Abstract
Purpose
Congenital cataract, opacification of the ocular lens, is clinically and genetically a heterogeneous childhood disease. In this study we aimed to identify the underlying genetic cause of isolated autosomal-dominant lamellar cataract in a multi-generation English family.
Methods
Whole-genome sequencing (WGS) was undertaken in two affected subjects and one unaffected individual. Segregation analysis was performed and a known cataract-causing mutation was identified. Segregation was further validated by sanger sequencing in the entire pedigree.
Results
A heterozygous mutation c.7 G > T; p.D3Y was identified in an NH2-terminal region of the gap junction protein GJA3 and found to co-segregate with disease.
Conclusion
We have identified a recurrent mutation in GJA3 in a large British pedigree causing the novel phenotype of autosomal-dominant congenital lamellar cataract. Previously, p.D3Y was found in a Hispanic family causing pulverulent cataract. WGS proved an efficient method to find the underlying molecular cause in this large family, which could not be mapped due to uninformative markers.
Similar content being viewed by others
Introduction
Lens opacity is widely considered to be the primary cause of blindness worldwide. Congenital cataracts are phenotypically and genetically heterogeneous. They are responsible for 1–6/10,000 births in the United Kingdom and 5–15/10,000 births in developing countries and are a pronounced factor of vision loss in infants and children [1].
Congenital cataract can occur in isolation, or in association with other non-ocular diseases. Most familial cataracts are associated with an autosomal-dominant mode of inheritance [2, 3]. Clinical classification depends on the position and type of the lens opacity, such as: blue-dot (cerulean), coralliform, nuclear, cortical, complete, pulverulent and anterior polar, posterior polar, posterior nuclear, polymorphic, and lamellar [4].
So far > 40 genes have been implicated in cataractogenesis, including crystallins encoding transparent intracellular lens proteins, water channel proteins (aquaporins), solute carrier protein, cytoskeletal proteins, chromatin-modifying protein-4B, transcription factors, transmembrane proteins, lens intrinsic membrane protein, receptor tyrosine kinase gene EPH receptor A2 [5], an endoplasmic reticulum membrane-embedded protein, Wolframin [6], and gap junction proteins including GJA8 and GJA3 [5].
Gap junction channels and hemichannels are made by connexins: they play an important role in intercellular communication. Each hemichannel is formed by six connexin units, called a connexon. Two connexons from neighboring cells dock to make a gap junction channel through the extracellular loops of connexins, which allows the exchange of ions and small molecules between cells [7]. In humans, at least 21 connexin genes have been associated with several different genetic defects including deafness, skin abnormalities, neurodegenerative diseases, cardiopathies, and cataracts [8,9,10,11].The lens expresses three discrete connexins: Cx43, Cx50, and Cx46, displaying various levels of expression and function in maintaining lens homeostasis (reviewed in ref. 12).
The lens is a transparent, avascular, and biconvex organ in the anterior chamber of the eye, situated behind the cornea. The cornea and lens transmit light onto the retina for fine focusing. The lens is comprised of two cell types: metabolically active epithelial cells that form a single layer along the anterior surface and fiber cells that form the main bulk of the lens. These fiber cells lose all of their intracellular organelles during differentiation and become metabolically inert. Using the gap junctions to maintain tissue homeostasis and transparency, the lens has therefore developed a substantial intercellular communication system [13]. Cx43 is expressed primarily in the lens epithelial cells, whereas Cx46 and Cx50 are extensively expressed in lens fiber cells [12]. Mutations in Cx50 and Cx46 lead to congenital cataracts in human and mice [14].
Here we report a recurrent mutation (p.D3Y) in GJA3 causing an isolated autosomal-dominant lamellar cataract in a five generation British family. Previously, this mutation has been found in a Hispanic family causing a different phenotype of pulverulent cataract [15].
Methods
Phenotyping
The family was identified through the proband attending the Genetic Service at Moorfields Eye Hospital, London, UK. Local ethics committee approval was obtained and all of the participants gave written informed consent. All the family members underwent full ophthalmic examination, including slit lamp examination; all affected individuals were diagnosed as having isolated lamellar cataract.
