Abstract
Objective
This study describes the imaging of the filtering area in CO2 laser-assisted sclerectomy surgery (CLASS) using ultrasound biomicroscopy (UBM) combined with the Indiana Bleb Appearance Grading Scale (IBAGS) and evaluates the mechanism by which CLASS lowers the intraocular pressure (IOP).
Methods
Twenty-eight cases (28 eyes) of primary open-angle glaucoma that could not be controlled by drugs underwent CLASS. At 1, 3, 6, 12, 18, and 24 months after surgery, IBAGS was used to evaluate the external morphology of the filtering blebs, and UBM was used to describe and measure their internal structure.
Results
During the early period after CLASS, most cases showed diffuse filtering blebs with a serious degree of congestion. At the end of follow-up, most cases did not present filtering blebs. All patients showed an intact and thin trabeculodescemetic membrane (TDM) with an average thickness of 0.094 ± 0.017 mm. The scleral reservoir size gradually decreased over time and tended to stabilize after 18 months. At 3 and 6 months after surgery, 53.57% of the patients had abnormalities in the TDM area, and after laser goniopuncture treatment, the scleral reservoir became slightly larger and the IOP decreased. The TDM thickness was not correlated with postoperative IOP, and the scleral reservoir size was negatively correlated with IOP.
Conclusion
During the early phase after CLASS, the subconjunctival and suprachoroidal pathways may be the main mechanisms lowering IOP; over time, internal drainage pathways such as the intrascleral, trabecular-meshwork, and suprachoroidal pathways play greater roles in lowering IOP.
Similar content being viewed by others
Introduction
CO2 laser-assisted sclerectomy surgery (CLASS) is a recent modification of nonpenetrating glaucoma filtration surgery that has the same effect as traditional nonpenetrating deep sclerectomy (NPDS) in lowering the IOP [1]. The occurrence of TDM perforation during deep scleral dissection in NPDS is closely related to the learning curve. During the early stage of learning, the incidence rate can reach 30–50%, while experienced surgeons have an incidence rate of 3% [1]. The need to dissect the deep sclera and preserve the intact and thin TDM during the operation increases the difficulty of surgery and the requirements for surgical experience, affecting the popularity of NPDS [2]. CLASS uses a CO2 laser that provides accurate ablation of dry tissues and is absorbed by fluid, ablates the deep sclera to form a scleral reservoir, and ablates the corneal sclera. During the CO2 laser ablation process, the aqueous humour percolates and absorbs the CO2 laser to prevent further ablation, avoiding the occurrence of TDM perforation and generating an intact and thin TDM [3]. CLASS creates the scleral reservoir and TDM easily and accurately, and rendering deep sclerectomy easier, conducive to the popularity of surgery [1].
The long-term success of glaucoma filtration surgery depends on the formation and maintenance of filtration channels. In both NPDS and CLASS, the aqueous humour seeps through the intact and thin TDM to the scleral reservoir, then through a variety of aqueous outflow channels, reducing the IOP. Formation and long-term maintenance of the scleral reservoir are thought to be important in facilitating aqueous humour drainage [4]. For the evaluation of the external filtering bleb morphology, the Indiana Bleb Appearance Grading Scale (IBAGS) and the Moorfields Bleb Grading System [5] are currently the two most widely used clinical grading systems. Ultrasound biomicroscopy (UBM) [6] inspection techniques can provide a good assessment of the features of the intrableb and intrascleral space in the surgical area, enabling a better understanding of the structural changes caused by filtration surgery. At present, there are few reports on long-term observations of CLASS filtration channels. We combined IBAGS and UBM to image filtration in the CLASS surgical area and evaluate the drainage mechanism of the aqueous humour in CLASS. We also studied the correlations between scleral reservoir measurement parameters and intraocular pressure (IOP).
Materials and methods
Research design
This study was approved by our institutional ethics committee and followed the Declaration of Helsinki. Written informed consent was obtained from all participants enroled in the study.
