Hum Genet. 2020;107:942–52.
Lynch F, Nisselle A, Gaff CL, McClaren B. Rapid acute care genomics: challenges and opportunities for genetic counselors. J Genet Couns. 2021;30:30–41.
ACMG Board of Directors. Points to consider in the clinical application of genomic sequencing. Genet Med. 2012;14:759–61.
Brett GR, Wilkins EJ, Creed ET, West K, Jarmolowicz A, Valente GM, et al. Genetic counseling in the Era of genomics: what’s all the Fuss about? J Genet Couns. 2018;27:1010–21.
Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104:510–20.
Roberts MC, Kennedy AE, Chambers DA, Khoury MJ. The current state of implementation science in genomic medicine: opportunities for improvement. Genet Med. 2017;19:858–63.
Best S, Brown H, Lunke S, Patel C, Pinner J, Barnett CP, et al. Learning from scaling up ultra-rapid genomic testing for critically ill children to a national level. NPJ Genom Med. 2021;6:5.
Franck LS, Kriz RM, Rego S, Garman K, Hobbs C, Dimmock D. Implementing rapid whole-genome sequencing in critical care: a qualitative study of facilitators and barriers to new technology adoption. J Pediatr. 2021;237:237–43.
Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50.
Greenhalgh T, Wherton J, Papoutsi C, Lynch J, Hughes G, A'Court C, et al. Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J Med Internet Res. 2017;19:e367.
Author information
Authors and Affiliations
Contributions
Both authors conceptualised and wrote the manuscript, critically revised it, and approved the final version for the publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stark, Z., Ellard, S. Rapid genomic testing for critically ill children: time to become standard of care?. Eur J Hum Genet 30, 142–149 (2022). https://doi.org/10.1038/s41431-021-00990-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-021-00990-y
This article is cited by
-
Construction and optimization of multi-platform precision pathways for precision medicine
Scientific Reports (2024)
-
Rapid genomic testing in critically ill patients with genetic conditions: position statement by the Human Genetics Society of Australasia
European Journal of Human Genetics (2024)
-
2022: the year that was in the European Journal of Human Genetics
European Journal of Human Genetics (2023)
-
Multi-omics for better and faster rare disease diagnosis
Nature Medicine (2023)
-
Genomic newborn screening for rare diseases
Nature Reviews Genetics (2023)