Abstract
Reproductive genetic carrier screening (RGCS) allows for the identification of couples who have an increased likelihood of conceiving a child with a particular autosomal recessive or X-linked condition. The aim of this study was to assess the level of satisfaction, anxiety, knowledge retention, psychosocial and counseling-related aspects among couples who chose to have RGCS. Participants were initially informed about their screening results by telephone. After obtaining a written report of test results, participants were asked to complete an individual self-administered questionnaire. All participants (n = 67) felt they had enough information to make an informed choice. None of the participants regretted their choice to have RGCS. Test results were most often shared with parents (61%) or siblings (37%). Our findings demonstrate that the information/counseling and reporting strategy that was used in the context of this study led to high participant satisfaction, an increase in knowledge over time and favorable psychosocial and counseling-related outcomes.
Similar content being viewed by others
Introduction
Reproductive genetic carrier screening (RGCS) allows for the identification of couples who have an increased likelihood of conceiving a child with a particular monogenic recessive condition. The information gained through RGCS can be used to make informed reproductive decisions when planning for a future pregnancy [1].
In 2019, a Belgian RGCS offer became available to couples considering having children in the future including more than 1000 genes associated with multiple autosomal recessive (AR) and X-linked conditions. The test is specifically intended for individuals who have no personal or family history of genetic conditions. Currently, the test has a cost price of 1400 euros per couple. All individuals who wish to undergo RGCS are asked to sign an informed consent form. Blood samples are taken from both reproductive partners simultaneously and the analysis is performed exclusively through the accredited laboratories of the Belgian genetic centers. Results are communicated as either a “normal couple result” which means that there is no demonstrable increased risk or as an “abnormal couple result” which entails that there is an increased risk of having a child with one of the genetic conditions screened for. In addition, patients obtain individual carrier status for seven of the most frequent AR conditions (ACADM, CFTR, DHRC7, GJB2, HBB, PAH, and SMN1) and the X-linked conditions (female) to allow for cascade testing.
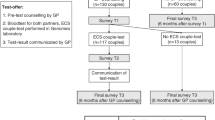
Following the introduction of the Belgian RGCS offer, we implemented a small-scale longitudinal study with three specific objectives. First, we wanted to assess the intention to have RGCS among non-pregnant couples in the general population recruited via a group practice of fourteen gynecologists located in a city in Flanders (Belgium). Secondly, we wanted to assess the extent to which couples make informed choices regarding participation in RGCS. Findings related to the first two objectives have been described elsewhere [2, 3]. Within our study, most nonpregnant women visiting their gynecologist (81%) showed the intention to have RGCS [2]. However, not everyone decided to accept the free RGCS offer. We have reported high rates of informed choice (82%) among couples who did choose to have RGCS [3].
Here, we present the results related to the third objective of the research project which assessed the level of satisfaction, anxiety, knowledge retention, and psychosocial and counseling-related aspects among couples who choose to have RGCS and obtained their screening test results.
As information gained through RGCS can have certain social consequences (e.g., informing family members) and could also have an impact on psychological well-being and health perception (e.g., feeling less healthy after being identified as a carrier) we aimed to learn from the lived experiences of couples who underwent RGCS and received their screening test results. Anxiety levels might for example increase while waiting for screenings results or after being identified as a carrier or an at-risk couple [1]. Earlier studies focusing on preconception RGCS for larger test panels reported favorable psychosocial outcomes and high satisfaction among participants who chose to have RGCS [4, 5]. Nevertheless, some studies did report increased anxiety levels like the American study by Kraft et al. [6] where participants had increased anxiety while waiting for screening results but no negative impact in the long term [6]. Another example is a Dutch study by van Dijke et al. [5] where high-risk individuals (with an a priori high risk of being a carrier or carrier couple) and pregnant women had significantly higher anxiety before receiving screening test results compared to individuals within the general-risk group and non-pregnant.