Whole-genome sequencing (WGS) and bioinformatics analysis
Genomic DNA was extracted from ethylenediaminetetraacetic acid-sequestered blood samples taken with informed consent and local ethical approval using the Nucleon II DNA extraction kit (ScotlabBioscience, Strathclyde, Scotland, UK). Genomic DNA was processed using the Illumina TruSeq DNAPCR-Free Sample Preparation kit (Illumina) and sequenced using an Illumina Hiseq 2500, generating mean genome coverage of 35 × . WGS was done by a service provider (Macrogen.Inc., Korea). As described in Berry et al. 2017 [16], raw data in fastq format was analyzed using the Phenopolis platform [17]. The short read sequence data were aligned using novoalign (version 3.02.08). Variants and indels were called according using GATK haplotype caller [18]. The variants were then annotated using the Variant Effect Predictor (VEP) [19]. Variants were then filtered to only contain variants not present in public control databases Kaviar (Glusman et al. 2011) [20] and gnomAD (http://gnomad.broadinstitute.org/), and predicted to be moderately or highly damaging according to the VEP. Cosegregation of the filtered variants in both affected individuals was then performed. Finally, the list of variants was further screened using Phenopolis, for genes associated with the Human Phenotype Ontology [21] term “lamellar cataract” (HP:0007971) according to OMIM [22]. The mutations were then ordered on CADD score with the highest-ranking mutations at the top.
Structural bioinformatics
The protein structure of GJA3 was analyzed using SWISS-MODEL https://swissmodel.expasy.org/repository/uniprot/Q9Y6H8.
The best PDB match, with a match of 49%, was the structure of 2ZW3 PDB ID, solved with X-ray crystallography (reference https://www.ncbi.nlm.nih.gov/pubmed/?term = 19340074).
All structures were downloaded in PDB format and analyzed using Pymol (version 1.8) locally.
Sanger sequencing
Bi-directional direct Sanger sequencing was performed to validate the variant identified by WGS. Genomic DNA was amplified by PCR using GoTaq 2 × master mix (AB gene; Thermo Scientific, Epsom, UK) and GJA3-specifc primers designed with Primer3 http://bioinfo.ut.ee/primer3-0.4.0/primer3/. PCR conditions were followed as: 94 °C for 10 min of initial denaturation followed by 30 cycles of amplification of 30 s at 94 °C, 30 s at 60 °C, and 45 s at 72 °C. After the PCR products were reacted with BigDye Terminator v3.1, they were run on ABI 3730 Genetic Analyzer (both from Applied Biosystems) and analyzed using SeqMan Pro (version 8.0.2 from DNASTAR) sequence analysis. After validating the variant, family segregation was performed in all the individuals.
Results
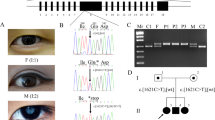
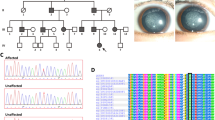
Sixteen members of a large five generation British family including 10 affected, 4 unaffected, and 2 spouses were examined (Fig. 1). All the affected family members had bilateral cataract and age of onset varied from birth to age 20 months. One Individual (III-10) was diagnosed at the age of 3 weeks and also had glaucoma. One of the patients (IV-2) had bilateral cataract at birth, surgery at age 11 years, and suffered bilateral retinal detachment.
WGS was undertaken in two affected (IV-5, V-1) and one unaffected (III-11) member of the family. Variant annotation and filtering was performed using the Phenopolis platform. From a total of 7,096,614 variants in the three individuals, 549,719 were found to co-segregate in the two affected individuals. After filtering for rare variants with a homozygous frequency of 0 and allele frequency < 0.01 in Gnomad and Kaviar, 33,310 variants remained. A gene list of 97 cataract-associated genes was used for gene panel screening, after which, 44 variants remained. The top scoring variant on CADD (score of 27.4) was a known rare heterozygous damaging variant, NM_021954.3:c.7 G > T; p.D3Y, in GJA3 gene on chromosome 13q11-q12 (reference). Direct sequencing confirmed that this missense mutation c.7 G > T in exon 2 of GJA3 co-segregated with all affected members of the family (Fig. 2).
