Abstract
Objectives
To document and characterise hyper- and hypo-reflective lesions, which we describe as ‘granular’ on short-wavelength autofluorescence (SW-AF) and near-infrared (NIR)-AF images in diabetic macular oedema (DMO).
Methods
Consecutive 103 eyes of 78 patients suffering from centre-involving DMO were reviewed retrospectively. Mosaics of hyper- and hypo-fluorescent dots on both SW-AF and NIR-AF signals were delineated and defined as granular lesions in the macula. We evaluated the association of such lesions with the logarithm of the minimum angle of resolution visual acuity (logMAR VA) and spectral-domain optical coherence tomography (SD-OCT) images.
Results
Diffuse mosaics of hyper- and hypo-fluorescent dots were delineated in 36 and 45 eyes on SW-AF and NIR-AF images, respectively, and both AF images defined granular lesions in 33 eyes. These lesions were delineated in both the fovea and extrafoveal areas on NIR-AF images but were limited to the parafoveal and perifoveal subfields on SW-AF images. There was a significant difference in logMAR VA between eyes with and without granular lesions (0.358 ± 0.269 vs. 0.185 ± 0.234; P = 0.001). Granular lesions were associated with the mosaic pattern on NIR-AF images (P < 0.001) but not with other parameters on SW-AF and NIR-AF images. The retinal thickness in the central subfield was greater in eyes with granular lesions (538.0 ± 163.6 μm vs. 448.8 ± 120.2 μm; P = 0.003). Granular lesions were associated with ELM disruption and hyper-reflective foci in the outer retinal layers (P = 0.004 and P = 0.037, respectively).
Conclusions
Granular lesions defined on both SW-AF and NIR-AF images were related to retinal oedema with photoreceptor damage and concomitant VA reduction in DMO.
Similar content being viewed by others
Introduction
Diabetic retinopathy (DR) is a leading cause of visual disturbance worldwide; in particular, diabetic macular oedema (DMO) is often refractory to intervention and concomitantly results in poor visual prognoses [1, 2]. The breakdown of the inner blood-retinal barrier (BRB) in the retinal vasculature induces the thickening of retinal parenchyma and a variety of macular morphological changes [3,4,5]. Basic research has shown that diabetes promotes pathological changes in the retinal pigment epithelium (RPE), which constitutes the outer BRB [6,7,8,9]. The mechanism by which the joint disruption of the inner and outer BRB exacerbates the pathogenesis of DMO is not fully understood.
Optical coherence tomography (OCT) enables us to measure retinal thickness and concomitantly to diagnose centre-involving DMO as proposed by the Diabetic Retinopathy Clinical Research Network (DRCR.net) [10]. OCT has also delineated the diversity of macular morphological changes, i.e., cystoid macular oedema (CMO), serous retinal detachment (SRD) and sponge-like retinal swelling, which might be associated with visual prognoses after the use of anti-vascular endothelial growth factor (VEGF) agents [4, 11]. Recent publications have demonstrated the clinical significance of photoreceptor damage at the fovea in macular oedema that is associated with retinal vascular diseases [12, 13]. OCT depicts the external limiting membrane (ELM) and the ellipsoid zone of photoreceptors (EZ) as markers of photoreceptor integrity [14, 15]. It has also been reported that hyper-reflective foci in the outer retinal layers are related to photoreceptor damage and concomitant visual disturbance in DMO [16,17,18].
Several publications have proposed the clinical feasibility of using fundus autofluorescence (FAF) to assess outer retinal layers [19, 20]. The use of short-wavelength autofluorescence (SW-AF), whose signals are derived mainly from lipofuscin and retinal fluorophores, has recently increased our understanding of the pathogenesis in photoreceptor–RPE interaction [19,20,21,22]. SW-AF images delineate oval or round lesions with hyperfluorescence in most areas with foveal cystoid spaces or in hypofluorescent areas in the parafovea or perifovea in eyes with DMO [23,24,25,26,27]. A recent publication reported the finding of granular hypofluorescence and/or variable hyperfluorescence on SW-AF images as diabetic retinal pigment epitheliopathy in DR [28]. NIR-AF signals represent the amount of melanin in the RPE. The signal levels are reduced, and mosaic patterns were delineated in DMO [29]. However, the correspondence between FAF findings and pathological changes of the photoreceptors or RPE in DMO has not yet been fully elucidated.