While the Belgian RGCS offer has some similarities with the RGCS offers that have been evaluated so far, there are also specific aspects that make this offer different from other RGCS offers (e.g., communication of screening results = couple-based + individual test results for some of the most frequent AR conditions, inclusion of X-linked conditions, RGCS related costs etc.). We argue that more evidence on the possible psychological and social impact of an RGCS offer is necessary to ensure a responsible implementation of RGCS both in Belgium and on an international level. A better comprehension of the lived experiences of couples who opted to have the Belgian RGCS offer could be most valuable to illustrate the possible impact of this kind of RGCS offer. Therefore collecting follow-up information on those taking part will be essential. In addition, these insights could also be used to improve future RGCS programs and policy by enabling the development of key frameworks in order to support informed reproductive decision-making.
Methods
Nonpregnant women of reproductive age visiting their gynecologist were invited to answer a self-administered questionnaire assessing the intention to have RGCS (objective 1). Prior to filling in the questionnaire, participants were briefly informed about RGCS. Participants who showed the intention to have RGCS were asked to consider participation in a follow-up clinical study where RGCS was offered free of charge. At least one week after the initial contact moment, the researcher re-contacted the female participants to inquire about their decision to accept or decline the RGCS offer. If participants (=two reproductive partners) were interested to participate, they were sent an extensive information leaflet prior to a pre-test counseling session. Both reproductive partners had to be present during this counseling session. If couples agreed to have RGCS they were asked to complete an individual self-administered questionnaire (objective 2) after their blood samples were taken and another individual self-administered questionnaire (objective 3) when receiving back their test results. Women who were pregnant, >40-year-old women, individuals with a history of bone marrow transplantation, minors, and those not able to read and write in Dutch or not able to give informed consent were excluded from participation in this study. A more detailed overview of the recruitment strategy and study set-up of this longitudinal study has been described earlier [2, 3].
Participants were initially informed about their RGCS screening results over the phone by a researcher (EVS) between September 2019 and January 2021. Subsequently, a written report of test results was sent by registered mail to all participants. Each participant received an individual report including their couple-result, their individual test results for seven autosomal recessive conditions (ACADM, CFTR, DHRC7, GJB2, HBB, PAH, and SMN1) and X-linked conditions (female participants). If there were any ambiguities or questions, participants were free to contact the researcher for further explanation. Together with the written report of test results, participants also received the final questionnaire of the research project. Participants were asked to return the completed questionnaires by using the prepaid envelope that was provided to them. A one-time reminder email was sent to all the participants to help improve the response rate.
The questionnaire assessed participants’ satisfaction, knowledge retention, anxiety, and psychosocial/counseling-related aspects (see supplementary material). To assess knowledge and anxiety we used the same measures that were used within the questionnaires that participants were asked to fill out at the end of the pre-test counseling session [3]. The knowledge scale including 14 knowledge items (score range 0–14, α = 0.729) that was specifically developed for this research project has been described elsewhere [7, 8]. Anxiety was measured using the State-Trait Anxiety Inventory (STAI-6) [9] (six items, α = 0.825) and transferred to prorated 20-item STAI scores (score range 20–80). A score ≥40 was considered clinically relevant [5, 10, 11]. The Dutch version of the STAI-6 has been validated before in a preconception setting and has shown good reliability and validity [12].
Data-analysis
Statistical analyses were performed using IBM SPSS® Statistics 28 for Windows. Descriptive analysis was used to report on all items included in the questionnaire. Analyses were based on complete case analysis. Each participant was treated as an individual study subject.
Ethics
The research was conducted in accordance with the Declaration of Helsinki and local statutory requirements. Approval to conduct this human subject’s research was obtained by the Research Ethics Committee UZ/KU Leuven (S63243). Written informed consent was obtained from all participants. Participation was voluntary and participants had the right to stop at any time.
Results
The mean turnaround time for reporting test results was 39 weeks (SD 9, IQR 38–42). Twelve study participants (n = 12/82; 15%) were identified as a carrier of one autosomal recessive (AR) condition and one female participant was identified to be carrier of an X-linked condition (see Table 1). In total, 67 out of 82 participants who obtained their screening test results returned a completed questionnaire by mail resulting in a response rate of 82% (n = 67/82). This group of 67 participants included nine participants who were identified as a carrier and eight participants whose partner was identified as a carrier of a genetic condition. Within the group of individuals that dropped out, there were four identified carriers. Seven female participants were pregnant when receiving their test results (n = 7/41; 17%) One couple broke up while waiting for results, all other participants were still in a relationship with the same partner.