This study was an uncontrolled retrospective study. In total, 28 patients (28 eyes) with medically uncontrolled primary open-angle glaucoma (POAG) underwent CLASS at the glaucoma department of our institution from November 2016 to December 2017. All participants were from the Han Chinese population with a heavily pigmented iris and were followed up postoperatively for 24 months. The inclusion criteria were as follows: patients aged 18 years or older, medically uncontrolled POAG that was defined as uncontrolled IOP (≥21 mmHg) under maximally tolerable hypotensive medications (three or more), and with progressive visual field defects and cup/disc ratio progression. The exclusion criteria were as follows: history of previous intraocular surgery and ocular laser procedures, other eye diseases, refractive stromal opacity that may interfere with optic nerve evaluation, and patient uncooperative or unable to complete the examination.
All patients underwent comprehensive ocular examinations before the surgery, including best-corrected visual acuity (BCVA), IOP (Goldmann applanation tonometer), slit-lamp biomicroscopy, fundus examination including optic disc evaluation (stereoscopic optic disc photography; Kowa Nonmyd WX, Kowa Company, Ltd., Japan), gonioscopy, visual field examinations (Humphrey Field Analyzer; 30-2; Carl Zeiss Meditec, Dublin, CA), and nerve fibre layer thickness (RNFL) (Optical Coherence Tomography; Heidelberg Engineering Inc., Heidelberg, Germany). The patients attended glaucoma clinic follow-up visits 1 week, 1 month, 3 months, 6 months, 12 months, 18 months, and 24 months after the surgery. Each visit was followed by an IOP measurement and a gonioscopic examination of the surgical area. At 1, 3, 6, 12, 18, and 24 months after the surgery, a UBM examination and slit-lamp photography were performed in the operation area. “Complete success” was defined as IOP values ranging between 5 and 18 mmHg without glaucoma medication, IOP reduction of ≥20% compared with baseline IOP, and without the need for additional glaucoma medication or reoperation (except for laser goniopuncture (LGP) therapy); “qualified success” was defined as when the IOP was achieved with glaucoma medication. Failure was defined as an IOP value <5 or >18 mmHg, IOP reduction of <20% compared with baseline IOP, or the need to undergo further glaucoma drainage surgery other than goniopuncture. Goniopuncture was not considered a failure or adverse event, as it is commonly used as a normal postoperative intervention to maintain or augment the operative results [7].
Surgical procedures
All lasers and surgeries were performed by an experienced glaucoma surgeon (GX Tang). All patients underwent laser peripheral iridotomy (LPI) and argon laser peripheral iridoplasty (ALPI) at the peripheral iris corresponding to the scleral reservoir ablation site 1 day before the surgery. ALPI settings of 250–300 mW power with a spot size of 500 μm for a duration of 0.2 s and LPI setting of 2–5 mJ were used. A neodymium:yttrium aluminium garnet (Nd:YAG) laser was used to create a peripheral iridotomy (minimum 0.2 mm in width) as close as possible to the root of the iris and centred in the expectant ablation area. Following the treatment, 1% prednisolone acetate drops (PredForte; Allergan, Irvine, CA) were applied four times daily.