In this study, we for the first time characterise the granular lesions on both SW-AF and NIR-AF images in DMO and compare them to photoreceptor integrity as measured using OCT images.
Methods
Patients
In this retrospective study, we reviewed 103 consecutive eyes of 78 patients who visited the Department of Ophthalmology at Kyoto University Hospital from June 2010 to June 2013. The major inclusion criterion was centre-involving DMO, for which spectral-domain (SD-)OCT and FAF images of sufficient quality were obtained on the same day. The exclusion criteria were as follows: (1) severe intraretinal lesions containing microaneurysms, retinal haemorrhage and hard exudates that blocked the signals of SW- or NIR-AF in any subfield of the Early Treatment Diabetic Retinopathy Study (ETDRS) grid; (2) any other chorioretinal diseases, including age-related maculopathy and age-related macular degeneration; (3) severe media opacity; (4) any previous treatment for DMO, including macular laser and intravitreal therapies; (5) cataract surgery within 3 months; and (6) any major surgery other than cataract extraction within 1 year. All research and measurements adhered to the tenets of the Declaration of Helsinki. The ethics committee of Kyoto University Graduate School of Medicine approved the study protocol. Written informed consent was obtained after the nature and possible consequences of this study had been fully explained to the patient.
Fundus autofluorescence
After comprehensive ophthalmologic examinations including measurement of the best-corrected VA and slit-lamp biomicroscopy, 45° colour fundus photographs (3216 × 2136 pixels) were acquired using a fundus camera (TRC-50LX, Topcon, Tokyo, Japan). Retinal sectional images of the macula were scanned using SD-OCT (Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany), after which qualitative and quantitative OCT parameters (i.e., the mean retinal thickness of the central subfield (CSF) and the presence of foveal cystoid spaces, foveal SRD, hyper-reflective foci in outer retinal layers and the disruption of foveal ELM) were determined [4, 10, 17, 30].
FAF images in the macula were obtained using a scanning laser ophthalmoscope (Heidelberg Retina Angiograph 2, Heidelberg Engineering). NIR-AF images were acquired using an 800-nm filter and excitation with a 787-nm diode laser and mosaic pattern; cystoid sign and the relative fluorescence intensity were evaluated as described previously [29].
For the SW-AF images, fluorescence signals acquired through a 500-nm filter after excitation with a 488-nm laser were detected in an area of 30° × 30° centred on the fovea. The gain level was adjusted to delineate the major vessels and the disc on a single-scan image; 20–80 scanned images were averaged to obtain sufficient quality. We evaluated the increase in SW-AF levels in areas corresponding to the cystoid spaces [23,24,25, 27]. Well-demarcated oval or round areas with higher levels of FAF signals around the macula were defined as ‘autofluorescent cystoid spaces’. In healthy eyes, the signal levels of SW-AF were gradually decreased at the fovea, whereas SW-AF often showed that the areas with hypofluorescence were extended to the parafovea or perifovea in eyes with DMO, a phenomenon termed ‘the enlargement of hypofluorescent areas’ in the current study.
We also defined ‘granular lesions’, in which diffuse mosaics of hyper- and hypo-fluorescent dots on both SW-AF and NIR-AF images were delineated (Fig. 1g–i). Such fluorescent dots on these FAF images partly corresponded to each other (Fig. 2). Highly hyperfluorescent dots were scattered on SW-AF images in several cases, whereas NIR-AF images did not show corresponding findings (Supplemental Figure). We did not include these dots in the granular lesions. We often observed that autofluorescence was blocked by intraretinal lesions, including microaneurysms, intraretinal haemorrhages and hard exudates; such lesions were carefully excluded in this study. Two independent retinal specialists (S.Y. and A.U.) evaluated these qualitative findings on SW-AF images in a masked fashion, and when they disagreed, a third, more experienced retinal specialist (T.M.) made the final decision.