Satisfaction, regret, and recommendation
None of the participants regretted their choice to have RGCS. The majority of participants also indicated that they would make the same choice to have RGCS if they had to decide again (n = 62/67; 92%) and that they would recommend RGCS to couples with a desire to have children (n = 63/67; 94%) (see supplementary materials). All test results were initially communicated over the phone by the researcher (E.V.S.) who performed the pre-test counseling sessions and who acted as the central contact person throughout the study. Thirty couples chose the female partner as the first point of contact (n = 30/41; 73%) to receive the test results and four couples (n = 4/41; 10%) the male partner. In addition, seven couples (n = 7/41; 17%) preferred to be informed individually. The vast majority of participants indicated to be (somewhat or completely) satisfied with the way results were communicated (n = 61/67; 91%). The turnaround time was considered to be (far) too long by 58% (n = 39/67) of participants (see supplementary material).
Psychosocial outcomes
If test results were shared with others (n = 51/67; 76%), this was mostly done with parents (n = 41/51; 80%), siblings (n = 25/51; 49%) or friends (n = 20/51; 39%). Some participants had communicated their test results to their gynecologist (n = 11/51; 22%), their general practitioner (n = 4/51; 8%), other family members (n = 6/51; 12%) or colleagues (n = 3/51; 6%) at the time of completing the questionnaire. All identified carriers shared their test results with someone else (n = 9; 100%) such as their parents (n = 8/9; 89%), their siblings (n = 6/9; 67%), friends (n = 5/9; 56%), their general practitioner (n = 3/9: 33%), their gynecologist (n = 3/9, 33%) or other family members (n = 1/9; 11%). Only a minority of participants (n = 5/67; 7%) indicated that they were concerned about the possibility that their family members could be carriers of the conditions that are included in the test. All participants (n = 67/67; 100%) reported that the decision to have RGCS did not impact their relationship with their partner. Similarly, most participants stated that the decision to have RGCS (n = 63/67; 94%) and the test results they received (n = 61/67, 91%) did not impact the (possible) desire to have children with their current partner. A small proportion of participants felt worried while waiting for the test results (n = 11/67; 16%). All participants felt confident that the screening results that they received were correct and 92% (n = 62/67) of participants indicated that they do not feel worried about their own screening results. None of the participants agreed with the statement that they feel less healthy after receiving their screening results (see supplementary material).
Counseling related aspects
The information brochure that study participants received through email before coming to the pre-test counseling session was completely read by 66% (n = 44/67) of participants and partly by 31% (n = 21/67). All study participants felt they had enough information to make an informed choice. Three participants (4%) looked up additional information before coming to the pre-test counseling session through the internet. Specifically, they searched for more information about the principles of inheritance (n = 2) and more information about reproductive options of couples with an increased likelihood of conceiving a child with a hereditary condition (n = 1).
Most participants indicated that based on the information they obtained, it was sufficiently clear what their own individual result (n = 64/67; 95%) and their couple result (n = 66/67; 98%) entailed. One-fifth of study participants (n = 14; 21%) looked up additional information after receiving their screening results. This group included nine individuals that were identified as a carrier of a monogenic condition and four partners of individuals that were identified as a carrier of a monogenic condition. Twelve of these participants looked up additional information through the internet, while two participants consulted their gynecologist and two other participants consulted their general practitioner. Participants specifically sought more information about the principles of inheritance (n = 1), more information on the condition of which they are a carrier (n = 6) and more information about the conditions included in the test panel (n = 8).
