Abstract
Purpose
To analyze the serum cytokines profile in patients with tubercular multifocal serpiginoid choroiditis (TB MSC) receiving anti-tubercular therapy (ATT) and oral corticosteroids.
Methods
In this prospective longitudinal study, patients with active TB MSC were included. Serum levels of interferon (IFN)-γ, interleukin (IL)-10, and tumor necrosis factor (TNF)-α were analyzed using bead-based immunoassay. The levels of transforming growth factor (TGF)-β were measured using cytokine bead array. Serial measurement was performed at baseline, 1, 3, and 6 weeks after initiation of therapy. Patients developing paradoxical worsening (PW) of TB MSC were identified and their serum levels of cytokines were compared with those patients who showed healing of lesions. Comparison of cytokine levels with baseline values was also performed.
Results
Twelve patients (three females) were included in the study. Four patients showed paradoxical worsening of TB MSC at 3.2 ± 1 weeks after initiation of therapy. Compared to patients who showed healing of lesions, patients with PW showed higher baseline IL-10 (not significant; p = 0.28). Among patients developing PW, levels of IFN-γ peaked at 1 week ((p = 0.01) and levels of TNF-α peaked at 3 weeks (p = 0.02) (coinciding with PW) compared to patients who showed healing. There was no significant difference in TGF-β levels at any time point in either group (p > 0.47).
Conclusions
Baseline and serial levels of inflammatory serum cytokines may help in predicting the response to ATT and corticosteroids in TB MSC. Patients with paradoxical worsening may show rise in pro-inflammatory cytokines after initiation of ATT indicating higher bacillary load.
Similar content being viewed by others
Introduction
Serpiginous-like choroiditis (recently termed as multifocal serpiginoid choroiditis; MSC) is a distinct variant of tubercular (TB) posterior uveitis that affects Asian Indian young adults [1,2,3]. The lesions are multifocal to begin with, involve both posterior pole and periphery, and may be associated with vitritis [3]. M. tuberculosis infection has been proposed as an etiologic agent for MSC and treatment with anti-tubercular therapy (ATT) has shown to reduce the number of recurrence of the disease [4]. In ~14% eyes with TB MSC, continuous progression of the lesions has been reported following initiation of ATT [5]. Patients with paradoxical worsening need therapy with high-dose corticosteroids in order to minimize the tissue secondary to inflammatory response. Despite advances in clinical and imaging studies, the exact biochemical pathways responsible for such paradoxical worsening have not been elucidated adequately in the literature.
In a study of 1817 patients with pulmonary/extrapulmonary TB, increased incidence of paradoxical worsening was associated with higher antigenic load, HIV positivity and peripheral lymphadenopathy. Thus, development of paradoxical worsening may be related to host immunity [6]. In the context of ocular TB, very few studies have shed light on the biochemical basis of the disease (but not on paradoxical worsening). In a study of TB-related uveitis that employed magnetic color-bead-based multiplex assay, aqueous analysis revealed elevated levels of interleukin (IL)-6, CXCL-8, and interferon gamma (IFN-γ)-induced CXCL-9 and CXCL-10 compared to healthy controls [7]. Another study revealed elevated levels of cytokines such, as IL-15, IL-17, IFN-γ, tumor necrosis factor (TNF)-α and IL-10, and chemokines, such as IL-8, CXCL-9 and CXCL-10 in TB-related uveitis compared to healthy controls [8]. Overexpression of transforming growth factor (TGF)-β is also an important component of pulmonary TB [9]. Thus, mediators such as IFN-γ, IL-10, TNF-α, and TGF-β may play a central role in the pathogenesis of TB including ocular TB.
Thus far, there are lack of studies that have analyzed the baseline serum cytokine profile and changes in their levels following initiation of anti-tubercular therapy (ATT) among patients with TB MSC [10]. Similarly, the immunochemical mechanisms that lead to the development of paradoxical worsening of TB MSC are not known. The index study was performed with an aim to monitor the cytokine levels in patients with TB MSC and determine characteristics of the cytokine signaling that leads to development of paradoxical worsening of the disease.