The surgical procedures were as follows. First, a conjunctival flap was generated using the upper fornix as the base. Tenon’s capsule was opened and separated to expose the scleral area. Electric coagulation was performed on the blood vessels on the scleral surface for full haemostasis. A scleral flap approximately 5 mm × 5 mm in size and approximately one-third of the thickness of the sclera was dissected at least 1 mm within the clear corneal limbus. A cotton sheet containing 0.04% mitomycin was placed under the conjunctival and scleral flaps for 2–5 min, and the area was washed with 50 ml normal saline. Second, the CO2 laser beam was then applied over the scleral bed to create a deep scleral lake. The ablation of the scleral lake was performed as follows: a rectangular ablation was selected—the size was 3 mm × 4 mm, the energy was 20–24 W, and the ablation depth was close to the suprachoroid lamina; 0.04% mitomycin was then applied on the scleral floor for 1 min, followed by washing out. The ablation of the corneoscleral limbus was performed as follows: arc ablation was selected, the size was 2 mm × 1 mm, and the energy was 20 W, and the ablation was performed until the outer wall of Schlemm’s canal was opened, abd aqueous humour percolation was observed. Finally, the superficial scleral flap was repositioned and closed with two interrupted 10-0 nylon sutures at the top corners, the knots were buried. No tension was applied to the two sutures as the aqueous outflow resistance was at the TDM. The conjunctival flap was closed tightly with interrupted 10-0 nylon sutures. The patients were postoperatively treated with tobramycin dexamethasone eye drops (Novartis Alcon, Switzerland) six times daily, and the dose was tapered for a minimum of 6 weeks; the patients also received 0.5% pilocarpine nitrate eye drops (Shandong Baofu Ruida Pharmaceutical Co. LTD, Shandong, China) three times daily for 3 months.
LGP was performed with a Microruptor II neodymium:yttrium aluminium garnet (Nd:YAG) laser when the IOP exceeded 18 mmHg; the scleral reservoir decreased, and the TDM area exhibited an anomaly due to insufficient aqueous percolation through the TDM. Tiny holes were created around the TDM to facilitate aqueous humour drainage from the anterior chamber to the sclera reservoir. A Lasag-15 gonioscopy contact glass CGAL (Haag-Streit AG, Switzerland) was used to create 2–6 spots around the TDM, with energy ranging from 2 to 4 mJ. Shooting was stopped and the IOP was checked as soon as a microhole was made in the membrane. LGP was considered a success when the final IOP was less than 18 mmHg. In cases of peripheral anterior synechiae (PAS) to TDM, ALPI was applied simultaneously to shrink the iris and separate the peripheral iris from TDM. ALPI settings included power 250–300 mW, spot size 300–500 μm, spot duration 0.2 s. After treatment, the patients were treated with tobramycin dexamethasone eye drops four times daily for 7 days and 0.5% pilocarpine nitrate eye drops three times daily for 1 month.
UBM inspection
We used an MD-300 L UBM (E1920NW Tianjin MEDA) to acquire images with a probe frequency of 50 MHz, inspection depth of 5 mm, and observation range displayed on the monitor (8 mm × 5.5 mm). All UBM inspections were performed by one inspector. All patients underwent a scan of the surgical area perpendicular to the limbus, and the scan was repeated three times to evaluate the following factors: quantitative indicators, including central anterior chamber depth; residual TDM thickness; scleral reservoir size with measures of the maximum anteroposterior length (MAPL) of the longitudinal scan and maximum height (MH); and qualitative indicators, including the reflective density and height of the filtering bleb, the integrity of the TDM, the visibility of the subscleral flap path, and the presence of a hyporeflexive suprachoroidal space.
Based on the parameters measured by UBM, the filtering blebs were divided into the L-type (low-reflective), H-type (highly reflective), E-type (encapsulated), and F-type (flattened) [8]. The largest and clearest images of the scleral reservoir in the scanned images were measured using a UBM calliper tool; each parameter in the same image was measured three times, and the average value was recorded. TDM was measured at the thinnest point.
Indiana bleb appearance grading scale
According to the IBAGS system [9], the external morphologies of the filtering bleb were scored, including the following four indicators: height (H0-3), range (E0-3), blood vessel distribution (V0-4), and the Seidel test (S0-2).
Statistical analysis
SPSS 17.0 statistical software was used for the data processing, and the general data are described by the mean ± standard deviation (SD), as medians and interquartile range for continuous variables, or as n (%) for categorical variables. The Shapiro–Wilk test was used to test normality, and parametric or nonparametric tests were then applied accordingly. Pearson’s correlation coefficient or Spearman’s correlation coefficient was used to evaluate the correlations between the IOP and scleral reservoir parameters. IOP, MAPL, and MH at different time points after the surgery were compared by a single-factor repeated-measures analysis of variance. Kaplan–Meier curves were created to present the duration of complete and qualified success. P < 0.05 was considered statistically significant.