Knowledge
The mean knowledge score for our study sample was 11.8 (SD 2.5, IQR 10–14) compared to a mean knowledge score of 10.4 (SD 1.8, IQR5–12) during the pre-test counseling session. Most participants (n = 55/65; 85%) answered at least 10 out of 14 knowledge questions. Most knowledge items on the knowledge scale were answered correctly by the vast majority of participants (84–98%), with the exception of the questions assessing participants understanding of autosomal recessive inheritance which were answered correctly by far fewer participants (K10 = 40%; K11 = 57%) (see Table 2). Knowledge scores improved over time for 45 participants and declined for six participants. In addition, no changes in knowledge score were observed for nine participants (see supplementary materials). Nine out of ten participants (n = 61/67; 91%) correctly answered that couples who receive a normal couple result still have a chance of conceiving a child with a hereditary condition and 97% (n = 65/67) of participants understood that the risk for a couple with an increased likelihood of conceiving a child with a hereditary condition is not absolute.
State-trait anxiety inventory
The mean STAI score for our study sample was 26.9 (SD 7.8, IQR 20–33.3). The STAI score increased over time for 28 participants and declined for 16 participants. In addition, no changes in the STAI score were observed for another 16 participants. Five participants (7%) had anxiety scores that are considered clinically relevant (score ≥40) (see Table 3). These participants did not have clinically relevant anxiety scores before the intervention. All other participants with an increase in STAI scores over time still had no clinically relevant anxiety score.
Discussion
Our study results demonstrate that most participants were satisfied with their choice to have RGCS and the way results were communicated. In addition, only a small proportion of participants felt worried while waiting for their screening results. These findings are in line with a previous Dutch study by van Dijke et al. [5] where couples’ experiences with an RGCS offer for 50 severe AR conditions were evaluated [5]. All study participants felt they had enough information to make an informed choice and that based on the information they obtained, it was sufficiently clear what their own individual result and their couples’ result entailed. We have previously shown that 82% of our study participants also made an informed choice with regard to RGCS according to our modified version of the Multidimensional measure of informed choice [3, 13]. The information brochure that was developed in the context of the research study was not completely read by all participants. We would therefore like to underline the added value of giving information at multiple time-points and through different ways (e.g., information brochure, pre-test counseling session, telephone reporting of results, written test report) like it was organized in our study setting. We believe that an information brochure could complement but not replace more in-depth counseling. Providing information in advance could facilitate efficient and effective pre-test counseling [11]. Interactive education tools like a patient decision aid could help clarify theoretical concepts in a non-directive way and stimulate a process of deliberation in settings with limited resources. If participants looked up additional information this was mostly done through the internet, which also demonstrates the need to offer good quality information via this route.
The turn-around time was found to be too long by our study participants. The initially set turn-around time of ±6 months was not achieved in the majority of cases because of multiple reasons (COVID-19 pandemic, difficulties encountered during the analysis). A quiet similar result was reported by van Dijke et al. [5] even though the turn-around time (±7 weeks) was considerably shorter within this study [5]. Even though the turn-around time for the Belgian RGCS offer has currently been reduced to ±3 months, this finding shows how important it is to inform couples with a desire to have children about the possibility to have RGCS in due time to allow for informed reproductive decision-making and to increase reproductive autonomy. Hereby, reproductive autonomy specifically refers to the capacity to reflect on one’s values and preferences (e.g., long-term goals) relevant to inform choices with regard to reproduction decision making (e.g., when to become pregnant, whether to continue a pregnancy, etc.) [14]. The current predominant focus on reproductive autonomy may lead to the idea that RGCS is a clinical intervention. However, RGCS could also be seen as a public health intervention because of common features with other screening offers available to the public (e.g., testing of individuals without an a priori risk). While acknowledging that prevention of certain genetic conditions as a main goal for RGCS is problematic because of the possibility of implicit judgements, it’s important to acknowledge social and relational factors (e.g., socio-economic conditions) beyond an individual’s sphere of control that can undermine or limit reproductive choices [14]. While RGCS doesn’t aim to change or improve the genetic composition of the whole population compared to some past eugenics programs it is important to acknowledge potentially eugenic effects at a societal level. Improving informed reproductive decision-making could result in a reduction of the prevalence of conditions screened for when at-risk couples opt to have prenatal diagnoses followed by pregnancy termination of an affected fetus, to undergo PGT-M, to use donor gametes, to adopt or to refrain from having biological children together [1].