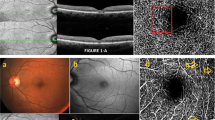
Granular lesions on SW-AF and NIR-AF images in DMO. Colour fundus photographs (left column), SW-AF images (centre column), and NIR-AF images (right column) in a healthy subject (a–c) and in DMO without (d–f) or with (g–i) granular lesions. Some eyes with DMO had granular lesions, which comprised a diffuse mosaic of hyper- and hypo-fluorescent dots in the extrafoveal areas on the SW-AF image and in the macula on the NIR-AF image
The correspondence of hyper- and hypo-fluorescent dots in granular lesions on SW-AF and NIR-AF images in DMO. a, b SW-AF and NIR-AF images. c Magnified SW-AF, NIR-AF, and their merged images often showed the coincidence of hyper- and hypo-fluorescent dots in the extrafoveal areas on SW-AF and NIR-AF images
Statistical nalysis
The results are expressed as the mean ± standard deviation. The Student’s t-test was used to compare quantitative data populations with normal distributions and equal variance. The data were analysed using the Mann–Whitney U test for populations with non-normal distributions or unequal variance. Significant differences in the sampling distributions were determined using the Fisher exact test. P < 0.05 was considered significant.
Results
Characteristics of granular lesions on SW-AF and NIR-AF images
The patient characteristics are shown in Supplemental Table 1. Hyperfluorescent dots on SW-AF images were delineated in 49 eyes. Diffuse mosaics of hyperfluorescent and hypo-fluorescent dots on SW-AF images were delineated in 36 eyes (Figs 1, 2). In contrast, highly hyperfluorescent dots were scattered on SW-AF images in 20 eyes, although we did not observe the corresponding NIR-AF findings (Supplemental Figure). The SW-AF mosaics were often depicted in the outer ring of the ETDRS grid (35 eyes) but were observed in the inner ring or the central subfield less frequently on SW-AF images (Supplemental Table 2).
NIR-AF images also depicted diffuse mosaics of hyper- and hypo-fluorescent dots in 45 of 49 eyes with mosaic pattern, as documented in a previous publication (Figs 1, 2) [29]. The mosaics of NIR-AF dots were present more frequently in the fovea or parafoveal areas than in the perifoveal areas (Supplemental Table 2). A comparative study revealed that the hyper- and hypo-fluorescent dots on SW-AF and NIR-AF images often corresponded to each other. The diffuse mosaic of fluorescent dots on both FAF images were delineated in 33 eyes and are defined as granular lesions in this study.
We further evaluated the relationship between granular lesions and other SW-AF or NIR-AF findings but failed to find any significant association between the granular lesions with autofluorescent cystoid spaces or enlarged hypofluorescence on SW-AF images (Table 1). The granular lesions were delineated more frequently in eyes with mosaic pattern in the NIR-AF images than in those without such findings (P < 0.001). On the contrary, we did not find an association between the granular lesions and the cystoid sign or the relative fluorescence intensity of NIR-AF (Table 1).
Association of granular autofluorescence with VA and SD-OCT findings
Twenty-two eyes suffering from NPDR (14 and 8 eyes with moderate and severe NPDR, respectively) were accompanied by granular lesions, and 62 eyes (2, 49 and 11 eyes with mild, moderate and severe NPDR) were not. Eleven eyes (33.3%) with the granular lesions had PDR, whereas only 8 eyes (11.4%) without the lesions suffered from PDR. Thirty-six eyes with the granular lesions in any areas had worse VA than those without the lesions (0.358 ± 0.269 vs. 0.185 ± 0.234; P = 0.001).
We further investigated the association between the granular autofluorescence and the SD-OCT findings. Eyes with the granular lesions had greater CSF thickness than those without the lesions (P = 0.003) but there was no association between the granular lesions and cystoid spaces or SRD at the fovea (Table 2). The ELM at the fovea was disrupted in 18 eyes (54.5%) with granular autofluorescence, whereas only 17 eyes (24.3%) without such lesions had ELM disruption (P = 0.004; Table 2). Similarly, a relationship between the granular lesions and hyper-reflective foci in the outer retinal layers was observed (P = 0.037; Fig. 3).