Results
In total, 28 consecutive POAG patients (28 eyes) were enroled in the study. All participants were from the Han Chinese population. We examined 22 males and six females with a mean age of 51.61 ± 14.73 years (range 29–82 years). The initial BCVA (LogMAR) was 0.25 ± 0.31 (range 0–1). The initial visual field mean deviation was −12.34 ± 7.56 dB (range −1.82 to −27.7 dB), and the mean pattern SD was 8.76 ± 3.69 dB (range 1.77–14.65 dB). The initial mean retinal nerve fibre layer thickness was 76.93 ± 16.93 μm (range 46–109 μm). The mean number of antiglaucoma medications before surgery was 3.29 ± 0.46 (range 3–4).
IOP
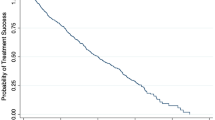
The average IOP significantly decreased from 26.96 ± 3.82 mmHg before surgery to 10.53 ± 1.40 mmHg 1 week after surgery (P < 0.001), 14.50 ± 3.17 mmHg 1 month after surgery (P < 0.001), 17.06 ± 4.31 mmHg 3 months after surgery (P < 0.001), 17.64 ± 4.66 mmHg 6 months after surgery (P < 0.001), 15.34 ± 3.16 mmHg 12 months after surgery (P < 0.001), 15.75 ± 3.27 mmHg 18 months after surgery (P < 0.001), and 15.97 ± 3.20 mmHg 24 months after surgery (P < 0.001). Thus, the IOP at each time point after surgery was significantly lower than that before surgery (Table 1). The mean number of antiglaucoma medications was 0.07 ± 0.26, 0.36 ± 0.62, 0.46 ± 0.79, 0.61 ± 1.03, and 0.71 ± 1.21 at 3, 6, 12, 18, and 24 months after surgery, respectively, and the difference was significant (χ2 = 31.397, P < 0.001), but there was no significant difference between 18 and 24 months (χ2 = 3.000, P = 0.083). The complete success rates were 71.4%, 67.9%, 64.3%, and 64.3% at 6, 12, 18, and 24 months after surgery, respectively, and the qualified success rates were 92.9%, 85.7%, 85.7%, and 85.7% at 6, 12, 18, and 24 months after the surgery, respectively (Fig. 1).
UBM findings (Fig. 2)
Anterior chamber depth
The central anterior chamber depth was reduced from 2.69 ± 0.38 mm before surgery to 2.55 ± 0.36 mm 1 month after surgery (P < 0.001), 2.61 ± 0.33 mm 3 months after surgery (P < 0.05), 2.61 ± 0.37 mm 6 months after surgery (P < 0.05), 2.66 ± 0.37 mm 12 months after surgery (P > 0.05), 2.67 ± 0.37 mm 18 months after surgery (P > 0.05), and 2.66 ± 0.37 mm 24 months after surgery (P > 0.05) (Table 2). At 1 month after surgery, a hypoechoic area in the suprachoroidal space was clearly observed posterior to the scleral reservoir site in four eyes (14.29%), which might represent ciliary body detachment or suprachoroidal drainage of the aqueous humour. UBM follow-up was conducted 1 month later, and all hypoechoic area disappeared.