Materials and methods
This prospective study was performed at the Retina and Uveitis services of the Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India. Institutional Ethics Committee approval was obtained for the protocol prior to the conduct of the study. Subjects diagnosed with TB MSC (in at least one eye) from July 2015 to September 2016 were enrolled in the study. The study adhered to the tenets of the Declaration of Helsinki and the rules laid down by Health Insurance Portability and Accountability Act of 1996 (HIPAA). All those patients where the physician decided to initiate ATT with corticosteroids were enrolled in the study.
Study subjects
The inclusion criteria of the study were presence of active TB MSC lesions (multifocal choroiditis lesions with central healing and active edges), and exclusion of other non-infectious uveitis entities (such as sarcoidosis) by clinical or relevant laboratory tests, along with one of the following criteria: (1) positive tuberculin skin test (positive induration of 10 × 10 mm after 48–72 h) or positive interferon gamma release assay (IGRA) test (QuantiFERON TB Gold/IGRA >0.35 IU/ml or positive T-SPOT.TB®); and/or (2) evidence of healed or active tubercular lesion on chest X-ray or CT chest [1, 11, 12].
The exclusion criteria were presence of media opacities such as dense cataract or vitreous hemorrhage, and poor fixation that did not allow acquisition of adequate quality of images. A minimum follow-up of 8 weeks was required from the initiation of ATT in order to detect paradoxical worsening. Patients diagnosed with any other confounding ocular pathology, such as diabetic retinopathy, hypertension, glaucoma, or optic neuropathy were excluded from the study.
All the patients underwent a thorough systemic evaluation by an internist (AS) to rule out the presence of pulmonary or any other site with extrapulmonary TB. Routinely, all the patients also underwent contrast-enhanced chest tomography prior to initiation of therapy. Additionally, all patients also had their laboratory investigations to rule out other possible etiologies and HIV test too were performed for all. The therapy with ATT and corticosteroids was initiated after close collaboration with the internist with specialized interest in ocular infections and inflammations.
Cytokine analysis
For the purpose of serum cytokine analyses, venous blood sample was drawn from the patients receiving ATT and corticosteroids. A baseline venous blood sample was taken from all the patients before starting the therapy followed by obtaining samples at 1 week, 3 weeks, and 6 weeks after initiation of treatment.
Serum was extracted from the blood samples and the sera were preserved at −80 °C. The cytokines that were measured and analyzed were TNF-α, IFN-γ, IL-10, and TGF-β. The preserved serum samples were thawed on ice and centrifuged at 5000 rpm for 5 min to remove flocculates. The levels of IFN-γ, TNF-α, and IL-10 were measured using LEGENDplexTM bead-based immunoassay (Biolegend Inc., San Diego, CA, USA). Briefly, the individual detection beads (13×) were mixed and diluted to 1X using assay buffer. The standards were prepared from top standard (10,000 pg/ml) using fourfold serial dilution to give final standards (0, 2.4, 9.8, 39.1, 156.3, 625, and 2500 pg/ml). The samples were diluted twofold before use. The samples and standards (25 μL) were mixed with 1X beads (25 μL) in flow tubes and incubated for 2 h in a shaking incubator at 500 rpm. Then detection antibody (25 μL) was added into each tube, followed by SA-PE reagent. The tubes were washed with 200 μL of wash buffer, followed by acquisition at BD FACS LSR Fortessa using FACS DIVA 7.0 software (BD Biosciences USA). The data were analyzed using LEGENDplexTM Data analysis software to get the levels of cytokines in serum samples. The levels of TGF-β were measured in serum from subjects using cytokine bead array (BD Biosciences). The tubes were washed with 200 μL of wash buffer, followed by acquisition at BD FACS LSR Fortessa using FACS DIVA 7.0 software (BD Biosciences USA). The data were analyzed using FCAP array software (BD Biosciences USA) to get the levels of TGF-β in serum samples.