Relationship between granular lesions on FAF images and photoreceptor damage as seen on SD-OCT images in DMO. a–c A representative case without granular lesions had an intact ELM (arrow) and no hyper-reflective foci in the outer retinal layers. d–f Another representative case with granular lesions on FAF images showed a disrupted ELM and hyper-reflective foci in the outer retinal layers (arrowheads). a D = SW-AF images. b E = NIR-AF images. c F = retinal sections on SD-OCT images
Discussion
In this comparative study, we documented the characteristics of granular lesions on SW-AF and NIR-AF images in eyes with centre-involving DMO for the first time. The mosaic of hyper- and hypo-fluorescent dots on SW-AF and NIR-AF images corresponded to each other in the granular lesions to some extent. NIR-AF signals are derived from melanin and represent changes in the RPE, whereas the increased accumulation of lipofuscin, a main source of SW-AF signals, depends on a poor interaction between the photoreceptors and RPE [31]. This may allow us to speculate that the granular lesions seen on FAF images indicate diabetic RPE changes. In addition, eyes with the granular lesions had poorer VA than those without the lesions, which might be consistent with the association of the granular lesions with retinal thickening and photoreceptor damage on SD-OCT images [30, 32, 33]. These results suggest that the granular lesions on FAF images and the pathogenesis at the interface between photoreceptor and RPE in DMO may be clinically relevant.
A recent publication reported the finding of diabetic retinal pigment epitheliopathy on SW-AF images [28]. Macular pigments block the SW-AF signals derived from the RPE in the macula; in particular, oedematous changes in the retinal parenchyma also result in the decreased SW-AF signals in DMO [34], which prevented us from correctly evaluating the SW-AF signals in the macula. In contrast, NIR-AF signals from melanin pigments are clearly delineated in the macula because the amount of melanin in the RPE is centrifugally increased, and the longer wavelength is not affected by macular pigments or oedematous changes. Indeed, the SW-AF images delineated mosaics of hyper- and hypo-fluorescent dots mainly in the perifovea, whereas the autofluorescent dots were present mainly in the central subfield on NIR-AF images in DMO (Supplemental Table 2). This suggests that it is feasible to determine differences between SW-AF and NIR-AF images and, and apply such changes in the assessment of centre-involving DMO.
Hyperfluorescent dots on both SW-AF and NIR-AF images often coincided with each other in the granular lesions. Since NIR-AF signals are derived from melanin, we speculated that the hyperfluorescent dots on the NIR-AF images might correspond to either RPE clumping or migrated macrophages containing melanolipofuscin rather than to the debris of photoreceptor components containing retinal fluorophores [17, 22, 31, 35, 36]. This notion is compatible with the association between the granular lesions and hyper-reflective foci in the outer retinal layers, which seems to correspond to lipid-laden macrophages or RPE hyperplasia [16, 17]. Hypo-fluorescent dots on the SW-AF and NIR-AF images also corresponded to each other. NIR-AF hypofluorescence might depend on the decreased amount of melanin and represent the dysfunction or atrophy of RPE, as shown in early age-related maculopathy [31, 37]. Since melanin has protective properties against cellular stresses, reduced amounts of melanin might promote intracellular damage in RPE with concomitant dysfunction in the nourishment of photoreceptor cells [38]. Degenerated or atrophic RPE might also not phagocytose the photoreceptor outer segments. These mechanisms would result in the decreased lipofuscin deposition and SW-AF signals.
The granular lesions on FAF images were associated with both visual impairment and photoreceptor damage as seen on SD-OCT images, which suggests their clinical relevance in DMO. We considered several possible pathological mechanisms in the outer retinal layers; migrated macrophages or RPE might change promote photoreceptor damage or vice versa [39]. Diabetes-induced RPE hyperplasia or clumping might disturb the exchange of nutrients and wastes through the RPE and concomitantly damage photoreceptor cells [7, 38, 40]. Extravasated macrophages or resident microglia might migrate into subretinal spaces and injure photoreceptor components via cytokines or excessive concentrations of reactive oxygen species. Otherwise, damaged photoreceptors might induce the overphagocytosis and nondigestion of photoreceptor components with the resultant precipitation of lipofuscin or melanolipofuscin in the RPE or macrophages [22].
Eyes with granular lesions on FAF images had greater retinal thickness than those without such lesions in DMO, suggesting their association with inner or outer BRB breakdown. The granular lesions might represent the breakdown of the outer BRB in the RPE monolayer [7, 29]. Hyperpermeability of the retinal vasculature might exacerbate retinal oedema, and the migration of blood components into the subretinal spaces might stimulate RPE proliferation and doubling [16, 17, 36, 41]. We found that temporal subfields had granular lesions more frequently than nasal subfields. The retinal vasculature in the temporal subfield was often accompanied by capillary dropout or vascular hyperpermeability when compared to that in the nasal subfield. These findings prompt us to speculate that the breakdown of the inner BRB might contribute to the pathogenesis of the granular lesions on FAF images, at least in part.