The p.D3Y mutation from aspartate (D3Y) to a tyrosine in the in the NH2-terminal (NT) cytoplasmic tail of the GJA3 protein is likely to affect the degree of metabolite cell-to-cell coupling and is essential for the voltage sensitivity. The aspartate is a negatively charged amino acid, whereas tyrosine is uncharged, which could have some effect on the hemichannel activity [23, 24] (Fig. 3).
Structure of the GJA3 protein. a Transmembrane view of GJA3 https://www.rcsb.org/pdb/explore/explore.do?structureId = 2zw3. b View of the GJA3 hemichannel https://swissmodel.expasy.org/repository/uniprot//Q9Y6H8 c Wild-type amino at position 3 (Aspartate) d Mutant amino acid at position 3 (Tyrosine). The side chain of the tyrosine interferes with the hemichannel activity
Discussion
Here we report a missense mutation c.7 G > T in the gap junction protein (GJA3) gene in a five generation English pedigree with autosomal-dominant congenital lamellar cataract. All the affected family members had bilateral cataract and age of onset varied from birth to age 20 months.
Lamellar cataract is also referred to as zonular cataract and is one of the most common phenotypes of autosomal-dominant congenital cataract. The inner fetal nucleus is made up of a clear lens surrounded by an opacified shell that is in turn surrounded by clear cortex, which may contain opacities referred to as “riders” or “cortical spokes”. Lamellar cataract represents a disturbance in the lens development at a particular time and the cataractous “shell” varies in size according to the stage of fetal development at which the disturbance occurs [4, 16]. The elongated fiber cells of the lens constitute the main bulk of the lens’ mass and represent the target cells for cataract formation owing to miscommunication; GJA3 protein mainly functions in gap junction communication between these cells [25]. Connexin 46 mutations are phenotypically highly heterogeneous [9] (summarized in Table 1).
In 1990, Willecke et al. [26, 27] were the first group to assign GJA3 to chromosome 13, and after 9 years, Mackay et al. found the first connexion 46 mutations in humans causing autosomal-dominant congenital cataract. Connexin 46 comprises two exons encoding a transmembrane protein of 435 amino acids, containing four transmembrane domains (TM1-TM4), two extracellular loops (E1, and E2), an intracellular loop (CL), and cytoplasmic NH2- and COOH termini. Connexins share the same membrane topology among all the family members. So far, 50 (novel and recurrent) cataract-causing mutations in GJA3 have been reported in various ethnic groups. Interestingly, half of the mutations are found in China, and the remainder have been found in other ethnic groups; 6 from India, 4 from Australia, 3 from Denmark, 10 from UK, 2 from USA, and 1 from Honduras; and exhibiting different phenotypes (Table 1).
In the present study, the recurrent p.D3Y(c.7G- > T) change in GJA3 gene resulted in an aspartate (a negatively charged amino acid) to tyrosine (an uncharged amino acid) at position 3 within the NT cytoplasmic tail. The Asp-3 residue of GJA3 is phylogenetically conserved, hence, this indicates aspartate is likely to be functionally important and that the mutation may therefore have a detrimental physiological effect. Several studies have suggested that the NT along with E1 and TM1 contribute to the pore lining region of the hemichannel and therefore any compromise in the amino-acid residues may interfere with the conformation and flexibility of NT and also with voltage gating [28,29,30,31,32]. Schlingmann and co-workers in 2012 has shown the involvement of Asp-3 (D3Y) in the determination of the cell-to-cell coupling and for the voltage dependent Cx46 hemichannels. This hypothesis is further supported by Tong et al. (2013); they demonstrated the effect of D3Y on reduced hemichannel activity and alterations in voltage gating and charge selectivity. Lens fiber cells are dependent on intercellular communication for their survival [33, 34].
Ebihara et al. 2010 [35] has reported the association of connexin 46 with calcium and sodium influx in fiber cells and their important role on the function and development of the lens. Further, the important role of Cx46 in the delivery of glutathione in the lens nucleus has been demonstrated. Cx46 not only have major role in congenital cataract but also age-related cataract, which may give rise to identify new therapeutic strategies [36].