Study treatment
All patients received four-drug ATT, including isoniazid 5 mg/kg daily, rifampicin 450 mg daily (if body weight <50 kg) and 600 mg daily (if body weight more than 50 kg), ethambutol 15 mg/kg daily, and pyrazinamide 25–30 mg/kg daily for first 2 months. Thereafter, rifampicin and isoniazid were continued for another 7 months. Systemic drug toxicity was monitored by doing liver function tests monthly. Oral corticosteroids were given to the patients in the dose of 1 mg/kg and tapered off over a period of 4–6 weeks. The treatment was under supervision of a senior internist who has special interest in systemic inflammatory diseases. The progression of lesions, if any, and response to therapy were documented using fundus drawings, color fundus photographs (Optos ultra-wide field retinal camera, Optos Inc., Scotland, UK) and Visupac FF450 fundus camera (Carl Zeiss Meditec, La Jolla, CA, USA) and fluorescein angiograms. Paradoxical worsening of lesions was defined clinically or on fluorescein angiogram as the development of new lesions in patients who were initiated on ATT. Paradoxical worsening also included lesions that continued to progress after initiation of ATT, as well as lesions that initially seemed to respond but showed worsening after initiating ATT [5, 13].
Statistical analysis
GraphPad Prism® (GraphPad Software Inc., La Jolla, CA) version 6.0 was used for analysis of data. Parametric and non-parametric tests were applied to ascertain significant difference in the pre and post measurements and between the two treatment groups. Non-parametric Mann–Whitney U rank test were used to analyze difference in means. Paired two tailed t-test were used to analyze difference between mean value of cytokines at different follow ups in the same group. The categorical variables were described in proportion. p-value <0.05 was considered statistically significant.
Results
Twelve patients with TB MSC were enrolled in the study which included three females and nine males. All the patients were Asian Indians. The mean age at the time of initial presentation was 32.58 ± 12.32 years. The lesions were bilateral in four patients and unilateral in eight patients. All the patients with active disease in one or both the eyes were started on both ATT and oral steroids (prednisolone acetate 1 mg/kg). All the study patients were Mantoux positive and four of the 12 patients had evidence of lymphadenopathy (mediastinal/pretracheal/paratracheal) on contrast-enhanced computerized chest tomography. None of the patients included in the study were diagnosed with HIV infection. Vitritis was observed in all the study patients (16 eyes) with active disease. Anterior chamber cells were seen in one eye. Patients were started on oral corticosteroids with mean dose of 61.82 ± 6.03 mg.
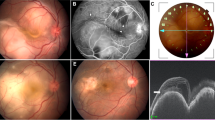
Four (33.3%) out of 12 patients developed paradoxical worsening after initiation of ATT. Paradoxical worsening occurred at a mean duration of 3.2 ± 1 weeks. Three out of four patients with paradoxical worsening had unilateral involvement. Patients with paradoxical worsening received intravenous methylprednisolone (1 g/day) for 3 days. The remaining patients in the study showed healing of TB MSC lesions with which was confirmed on FAF; seen as hyper-autofluorescent edges of the lesions turning hypo-autofluorescent. All the patients with paradoxical worsening who received intravenous methylprednisolone demonstrated healing response and improvement in the lesions after high-dose corticosteroid therapy. Figure 1 (supplemental data) demonstrates paradoxical worsening of a patient with TB MSC requiring intravenous methylprednisolone therapy, compared to another patient (Figure 2; supplemental data) who showed a good healing response to ATT and corticosteroids without paradoxical worsening. The baseline demographic, clinical, and laboratory profile of the patients is described in Table 1.
Cytokine profile analysis
Since the patients who showed paradoxical worsening of TB MSC lesions showed a characteristically different cytokine profile compared to the patients who did not show such worsening on ATT, we have analyzed the cytokine profile separately for the two subsets of patients.
IL-10
The mean value of IL-10 at baseline among the patients developing paradoxical worsening group was 17.54 ± 22.79 pg/ml (Table 2). The baseline IL-10 values in the no worsening group was 7.60 ± 8.4 pg/ml. Thus, the mean IL-10 value at baseline was higher in paradoxical worsening group as compared to the non-paradoxical worsening group. The value of IL-10 increased from baseline to 1 week after initiation of treatment and later decreased in both the groups.
IFN-ϒ
The mean value of IFN-ϒ in patients with paradoxical worsening at baseline was 123.07 ± 61.44 pg/ml while in the no worsening group was 186.21 ± 169.79 pg/ml (Table 3). There was sharp rise in levels of IFN-ϒ between baseline to 1 week in patients who showed paradoxical worsening (p = 0.01). However, there was no significant change in the levels of IFN-ϒ among patients who did not show paradoxical worsening. Those patients who responded well to treatment had progressive decrease in the value of IFN-ϒ with time. The levels of IFN-ϒ fell after pulse steroid therapy during the period of paradoxical worsening until their last follow-up visit at 6 weeks.