We further considered the relationship between the granular lesions seen on SW-AF images and foveal pathomorphologies. The granular lesions were not related to either autofluorescent cystoid spaces as seen on SW-AF images or foveal cystoid spaces as seen on SD-OCT images in the current study. A recent publication has reported that foveal cystoid spaces are related to vascular changes around them in DMO [42]. As discussed above, the granular lesions on FAF images might depend on the pathogenesis in the outer retinal layers but not in the inner retinal layers. Another morphological finding, SRD, was also not related to granular lesions in this study. Central serous chorioretinopathy sometimes exhibits irregular increased autofluorescence or discrete granules with increased intensity within the detached areas [43]. In contrast, the areas with granular lesions did not match those of SRD in this study, suggesting that differences exist in the pathogenesis of these two diseases. It has been reported that foveal SRD is accompanied by vascular hyperpermeability in the perifoveal areas in DMO [44]. Taking these findings together, we might speculate that the location of vascular permeability rather than the disruption of the outer BRB contribute to the development of SRD.
Several findings on SW-AF images have been reported in eyes with age-related macular degeneration. RPE clumping or hyperpigmentation in early age-related maculopathy corresponds to the mosaic of hyperfluorescence and hypofluorescence of SW-AF signals, which is similar to the granular lesions seen in DMO [45, 46]. We carefully excluded these diseases in this study and consequently did not find drusen or hypofluorescent lesions, such as geographic atrophy. In addition, the patients with granular lesions were younger in this study (data not shown). These data suggest that granular lesions in diabetic patients are different from changes in patients with early or late age-related maculopathy. In contrast, eyes with PDR had granular lesions more frequently than those with NPDR. This finding might be consistent with several pathological mechanisms (i.e., vascular hyperpermeability, photoreceptor damage and RPE changes) in accordance with DR progression [3, 7, 47].
This study has several limitations. Since the pathohistological study did not elucidate the origin of abnormal autofluorescence in DMO, further study should determine the clinicopathological correlations with this FAF finding. The comparative study demonstrated these granular lesions as a novel FAF finding, although the qualitative lesions were subjectively evaluated. Further investigation is needed to develop methods for the automatic detection of this clinically relevant finding. The correlation between the granular lesions and the en-face OCT findings would promote the interpretation of photoreceptor–RPE interaction [48]. In addition, the clinicopathological relevance of this FAF finding in DMO is not fully understood. The participants in this single-centre study were all Asians, whose RPE contains greater amounts of melanin; thus, further study should be undertaken to ensure the generalizability of the results.
In this comparative study, we documented granular lesions in both SW-AF and NIR-AF images and observed their association with visual impairment, greater retinal thickness and photoreceptor damage in DMO, suggesting that these lesions have clinical relevance and may be important to the pathogenesis in photoreceptor–RPE interaction.
Summary
What was known before
-
Diabetes induces disturbances of the retinal pigment epithelium.
-
Short-wavelength autofluorescence is reduced in diabetic macular oedema.
-
Near-infrared autofluorescence delineates a mosaic pattern in diabetic macular oedema.
What this study adds
-
Granular lesions of short-wavelength and near-infrared autofluorescence correspond to each other in diabetic macular oedema.
-
Visual acuity is poorer in eyes with granular lesions.
-
Eyes with granular lesions have a greater central subfield thickness and exhibit a disruption of the external limiting membrane.
References
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64.
Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–39.
Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Levison SW. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002;47(Suppl 2):S253–62.
Otani T, Kishi S, Maruyama Y. Patterns of diabetic macular edema with optical coherence tomography. Am J Ophthalmol. 1999;127:688–93.
Murakami T, Yoshimura N. Structural changes in individual retinal layers in diabetic macular edema. J Diabetes Res. 2013;2013:920713.
Cunha-Vaz J. The blood-ocular barriers. Surv Ophthalmol. 1979;23:279–96.
Simo R, Villarroel M, Corraliza L, Hernandez C, Garcia-Ramirez M. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier--implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol. 2010;2010:190724.
Decanini A, Karunadharma PR, Nordgaard CL, Feng X, Olsen TW, Ferrington DA. Human retinal pigment epithelium proteome changes in early diabetes. Diabetologia. 2008;51:1051–61.