Filtering bleb morphology and scleral reservoir size
Morphology of filtering blebs: After 24 months of follow-up, the rate of low reflection under filtering blebs and the height of the filtering blebs showed downward trends, and the rate of high reflection under filtering blebs showed an upward trend. At 1 month after surgery, 21 eyes (75%) had L-type filtering blebs, and seven eyes (25%) had H-type filtering blebs. At 12 months after surgery, 12 eyes (43%) had L-type filtering blebs, and 16 eyes (57%) had H-type filtering blebs. At 24 months after surgery, 11 eyes (39%) had L-type filtering blebs, and 17 eyes (61%) had H-type filtering blebs; additionally, the L-type filtering blebs showed a downward trend, while the H-type filtering blebs showed an upward trend. No E-type or F-type filtering blebs were found at 24 months. Regarding the filtering bleb height, at 1 month after surgery, five eyes (18%) had a filtering bleb height less than 1 mm, 18 eyes (64%) had a filtering bleb height of 1–2 mm, and five eyes (18%) had a filtering bleb height >2 mm. At 24 months after surgery, 22 eyes (79%) had a filtering bleb height less than 1 mm, six eyes (21%) had a filtering bleb height of 1–2 mm, and zero eyes (0%) had a filtering bleb height >2 mm (Fig. 3).
Scleral reservoir size (Fig. 4): After 24 months of follow-up, all 28 eyes (100%) had a visible subscleral flap path. A complete TDM was observed in all cases; at 1, 3, 6, 12, 18, and 24 months after surgery, there was no statistically significant difference in the TDM thickness (F = 1.551, P > 0.224), and there was no statistically significant correlation with the postoperative IOP. The MAPL values of the scleral reservoir 1, 3, 6, 12, 18, and 24 months after surgery were 2.180 ± 0.924 mm, 1.783 ± 0.870 mm, 1.659 ± 0.842 mm, 1.701 ± 0.851 mm, 1.636 ± 0.802 mm, and 1.611 ± 0.775 mm, respectively (F = 40.217, P < 0.05). The MH values of the scleral reservoir 1, 3, 6, 12, 18, and 24 months after surgery were 0.528 ± 0.226 mm, 0.441 ± 0.188 mm, 0.379 ± 0.178 mm, 0.388 ± 0.1901 mm, 0.374 ± 0.179 mm, and 0.381 ± 0.185 mm, respectively (F = 28.054, P < 0.05). There was no statistical correlation between the MAPL or MH of the scleral reservoir and the IOP 1 month after surgery, but they were negatively correlated with the IOP at 3, 6, 12, 18, and 24 months after surgery (Tables 3, 4, Fig. 5).
Gonioscopy
The gonioscopy after CLASS clearly revealed the TDM area (Fig. 6). At 1, 3, and 6 months after surgery, an IOP level higher than the target level was observed in four, seven, and four eyes, respectively; furthermore, abnormalities in the TDM area were observed, including PAS to the TDM, pigmentation of the TDM area, and adhesion of blood cells. The corresponding UBM images also clearly showed the area of peripheral iris synechiae to the TDM (Fig. 6). LGP was performed in these 15 patients (53.57%). The mean amount of time between LGP and CLASS was 3.27 ± 1.91 months. We also compared the scleral reservoirs treated with LGP with those not treated with LGP and found that the size of the scleral reservoir with the LGP treatment was significantly smaller than that without the LGP treatment (P < 0.05) (Fig. 7).
a The gonioscopy of CLASS operation field (the red box is the TDM area); b Pigmentation of the TDM area; c Adhesion of blood cells of the TDM area; d Peripheral iris synechiae to the whole TDM area; e The corresponding UBM image of figure d; f Peripheral iris synechiae to the part TDM area; g The corresponding UBM image of figure f; h Peripheral iris synechiae to the back TDM area; i: The corresponding UBM image of figure h.
IBAGS scoring
IBAGS was used to score the external morphology of the filtering blebs. At 1 month after surgery, three eyes (11%) were H0, 20 eyes (71%) were H1, and five eyes (18%) were H2; three eyes (11%) were E0, 18 eyes (64%) were E1, and seven eyes (25%) were E2; and two eyes (7%) were V1, 12 eyes (43%) were V2, 11 eyes (39%) were V3, and three eyes (11%) were V4. At 24 months after surgery, 19 eyes (68%) were H0, nine eyes (32%) were H1, and zero eyes (0%) were H2; 21 eyes (75%) were E0, five eyes (18%) were E1, and two eyes (7%) were E2; and 25 eyes (89%) were V1, three eyes (11%) were V2, zero eyes (0%) were V3, and zero eyes (0%) were V4. The Seidel tests were all negative. After 24 months of follow-up, the height of the filtering blebs progressively decreased, the range of the filtering blebs progressively shrank, and the degree of vascular congestion became progressively lighter (Fig. 8).