TNF-α
The mean value of TNF-α at baseline was 91.6 ± 213.7 pg/ml in the no worsening group and 23.06 ± 28.86 pg/ml at 3 weeks (Table 4). In this group, a sharp decrease in TNF- α levels were observed beginning at 1 week after initiation of ATT and corticosteroids. On the other hand, the levels of TNF-α in the paradoxical worsening group at baseline was 56.9 ± 76.97 pg/ml and at 3 weeks was 87.62 ± 56.98 pg/ml. At 3 weeks, the levels of TNF-α were significantly higher in the paradoxical worsening group compared to the no worsening group (p = 0.02). Among patients who showed paradoxical worsening, the levels of TNF-α decreased to below baseline levels at 6 weeks after pulse steroid therapy (p = 0.03 compared to baseline TNF-α levels in the paradoxical worsening group).
TGF- β
The mean baseline value of TGF-β in patients with paradoxical worsening was 1986.15 ± 1373.62 pg/ml, while in non-paradoxical worsening group was 1669.82 ± 1286.72 pg/ml (Table 5). After initiation of therapy, there was a slight increase in the values of TGF-β in both the groups but it did not reach statistical significance. The levels of TGF-β were highest at 3 weeks after initiation of therapy in the group of patients who showed paradoxical worsening. At 6 weeks after initiation of therapy, there was no significant difference in the levels of TGF-β compared to the baseline values in either group (2353.35 ± 1350.66 pg/ml in the paradoxical worsening group and 1883.23 ± 842.45 pg/ml in the non-paradoxical worsening group) (p > 0.47).
Discussion
There are a number of lacunae in the knowledge of the immunological effects of M. tuberculosis in the serum levels of cytokines among patients with ocular TB, in particular TB MSC. The role of IL-10, TNF-α, and IFN-ϒ have been extensively studied in patients with pulmonary tuberculosis [10, 14]. In the index study, we aimed to analyze the serial levels of inflammatory cytokines in patients of TB MSC focusing on the levels among patients developing paradoxical worsening of the disease upon initiation of ATT and corticosteroids. As it is evident from the literature, there is complex immune interaction between the tubercular bacilli and the human immune system influencing the natural history of the disease.
IL-10 is also known as ‘cytokine synthesis inhibitory factor’ and is released by TH2 cells following antigen stimulation leading to blockade of the cytokine production from TH2 cells [15]. IL-10 achieves this effect by inhibiting the ability of macrophages and dendritic cells to activate TH1 cells. IL-10 acts by inhibiting the production of pro-inflammatory cytokines (IFN-γ, TNF-α, and IL-12) and also the action of antigen-presenting cells, blocking the activation of T-lymphocytes by inhibiting the expression of MHC class II molecule [16]. Those patients with high IL-10 values have low bacilli clearance rates and more chances of persistence of infection. M. tuberculosis itself is capable of stimulating the release of IL-1016. Thus, higher mean levels of IL-10 at baseline may indicate higher tubercular antigenic load in the body. In our study, higher IL-10 value was seen in patients who showed paradoxical worsening compared to those who showed resolution of the lesions (Table 2). When such patients are initiated on ATT, there may be an increased release of tubercular antigens. High load of these antigens released in the blood may stimulate the release of TNF- α and IFN-ϒ. As the inflammation is brought under control by the use of corticosteroids, the levels of IL-10 may show a decline in all the patients during the course of therapy.
Similar to the biological behavior of IL-10, TGF-β also inhibits T-cell proliferation and differentiation, and the production of TNF-α and IFN-ϒ. M. tuberculosis can also directly induce antigen-presenting cells (APC), TGF-β production [17] and the ability of infected macrophages to restrict the growth of M. tuberculosis has correlated inversely with the amount of stimulated TGF-β production [18]. In our study, the patients with paradoxical worsening had higher values of TGF-β at baseline as compared to the non-paradoxical group (Table 5). This may also be related to the higher bacillary load among patient developing paradoxical worsening. Upon initiation of therapy, there was an initial progressive rise in the TGF-β levels. Such an initial rise may play a role in modulating the inflammatory response. Patients in the non-paradoxical worsening group also showed a rising trend of TGF-β at the end of 1 week after initiation of treatment and later showed a decreasing trend. Similarly, the levels of TGF-β among patients who showed paradoxical worsening were highest at 3 weeks after initiation of therapy (though not statistically significant). This peak coincided with the development of paradoxical worsening. At 6 weeks of therapy, the levels of TGF-β returned to baseline levels in both the groups.