Here, we have found the recurrent p.D3Y (c.7G- > T) mutation in the GJA3 gene in a British family with a different phenotype, lamellar cataract; where previously this variant has only been reported in association with pulverulent cataract. These results show further heterogeneity in inherited cataract, with the same mutation, on a different genetic background, causing a different phenotype, presumably through diverse mechanisms.
Summary
What was known before
-
• Opacification of the ocular lens is clinically and genetically a heterogeneous childhood disease.
-
• Previously, p.D3Y mutation in GJA3 gene was found in a Hispanic family causing pulverulent cataract.
What this study adds
-
• In this study we have identified a recurrent mutation in GJA3 in a large British pedigree causing the novel phenotype of autosomal-dominant congenital lamellar cataract.
-
• Our study show further heterogeneity in inherited cataract, with the same mutation, on a different genetic background, causing a different phenotype, presumably through diverse mechanisms.
References
Rahi JS, Dezateux C, British Congenital Cataract Interest Group. Measuring and interpreting the incidence of congenital ocular anomalies: lessons from a national study of congenital cataract in the UK. Invest Ophthalmol Vis Sci. 2001;42:1444–8.
Reddy MA, Francis PJ, Berry V, Bhattacharya SS, Moore AT. Molecular genetic basis of inherited cataract and associated phenotypes. Surv Ophthalmol. 2004;49:300–15.
Krumpaszky HG, Klauss V. Epidemiology of blindness and eye disease. Ophthalmologica. 1996;210:1–84.
Ionides A, Francis P, Berry V, Mackay D, Bhattacharya S, Shiels A, et al. Clinical and genetic heterogeneity in autosomal dominant cataract. Br J Ophthalmol. 1999;83:802–8.
Shiels A, Bennett TM, Hejtmancik JF. Cat-Map: putting cataract on the map. Mol Vis. 2010;16:2007–15.
Berry V, Gregory-Evans C, Emmett W, Waseem N, Raby J, Prescott D, et al. Wolfram gene (WFS1) mutation causes autosomal dominant congenital nuclear cataract in humans. Eur J Hum Genet. 2013;21:1356–60.
Kumar NM, Gilula NB. The gap junction communication channel. Cell. 1996;84:381–8.
Beyer EC, Berthoud VM. The Family of Connexin Genes. In Book: Connexins. Humana Press; 2009. p. 3–26.
Beyer EC, Ebihara L, Berthoud VM. Connexin mutants and cataracts. Front Pharmacol. 2013;4:43.
Gerido DA, White TW. Connexin disorders of the ear, skin, and lens. Biochim Biophys Acta. 2004;1662:159–70.
Srinivas M, Verselis VK, White TW. Human diseases associated with connexin mutations. Biochim Biophys Acta . 2018;1860:192–201.
Berthoud VM, Minogue PJ, Osmolak P, Snabb JI, Beyer EC. Roles and regulation of lens epithelial cell connexins. FEBS Lett. 2014;588:1297–303.
White TW. Unique and redundant connexin contributions to lens development. Science. 2002;295:319–20.
Gong X, Li E, Klier G, Huang Q, Wu Y, Lei H, et al. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91:833–43.
Addison PKF, Berry V, Holden KR, Espinal D, Rivera B, Su H, et al. A novel mutation in the connexin 46 gene (GJA3) causes autosomal dominant zonular pulverulent cataract in a Hispanic family. Mol Vis. 2006;12:791–5.
Berry V, Pontikos N, Moore A, Ionides A C W, Plagnol V, Cheetham M E and Michaelides M. A novel missense mutation in HSF4 causes autosomal-dominant congenital lamellar cataract in a British family. Eye. 2018 32:806–12.
Pontikos N, Yu J, Moghul I, Withington L, Blanco-Kelly F, Vulliamy T, et al. Phenopolis: an open platform for harmonization and analysis of genetic and phenotypic data. Bioinformatics. 2017;33:2421–3.
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–303.
McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GRS, Thormann A, et al. The ensembl variant effect predictor. Genome Biol. 2016;17:122.
Glusman G, Caballero J, Mauldin DE, Hood L, Roach JC. Kaviar: an accessible system for testing SNV novelty. Bioinformatics. 2011;27:3216–7.
Köhler S, Vasilevsky NA, Engelstad M, Foster E, McMurry J, Aymé S, et al. The human phenotype ontology in 2017. Nucleic Acids Res. 2017;45:D865–76.
Hamosh A, Scott AF, Amberger JS, Bocchini CA, McKusick VA. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005;33:D514–7.
Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–5.
Berman HM,Westbrook J,Feng Z,Gilliland G,Bhat TN, The Protein Data Bank. Nucleic Acids Res. 2000;28:235–42.
Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol. 1991;115:1077–89.
Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Güldenagel M, et al. Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem. 2002;383:725–37.
Mackay D, Ionides A, Kibar Z, Rouleau G, Berry V, Moore A, et al. Connexin46 mutations in autosomal dominant congenital cataract. Am J Hum Genet. 1999;64:1357–64.
Dong L, Liu X, Li H, Vertel BM, Ebihara L. Role of the N-terminus in permeability of chickenconnexin45.6 gap junctional channels. J Physiol. 2006;576:787–99.
Maeda S, Nakagawa S, Suga M, Yamashita E. Structure of the connexin 26 gap junction channel at 3.5 Å resolution. Nature. 2009;458:597–02.
Verselis VK, Ginter CS, Bargiello TA. Opposite voltage gating polarities of two closely related connexins. Nature. 1994;368:348–51.
Oh S, Rubin JB, Bennett MV, Verselis VK, Bargiello TA. Molecular determinants of electrical rectification of single channel conductance in gap junctions formed by connexins 26 and 32. J Gen Physiol. 1999;114:339–64.
Purnick PE, Benjamin DC, Verselis VK, Bargiello TA, Dowd TL. Structure of the amino terminus of a gap junction protein. Arch Biochem. 2000;381:181–90.
Schlingmann B, Schadzek P, Busko S, Heisterkamp A, Ngezahayo A. Cataract-associated D3Y mutation of humanconnexin46 (hCx46) increases the dye coupling of gap junction channels and suppresses the voltage sensitivity of hemichannels. J Bioenerg Biomembr. 2012;44:607–14.
Tong J-J, Minogue PJ, Kobeszko M, Beyer EC, Berthoud VM, Ebihara L. The connexin46 mutant, Cx46T19M, causes loss of gap junction function and alters hemi-channel gating. J Membr Biol. 2015;248:145–55.
Ebihara L, Tong J J, Vertel B, White T W, and Chen T L. Properties of connexin 46 hemichannels in dissociated lens fiber cells. Invest. Ophthalmol. Vis. Sci. 2010. 52, 882–889. https://doi.org/10.1167/iovs.10-6200.36
Slavi N, Rubinos C, Li L, Sellitto C, White TW, Mathias R, et al. Connexin 46 (cx46) gap junctions provide a pathway for the delivery of glutathione to the lens nucleus. J Biol Chem. 2014;289:32694–702.
Acknowledgements
The present work is supported by grants from the National Institute for Health Research Biomedical Research Center at Moorfields Eye Hospital National Health Service Foundation Trust and UCL Institute of Ophthalmology (UK), Moorfields Eye Hospital Special Trustees (UK), Moorfields Eye Charity (UK), and the Foundation Fighting Blindness (USA). Michel Michaelides is supported by an FFB Career Development Award. We thank the members of the family for taking part in this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Berry, V., Ionides, A.C.W., Pontikos, N. et al. Whole-genome sequencing reveals a recurrent missense mutation in the Connexin 46 (GJA3) gene causing autosomal-dominant lamellar cataract. Eye 32, 1661–1668 (2018). https://doi.org/10.1038/s41433-018-0154-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-018-0154-8
This article is cited by
-
In silico analysis of non-synonymous single nucleotide polymorphisms (nsSNPs) in the human GJA3 gene associated with congenital cataract
BMC Molecular and Cell Biology (2020)