Surgical complications
During the 24-month follow-up period, UBM and gonioscopy revealed inaccurate ablation (one patient) and severe PAS (three patients). No hyphema, hypotony, bleb leakage, bleb infection, wound dehiscence, iris incarceration, or aqueous misdirection was observed.
Discussion
In the patients with POAG, we found that after the treatment with CLASS, the depth of the central anterior chamber 1, 3, and 6 months after surgery was slightly shallower than that before surgery, and the depth of the central anterior chamber 12, 18, and 24 months after surgery did not significantly change from that before surgery. The IOP at each time point after surgery significantly differed from that before surgery; the mean number of antiglaucoma medications decreased from 3.29 ± 0.46 before surgery to 0.71 ± 1.21 24 months after surgery; the complete success rate was 64.3%, and the qualified success rate was 85.7%. This result is similar to previous findings [10]. Therefore, we believe that CLASS is a simple, safe, and effective treatment [11, 12]. CLASS can not only significantly reduce the postoperative IOP and the use of antiglaucoma drugs [7, 10, 12] but also significantly reduce complications associated with penetrating trabeculectomy [2, 13], CLASS may be an alternative to trabeculectomy [14].
In both CLASS and NPDS, compared with classic trabeculectomy, the greatest advantage is the unique drainage route of the aqueous humour. The aqueous humour passes through an intact TDM and gradually seeps from the anterior chamber to the scleral reservoir, thus avoiding the sudden decrease in IOP after trabeculectomy. The key to surgical success is retaining a thin TDM during the operation to achieve effective aqueous humour penetration and persistence of the scleral reservoir after surgery. CLASS uses a carbon dioxide laser ablation system to generate the TDM easily and accurately. We observed that an intact and thin TDM was formed after CLASS, and no microperforation occurred intraoperatively; at 1 month after surgery, the TDM thickness was 0.094 ± 0.017 mm, and after 24 months of follow-up, the TDM thickness was 0.097 ± 0.014 mm. There were no significant differences across different time frames after surgery, and the postoperative TDM thickness was significantly lower than that reported after NPDS [15, 16].
The mechanism by which nonpenetrating glaucoma filtering surgery lowers the IOP is very complicated. It has been reported that the aqueous humour can be drained through the subconjunctival, trabecular-meshwork, intrascleral, and suprachoroidal-space pathways [17]. We used IBAGS to observe the external morphology of the filtering blebs and used a UBM examination to evaluate the internal structure of the operation area, thus performing a complete evaluation of the external and internal filtering blebs after CLASS. At 1 month after surgery, most filtering blebs had an external morphology that was diffuse, and the incidence of H1 was 71%, but the degree of congestion of the filtering blebs was severe, and the incidence of V2-V3 was 82%. At 24 months after surgery, most cases did not show filtering blebs; the occurrence rate of H0 was 68%, and the occurrence rate of E0 was 75%. After 24 months of follow-up, we found that the height of the filtering blebs was lower, the scope of the filtering blebs gradually decreased, and the degree of vascular congestion gradually lowered. These changes are consistent with our classification of filtering blebs under UBM examination. At 1 month after surgery, most filtering blebs appeared as L-type filtering blebs, and 64% of the filtering blebs had a height of 1–2 mm. Over time during the follow-up period, the L-type filtering blebs gradually changed into H-type filtering blebs, and the height of the filtering blebs gradually decreased. At 18 months after surgery, the proportion of L-type filtering blebs, the proportion of H-type filtering blebs, and the height of the filtering blebs tended to be stable. In 1995, Yamamoto et al. [8] studied UBM images of filtering blebs after trabeculectomy; classified the filtering blebs into the L-type (low-reflective), H-type (highly reflective), E-type (encapsulated), and F-type (flattened); and found that the L-type filtering blebs were the main type in eyes with good IOP control. Oh et al. [18] used IBAGS and AS-OCT to compare the morphology of filtering blebs 3 months after trabeculectomy and deep sclerectomy and found that the height of the filtering blebs was lower, the scope was narrower, and vascular congestion was more severe in the deep sclerectomy group. Jankowska-Szmul et al. [19] used IBAGS and AS-OCT to observe the morphology of filtering blebs 1 year after CLASS. The cases in the successful groups mostly showed shallow filtering blebs, and the incidence of H1 decreased from 91% 1 month after surgery to 52% 12 months after surgery; the incidence of H0 increased from 0% 1 month after surgery to 48% 12 months after surgery. These results are consistent with our observations. Therefore, we believe that most patients have a subconjunctival drainage route early after CLASS and that this route slowly diminishes and blebs continue to flatten over time and tend to stabilize by 18 months after surgery.