Vinores SA, Gadegbeku C, Campochiaro PA, Green WR. Immunohistochemical localization of blood-retinal barrier breakdown in human diabetics. Am J Pathol. 1989;134:231–5.
Danis RP, Glassman AR, Aiello LP, Antoszyk AN, Beck RW, Browning DJ, et al. Diurnal variation in retinal thickening measurement by optical coherence tomography in center-involved diabetic macular edema. Arch Ophthalmol. 2006;124:1701–7.
Shimura M, Yasuda K, Yasuda M, Nakazawa T. Visual outcome after intravitreal bevacizumab depends on the optical coherence tomographic patterns of patients with diffuse diabetic macular edema. Retina. 2013;33:740–7.
Murakami T, Tsujikawa A, Ohta M, Miyamoto K, Kita M, Watanabe D, et al. Photoreceptor status after resolved macular edema in branch retinal vein occlusion treated with tissue plasminogen activator. Am J Ophthalmol. 2007;143:171–3.
Sakamoto A, Nishijima K, Kita M, Oh H, Tsujikawa A, Yoshimura N. Association between foveal photoreceptor status and visual acuity after resolution of diabetic macular edema by pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2009;247:1325–30.
Murakami T, Nishijima K, Akagi T, Uji A, Horii T, Ueda-Arakawa N, et al. Optical coherence tomographic reflectivity of photoreceptors beneath cystoid spaces in diabetic macular edema. Invest Ophthalmol Vis Sci. 2012;53:1506–11.
Spaide RF, Curcio CA. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina. 2011;31:1609–19.
Bolz M, Schmidt-Erfurth U, Deak G, Mylonas G, Kriechbaum K, Scholda C. Optical coherence tomographic hyperreflective foci: a morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology. 2009;116:914–20.
Uji A, Murakami T, Nishijima K, Akagi T, Horii T, Arakawa N, et al. Association between hyperreflective foci in the outer retina, status of photoreceptor layer, and visual acuity in diabetic macular edema. Am J Ophthalmol. 2012;153:710–7 e1.
Nishijima K, Murakami T, Hirashima T, Uji A, Akagi T, Horii T, et al. Hyperreflective foci in outer retina predictive of photoreceptor damage and poor vision after vitrectomy for diabetic macular edema. Retina. 2014;34:732–40.
Delori FC, Dorey CK, Staurenghi G, Arend O, Goger DG, Weiter JJ. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci. 1995;36:718–29.
Delori FC, Fleckner MR, Goger DG, Weiter JJ, Dorey CK. Autofluorescence distribution associated with drusen in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2000;41:496–504.
Murakami T, Akimoto M, Ooto S, Suzuki T, Ikeda H, Kawagoe N, et al. Association between abnormal autofluorescence and photoreceptor disorganization in retinitis pigmentosa. Am J Ophthalmol. 2008;145:687–94.
Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: review and perspectives. Retina. 2008;28:385–409.
Bessho K, Gomi F, Harino S, Sawa M, Sayanagi K, Tsujikawa M, et al. Macular autofluorescence in eyes with cystoid macula edema, detected with 488 nm-excitation but not with 580 nm-excitation. Graefes Arch Clin Exp Ophthalmol. 2009;247:729–34.
Vujosevic S, Casciano M, Pilotto E, Boccassini B, Varano M, Midena E. Diabetic macular edema: fundus autofluorescence and functional correlations. Invest Ophthalmol Vis Sci. 2011;52:442–8.
Chung H, Park B, Shin HJ, Kim HC. Correlation of fundus autofluorescence with spectral-domain optical coherence tomography and vision in diabetic macular edema. Ophthalmology. 2012;119:1056–65.
Reznicek L, Dabov S, Haritoglou C, Kampik A, Kernt M, Neubauer AS. Green-light fundus autofluorescence in diabetic macular edema. Int J Ophthalmol. 2013;6:75–80.
Pece A, Isola V, Holz F, Milani P, Brancato R. Autofluorescence imaging of cystoid macular edema in diabetic retinopathy. Ophthalmologica. 2010;224:230–5.
Kang EC, Seo Y, Byeon SH. Diabetic retinal pigment epitheliopathy: fundus autofluorescence and spectral-domain optical coherence tomography findings. Graefes Arch Clin Exp Ophthalmol. 2016;254:1931–40.