Some of the couples who participated in the study did not wait for their screening test results to become pregnant. This result might even be an underestimation given the fact that couples were also eligible to participate in this study when they weren’t actively planning for a family and the drop-out we encountered due to noncooperation of certain participants. At the moment of the pre-test counseling session, 64 study participants (n = 64/82, 78%) indicated a desire to have children, of which 39% (n = 25/64; 13 females and 12 males) within the timeframe of the next year [3]. The seven women that became pregnant while waiting for the test results were indeed part of this group (n = 7/13; 54%), whereas the six other female participants stated they were not pregnant at the time of filling out the questionnaire.
The mean knowledge score among study participants increased from pre-intervention (M 10,.4, SD 1.8, IQR 5–12) to post-intervention (M 11.8, SD 2.5, IQR 10–14). While it is possible that participants looked up information while completing the questionnaire or discussed the knowledge questions with their partner in their home environment, this finding could also be due to the fact that participants received information multiple times. Another plausible explanation could be that participants accumulated knowledge over time by filling in the same knowledge items in the two questionnaires. In the Dutch study by van Dijke et al. [5] which mainly included both high-risk individuals (e.g., positive family history, consanguinity, etc.) and individuals from the general population knowledge increased slightly increased over time, but this difference was not found to be significant [5]. Previous studies focusing on screening for single gene conditions (e.g., Cystic Fibrosis, Tay-Sachs disease) have also reported reasonable retention of knowledge among those who had screening [15,16,17]. Noteworthy, is the study of Ioannou et al. (2010) where knowledge decreased among Ashkenazi Jewish high schools student following the expansion of a screening program for Tay Sachs disease with six additional conditions. The authors indicated that the increase in provided information on multiple conditions might have resulted in a lower level of genetic knowledge.
Only a small proportion of our study participants had STAI scores that were clinically relevant (≥40) after receiving their screening test results. These results are in line with the findings of a Dutch study by Birnie et al. [4] where no significant differences in mean STAI scores were found over time among couples from the Dutch general population who accepted a couple-based RGCS offer for 50 AR conditions provided by GP’s [4]. Likewise, an American study by Kraft et al. [6] where couples took part in a clinical study of preconception carrier screening using genome sequencing reported similar findings [6]. Within the Dutch study of Birnie et al. [4], 13% of test-acceptors had clinically relevant anxiety levels at 6 months after the counseling session with their GP [4]. As Birnie et al. [4] have pointed out, the absence of adverse psychological outcomes on a group level doesn’t mean that the RGCS offer was anxiety-free for everyone. Our results have also shown that an RGCS offer can still potentially lead to increased anxiety for some individuals. Van Dijke et al. [5] reported a significant (p < 0.001) decrease in STAI scores after participants had received their screening results. Within this study also no significant differences in anxiety were found between those that were identified as carriers and those that were identified as non-carriers for the conditions screened for.
About three-quarters of the participants shared their screening test results with others, and this mainly with parents and/or siblings. Test results were only shared to a very limited extent with health care providers like the gynecologist and/or the general practitioner. Participants that were identified as a carrier most often shared results with parents and siblings but only one identified carrier shared this information with other family members. These results may be explained by the fact that the questionnaires were sent out together with the written report of test results and that therefore participants might not yet have had the opportunity to share their screening results with their healthcare providers or other family members. Carriers identified through CF population screening in an Australian study by Gorrie et al. [18] most often reported speaking with a sibling and/or parent about their increased risk of being a carrier of CF [18] and much less with those outside the immediate family which is in line with our study results. It has been suggested that family members don’t always receive sufficient information to be able to make an informed choice with regard to carrier screening [19]. We believe that family communication after carrier identification through reproductive genetic carrier screening needs further investigation to assess to what extent cascade screening is being used in this context and which factors influence the decisions of family members. This would allow a more critical reflection on the desirability and utility of reporting individual test results for the opportunity to offer cascade screening in a context with limited resources for follow-up. In addition, it is noteworthy that some participants within the American study by Kraft et al. [6] did not share their negative test results with their health care provider because they did not see the need to do so or because they assumed results were already included in their medical record [6]. Healthcare providers should be aware of their responsibility for proper follow-up of patients to avoid that the implications of negative results are being misunderstood by their patients.