By observing the evolution of the filtration channel under UBM in 28 eyes over 24 months, we found that the scleral reservoir persisted in all cases; the scleral reservoir size gradually decreased over time, although it was slightly increased at 12 months after surgery, subsequently shrank, and stabilized at 18 months after surgery. Combined with the gonioscopic examination of the surgical site, we found that at 3 and 6 months after surgery, 15 eyes (53.57% of the patients) showed obvious pigmentation in the TDM area or partial or complete peripheral iris synechiae to the TDM, resulting in insufficient penetration of aqueous humour and an increased IOP. After the LGP treatment, the scleral reservoir became larger; the IOP was controlled in five eyes, and antiglaucoma drugs were still needed to reduce the IOP in 10 eyes. We compared the size of the scleral reservoir with and without LGP and found that the size in the LGP treatment group was significantly smaller than that in the non-LGP treatment group. We also found that in all cases without the LGP treatment, the scleral reservoir size gradually decreased with a longer follow-up time, while in the LGP treatment group, the size of the scleral reservoir was slightly increased 12 months after the surgery. This finding further shows that anterior adhesion or pigmentation in the TDM area can lead to insufficient aqueous humour penetration and an increased IOP, and after the treatment with LGP, the scleral reservoir becomes larger, and the IOP decreases. This finding is consistent with the observations reported by Cabrejas Laura et al. [20], who found that 6 months after NPDS, the eyes without the LGP treatment had a lower IOP and thinner TDM than those with the LGP treatment. Therefore, the existence and maintenance of the scleral reservoir are very important for postoperative aqueous drainage, which is the basis of aqueous drainage to the suprachoroidal, trabecular meshwork, intrasclera, and subconjunctiva.
Chinese patients possess a thicker iris thickness and narrower anterior chamber compared with those of Caucasian or African patients, so they typically develop a crowded anterior segment [21]. Furthermore, the incidence of PAS has reportedly been relatively high after CLASS [1]. Thus, preoperative iris management is necessary to improve the long-term surgical outcomes, and such modifications should be made considering the specific characteristics of Chinese patients with glaucoma [22]. We performed an LPI and an ALPI in the area facing the scleral reservoir ablation site to neutralize the pressure between the posterior and anterior chamber and to keep the iris away from the ablation site. After these treatments, we found a substantial reduction in the incidence of LGP at 1 month after surgery. Laser goniopuncture is considered a routine procedure in nonpenetrating glaucoma surgery and is used as normal postoperative intervention to maintain or augment the filtration and to decrease the IOP and avoid additional filtering surgery [1, 23]. In recent studies, the LGP reported frequency ranged from 45.7% to 65% in NPDS [24, 25] and from 18.5% to 85% in CLASS [1, 7, 12]. Although some authors did not use LGP in NPDS or CLASS to avoid goniopuncture complications such as wide rupture of TDM, hyphema, IOP spike, and choroidal detachment [11, 26], the complete success rate dropped precipitously compared with that achieved with the use of LGP [1, 24]. In this study, there were no goniopuncture complications, and the mean interval between LGP and CLASS was 3.27 ± 1.91 months, similar to the findings reported by Greifner et al. [1] with a mean time of 82.5 ± 107 days after surgery. Therefore, when the IOP increases after 2–3 months, sometimes rather abruptly, and the scleral reservoir gradually decreases on the UBM, these phenomena suggest the progressive reduction in permeability of the intact TDM, and it is an indication for LGP.