There was progressive rise in the levels of IFN-ϒ after initiation of ATT and corticosteroids, especially among patients who showed paradoxical worsening. This suggests heightened immune response in these patients. There was significant difference (p < 0.05) in the mean value of IFN- ϒ as early as 1 week following ATT in patients who showed paradoxical worsening compared to those who did not show such worsening (Table 3). In fact, patients with a good response and healing lesions showed progressive decrease in the levels of IFN-ϒ on treatment. This finding was the highlight of our study, indicating that rising levels of IFN-ϒ detectable at 1 week (prior to clinically detectable paradoxical worsening at a mean of 3.2 weeks) may be an important predictor of development of disease worsening and sight-threatening manifestations. Such patients may need a closer follow-up and serial comparison to detect any increase in the size of the lesions or new lesions developing especially in the posterior pole.
Analysis of the TNF-α levels indicated that the levels of this pro-inflammatory cytokine rapidly rise and peak at 3 weeks after initiation of therapy in patients who develop paradoxical worsening. Elevated levels of TNF-α are related to an excessive inflammation with necrosis and cachexia in patients with pulmonary tuberculosis [19]. The levels of TNF-α showed rapid decline at 6 weeks after treatment with pulse doses of corticosteroids among subjects who showed paradoxical worsening (Table 4). The decline in the levels of TNF-α (as well as IFN-ϒ correlated well with the healing of lesions on FAF imaging.
In comparing the two groups (paradoxical worsening versus no worsening), statistical analysis of IL-10 and TGF-β at similar time intervals did not show any statistical significance. This may be attributed to the fewer number of patients in our study, which is a limitation of the index analysis. In addition, the lack of significance may be attributed to the individual differences in the quantum of immune response to the tubercular bacilli. However, all the patients showed a similar trend based on their biological behavior (paradoxical or no paradoxical worsening). From our index analyses, it may not be possible to define cutoff values of TNF-α or IFN-ϒ levels beyond which paradoxical worsening is expected to occur. However, it may be concluded that patients who show progressive rise of TNF-α and rapid rise of IFN-ϒ as early as 1 week after initiation of ATT and corticosteroids, and high-baseline levels of IL-10 may be at a higher risk of paradoxical worsening and sight-threatening manifestations. The major limiting feature of our study was the modest sample size and relatively shorter follow-up. We did not measure systemic inflammatory parameters, such as C-reactive protein (before and after) in our patients. A larger sample size and longer follow-up may help define such cutoff values. Similarly, such an analyses may aid in evaluating the cytokine levels in patients who show disease recurrence.
In summary, levels of serum cytokines among patients with TB MSC may aid in improving our understanding of the disease pathogenesis and the natural course of the disease. Our study is a pilot analysis with few patients and larger cohort studies with more comprehensive methodologies are needed to validate our results. Such studies may focus on additional inflammatory biomarkers that may be relevant in the pathogenesis of TB-related uveitis. Detection of newer inflammatory cytokines may help in understanding of cytokine signatures of this entity. Identification of cytokine levels may aid in the development of newer and individualized therapeutic strategies for the treatment of patients. Paradoxical worsening may occur among patients who have a higher tubercular antigenic load. These patients may show a heightened immune response with higher baseline IL-10 values, early rising levels of IFN-ϒ, progressive increase in TGF-β, and rising levels of TNF- α after initiation of ATT and corticosteroids.
Summary
What was known before
There is limited information about the serum cytokine levels among patients with paradoxical worsening in tuberculosis-related uveitis.
What this study adds
Patients with paradoxical worsening in tuberculosis-related uveitis may show a heightened immune response with higher baseline IL-10 values, early rising levels of IFN-ϒ, progressive increase in TGF-β, and rising levels of TNF-α after initiation of ATT and corticosteroids.
References
Gupta V, Gupta A, Rao NA. Intraocular tuberculosis--an update. Surv Ophthalmol. 2007;52:561–87.