Future RGCS screening programs should ideally include a long-term monitoring and evaluation (M&E) process that helps to gain more insights into the potential impact of RGCS on the subsequent reproductive decision-making of couples with an increased likelihood of conceiving a child with a genetic condition. In addition, there should also be a close M&E of the clinical utility of the RGCS offer. Especially considering that to date the clinical significance of pathogenic variants rests incomplete [20]. Future research projects should also focus on the decision-making process of individuals/couples who are uncertain/undecided about RGCS or who decline RGCS. This would allow to gain more in-depth insights into the reasoning of these particular groups, to understand their concerns, their doubts/remaining questions, etc.
Study strengths and limitations
One of the strengths of this study is that we recruited couples from the general population in a setting where RGCS will most likely be offered in the near future. In addition, the counseling sessions weren’t performed by a trained genetics professional but by a researcher with a background in midwifery and health promotion. Within this study, we focused on test acceptors. As a result, we are not able to report on the views/experiences of test-decliners or those who initially showed the intention to have RGCS but finally decided not to participate in our study. Future research should pay specific attention to these specific groups. The last survey of our implementation study was also sent out together with the written report of test results immediately after participants received their screening results over the phone. Therefore, we are not able to report long-term impact of receiving screening results. The measures used to assess participants' satisfaction and psychological/counseling aspects were not validated for the Belgian setting. Finally, results should be interpreted with caution due to the limited sample size and the drop-out we encountered due to non-cooperation of certain participants.
Conclusion
Our results show that most participants were satisfied with their choice to have RGCS. Overall, anxiety levels were low while knowledge levels were generally high. The decision to have RGCS did not impact the relationship of participants or their desire to have children in the future. Only a small proportion of participants felt worried while waiting for the test results. Most participants positively evaluated the information/counseling and reporting strategy that was used in the context of this study.
Human studies and subjects
Approval to conduct this human subjects research was obtained by the Research Ethics Committee UZ/KU Leuven (S62558, S63243). All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Written informed consent for genetic testing was obtained from all individuals undergoing testing.
Animal studies
No non-human animal studies were carried out by the authors of this article.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Henneman L, Borry P, Chokoshvili D, Cornel MC, van El CG, Forzano F, et al. Responsible implementation of expanded carrier screening. Eur J Hum Genet. 2016;24:e1–e12.
Van Steijvoort E, Demuynck R, Peeters H, Vandecruys H, Verguts J, Peeraer K, et al. Reasons affecting the uptake of reproductive genetic carrier screening among nonpregnant reproductive-aged women in Flanders (Belgium). J Genet Coun. 2022;31:1043–53. n/a(n/a)
Van Steijvoort E, Peeters H, Vandecruys H, Verguts J, Peeraer K, Matthijs G, et al. Exploring informed choice in preconception reproductive genetic carrier screening by using a modified Multidimensional Measure of Informed Choice. Patient Educ Couns. 2022;105:3313–8.
Birnie E, Schuurmans J, Plantinga M, Abbott KM, Fenwick A, Lucassen A, et al. Couple-based expanded carrier screening provided by general practitioners to couples in the Dutch general population: psychological outcomes and reproductive intentions. Genet Med. 2021;23:1761–8.
van Dijke I, Lakeman P, Sabiri N, Rusticus H, Ottenheim CPE, Mathijssen IB, et al. Couples’ experiences with expanded carrier screening: evaluation of a university hospital screening offer. Eur J Hum Genet. 2021;29:1252–8.
Kraft SA, Schneider JL, Leo MC, Kauffman TL, Davis JV, Porter KM, et al. Patient actions and reactions after receiving negative results from expanded carrier screening. Clin Genet. 2018;93:962–71.