Yoshitake S, Murakami T, Horii T, Uji A, Ogino K, Unoki N, et al. Qualitative and quantitative characteristics of near-infrared autofluorescence in diabetic macular edema. Ophthalmology. 2014;121:1036–44.
Murakami T, Nishijima K, Sakamoto A, Ota M, Horii T, Yoshimura N. Association of pathomorphology, photoreceptor status, and retinal thickness with visual acuity in diabetic retinopathy. Am J Ophthalmol. 2011;151:310–7.
Keilhauer CN, Delori FC. Near-infrared autofluorescence imaging of the fundus: visualization of ocular melanin. Invest Ophthalmol Vis Sci. 2006;47:3556–64.
Browning DJ, Glassman AR, Aiello LP, Beck RW, Brown DM, Fong DS, et al. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114:525–36.
Alasil T, Keane PA, Updike JF, Dustin L, Ouyang Y, Walsh AC, et al. Relationship between optical coherence tomography retinal parameters and visual acuity in diabetic macular edema. Ophthalmology. 2010;117:2379–86.
Yoshitake S, Murakami T, Uji A, Unoki N, Dodo Y, Horii T, et al. Clinical relevance of quantified fundus autofluorescence in diabetic macular oedema. Eye (Lond). 2015;29:662–9.
Feeney L. Lipofuscin and melanin of human retinal pigment epithelium. Fluorescence, enzyme cytochemical, and ultrastructural studies. Invest Ophthalmol Vis Sci. 1978;17:583–600.
Rudolf M, Vogt SD, Curcio CA, Huisingh C, McGwin G Jr., Wagner A, et al. Histologic basis of variations in retinal pigment epithelium autofluorescence in eyes with geographic atrophy. Ophthalmology. 2013;120:821–8.
Green WR, Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100:1519–35.
Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–81.
Sparrow JR, Yoon KD, Wu Y, Yamamoto K. Interpretations of fundus autofluorescence from studies of the bisretinoids of the retina. Invest Ophthalmol Vis Sci. 2010;51:4351–7.
Bressler NM, Silva JC, Bressler SB, Fine SL, Green WR. Clinicopathologic correlation of drusen and retinal pigment epithelial abnormalities in age-related macular degeneration. Retina. 1994;14:130–42.
Ota M, Nishijima K, Sakamoto A, Murakami T, Takayama K, Horii T, et al. Optical coherence tomographic evaluation of foveal hard exudates in patients with diabetic maculopathy accompanying macular detachment. Ophthalmology. 2010;117:1996–2002.
Murakami T, Nishijima K, Sakamoto A, Ota M, Horii T, Yoshimura N. Foveal cystoid spaces are associated with enlarged foveal avascular zone and microaneurysms in diabetic macular edema. Ophthalmology. 2011;118:359–67.
von Ruckmann A, Fitzke FW, Fan J, Halfyard A, Bird AC. Abnormalities of fundus autofluorescence in central serous retinopathy. Am J Ophthalmol. 2002;133:780–6.
Murakami T, Uji A, Ogino K, Unoki N, Horii T, Yoshitake S, et al. Association between Perifoveal Hyperfluorescence and Serous Retinal Detachment in Diabetic Macular Edema. Ophthalmology. 2013;120:2596–603.
von Ruckmann A, Fitzke FW, Bird AC. Fundus autofluorescence in age-related macular disease imaged with a laser scanning ophthalmoscope. Invest Ophthalmol Vis Sci. 1997;38:478–86.
Spaide RF. Fundus autofluorescence and age-related macular degeneration. Ophthalmology. 2003;110:392–9.
Du Y, Veenstra A, Palczewski K, Kern TS. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc Natl Acad Sci USA. 2013;110:16586–91.
Paavo M, Lee W, Merriam J, Bearelly S, Tsang S, Chang S, et al. Intraretinal Correlates of Reticular Pseudodrusen Revealed by Autofluorescence and En Face OCT. Invest Ophthalmol Vis Sci. 2017;58:4769–77.
Funding
This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (17K11423).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yoshitake, S., Murakami, T., Uji, A. et al. Granular lesions of short-wavelength and near-infrared autofluorescence in diabetic macular oedema. Eye 33, 564–571 (2019). https://doi.org/10.1038/s41433-018-0256-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-018-0256-3