Gupta V, Gupta A, Arora S, Bambery P, Dogra MR, Agarwal A. Presumed tubercular serpiginous like choroiditis: clinical presentations and management. Ophthalmology. 2003;110:1744–9.
Bansal R, Gupta A, Gupta V, Dogra MR, Sharma A, Bambery P. Tubercular serpiginous-like choroiditis presenting as multifocal serpiginoid choroiditis. Ophthalmology. 2012;119:2334–42.
Gupta V, Shoughy SS, Mahajan S, et al. Clinics of ocular tuberculosis. Ocul Immunol Inflamm. 2015;23:14–24.
Gupta V, Bansal R, Gupta A. Continuous progression of tubercular serpiginous-like choroiditis after initiating antituberculosis treatment. Am J Ophthalmol. 2011;152:857–63. e852
Brown CS, Smith CJ, Breen RA, et al. Determinants of treatment-related paradoxical reactions during anti-tuberculosis therapy: a case control study. BMC Infect Dis. 2016;16:479.
Ang M, Cheung G, Vania M, et al. Aqueous cytokine and chemokine analysis in uveitis associated with tuberculosis. Mol Vis. 2012;18:565–73.
Abu El-Asrar AM, Struyf S, Kangave D, et al. Cytokine and CXC chemokine expression patterns in aqueous humor of patients with presumed tuberculous uveitis. Cytokine. 2012;59:377–81.
Toossi Z, Ellner JJ. The role of TGF beta in the pathogenesis of human tuberculosis. Clin Immunol Immunopathol. 1998;87:107–14.
Clifford V, Zufferey C, Street A, Denholm J, Tebruegge M, Curtis N. Cytokines for monitoring anti-tuberculous therapy: a systematic review. Tuberculosis. 2015;95:217–28.
Gupta A, Sharma A, Bansal R, Sharma K. Classification of intraocular tuberculosis. Ocul Immunol Inflamm. 2015;23:7–13.
Gupta A, Gupta V. Tubercular posterior uveitis. Int Ophthalmol Clin. 2005;45:71–88.
Agarwal A, Aggarwal K, Deokar A, et al. Optical coherence tomography angiography features of paradoxical worsening in tubercular multifocal serpiginoid choroiditis. Ocul Immunol Inflamm. 2016;24:621–30.
Choi R, Kim K, Kim MJ, et al. Serum inflammatory profiles in pulmonary tuberculosis and their association with treatment response. J Proteom. 2016;149:23–30.
Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–95.
Redford PS, Murray PJ, O’Garra A. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol. 2011;4:261–70.
Fulton SA, Cross JV, Toossi ZT, Boom WH. Regulation of interleukin-12 by interleukin-10, transforming growth factor-beta, tumor necrosis factor-alpha, and interferon-gamma in human monocytes infected with Mycobacterium tuberculosis H37Ra. J Infect Dis. 1998;178:1105–14.
Hirsch CS, Yoneda T, Averill L, Ellner JJ, Toossi Z. Enhancement of intracellular growth of Mycobacterium tuberculosis in human monocytes by transforming growth factor-beta 1. J Infect Dis. 1994;170:1229–37.
Bekker LG, Moreira AL, Bergtold A, Freeman S, Ryffel B, Kaplan G. Immunopathologic effects of tumor necrosis factor alpha in murine mycobacterial infection are dose dependent. Infect Immun. 2000;68:6954–61.
Acknowledgements
This work was partly supported by a grant from the Department of Biotechnology (DBT), India for the development of Centre of Excellence at the Advanced Eye Centre, PGIMER Chandigarh.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Agarwal, A., Deokar, A., Sharma, R. et al. Longitudinal analysis of serum cytokine profile among patients with tubercular multifocal serpiginoid choroiditis: a pilot study. Eye 33, 129–135 (2019). https://doi.org/10.1038/s41433-018-0157-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-018-0157-5
This article is cited by
-
Tubercular serpiginous choroiditis
Journal of Ophthalmic Inflammation and Infection (2022)
-
Automated lesion segmentation and quantification for prediction of paradoxical worsening in patients with tubercular serpiginous-like choroiditis
Scientific Reports (2022)
-
Paradoxical reactions in ocular tuberculosis
Journal of Ophthalmic Inflammation and Infection (2019)
-
Antituberculars
Reactions Weekly (2019)