Van Steijvoort E, Devolder H, Geysen I, Van Epperzeel S, Peeters H, Peeraer K, et al. Expanded carrier screening in Flanders (Belgium): an online survey on the perspectives of nonpregnant reproductive-aged women. Personal Med. 2021;18:361–73.
Van Steijvoort E, Devolder H, Geysen I, Van Epperzeel S, Peeters H, Peeraer K, et al. Knowledge, attitudes and preferences regarding reproductive genetic carrier screening among reproductive-aged men and women in Flanders (Belgium). Eur J Hum Genet. 2022;30:1255–61.
Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol. 1992;31:301–6.
Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res (Hoboken). 2011;63:S467–72.
Schuurmans J, Birnie E, van den Heuvel LM, Plantinga M, Lucassen A, van der Kolk DM, et al. Feasibility of couple-based expanded carrier screening offered by general practitioners. Eur J Hum Genet. 2019;27:691–700.
van der Bij AK, de Weerd S, Cikot RJ, Steegers EA, Braspenning JC. Validation of the dutch short form of the state scale of the Spielberger State-Trait Anxiety Inventory: considerations for usage in screening outcomes. Community Genet. 2003;6:84–7.
Marteau TM, Dormandy E, Michie S. A measure of informed choice. Health Expect. 2001;4:99–108.
Dive L, Newson AJ. Ethics of reproductive genetic carrier screening: from the clinic to the population. Public Health Ethics. 2021;14:202–17.
Barlow-Stewart K, Burnett L, Proos A, Howell V, Huq F, Lazarus R, et al. A genetic screening programme for Tay-Sachs disease and cystic fibrosis for Australian Jewish high school students. J Med Genet. 2003;40:e45.
Gordon C, Walpole I, Zubrick SR, Bower C. Population screening for cystic fibrosis: knowledge and emotional consequences 18 months later. Am J Med Genet Part A. 2003;120A:199–208.
Henneman L, Bramsen I, van der Ploeg HM, ten Kate LP. Preconception cystic fibrosis carrier couple screening: impact, understanding, and satisfaction. Genet Test. 2002;6:195–202.
Gorrie A, Archibald AD, Ioannou L, Curnow L, McClaren B. Exploring approaches to facilitate family communication of genetic risk information after cystic fibrosis population carrier screening. J Commun Genet. 2018;9:71–80.
McClaren BJ, Aitken M, Massie J, Amor D, Ukoumunne OC, Metcalfe SA. Cascade carrier testing after a child is diagnosed with cystic fibrosis through newborn screening: investigating why most relatives do not have testing. Genet Med. 2013;15:533–40.
Silver J, Norton ME. Expanded carrier screening and the complexity of implementation. Obstet Gynecol. 2021;137:345–50.
Acknowledgements
The authors would like to thank all individuals that participated in this study. In addition, they would like to express their gratitude to the team of gynecologists who have contributed to this study.
Funding
This project was financially supported by the Research Fund Flanders (FWO) (G094518N).
Author information
Authors and Affiliations
Contributions
EVS, HP, HV, JV, KP, GM, and PB designed the study. The data collection was carried out by EVS. The data analysis was performed by EVS. A first draft of the manuscript was written by EVS and critically discussed and revised by PB, HP, HV, JV, KP, and GM. PB coordinated the study. All the authors have approved the final version.
Corresponding author
Ethics declarations
Competing interests
EVS, HP, HV, JV, KP, GM, and PB declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Van Steijvoort, E., Peeters, H., Vandecruys, H. et al. Experiences of nonpregnant couples after receiving reproductive genetic carrier screening results in Belgium. Eur J Hum Genet 31, 696–702 (2023). https://doi.org/10.1038/s41431-023-01310-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-023-01310-2
This article is cited by
-
Exploring attitudes and experiences with reproductive genetic carrier screening among couples seeking medically assisted reproduction: a longitudinal survey study
Journal of Assisted Reproduction and Genetics (2024)
-
The complex genomics of single gene disorders
European Journal of Human Genetics (2023)