Emi et al. [27] reported that when the IOP was 15 mmHg, the posterior suprachoroidal pressure was 3.7 ± 0.4 mmHg lower than the IOP. These authors found that at a higher IOP, the pressure difference between the anterior chamber and the suprachoroidal space was increased; this pressure difference was the driving force of the uveal sclera outflow. In CLASS, i.e., the ablation of the scleral lake, the ablation depth is close to the suprachoroid lamina; the aqueous humour under the scleral reservoir is separated from the choroid by a thin scleral wall and might be filtered to the suprachoroidal space, causing detachment of the ciliary body [16]. In long-term follow-up after NPDS, more than half of cases presented a hypoechoic area in the suprachoroidal space which was associated with lower IOP and was statistically significant at 2 and 3 months after surgery. This effect was caused either by ciliary body detachment with a subsequent decrease in aqueous production or by choroidal resorption. Moreover, the authors considered that the choroidal resorption of the aqueous humour also took place in eyes without a hypoechoic area in the suprachoroidal space [4, 15]. We only observed this phenomenon in four eyes at 1 month after surgery, which could further verify the drainage of the aqueous humour through the thin deep scleral wall to the suprachoroidal space. A hypoechoic area in the suprachoroidal space may be seen in normal eyes, but this normal anatomic variant was not observed preoperatively and was much smaller than observed in our study. We observed fissures in the suprachoroidal space of four cases that did not receive special treatment. UBM follow-up was conducted 1 month later, and the entire hypoechoic area disappeared. Therefore, the suprachoroidal outflow pathway after CLASS may play a fairly major role in lowering the IOP.
Many studies have reported the relationship between scleral reservoir measurement parameters and IOP after NPDS. Some scholars believe that the scleral reservoir size is negatively correlated with IOP and that scleral filtration plays an important role in reducing the IOP [20, 28]. Other scholars believe that the correlation among the scleral reservoir size, TDM, and IOP is weak [19, 29]. In our study, there was no correlation between the TDM and IOP, but we found a negative correlation between the length and height of the scleral reservoir and the IOP during all time periods except for 1 month after surgery. Thus, the larger the scleral pool, the lower the IOP. At 1 month after surgery, there was no correlation between the size of the scleral reservoir and IOP. This finding may have been due to the action of subconjunctival filtration (external filtration) during the first month after surgery. Therefore, by observing the evolution of the filtration channel after CLASS through UBM, we believe that the mechanism of the long-term reduction in the IOP in CLASS seems to be less dependent on external filtering blebs and more dependent on the internal drainage pathways. To a certain extent, CLASS overcomes the problem of maintaining filtering blebs after filtration surgery.
With the advancement of glaucoma surgery and the improvement in surgical methods, we hope to increase the use of the Schlemm tube or the uveal choroid route to drain the aqueous humour and reduce the IOP through nonfiltering bleb surgery to avoid the complications caused by subconjunctival filtering blebs. The advancement of nonpenetrating glaucoma surgery has led to the current application of CLASS, which is a simple, safe, and effective method of lowering the IOP. By observing the morphology of the filtering blebs combined with the visualization of the scleral pathway under UBM, we believe that during the early stage after CLASS, the subconjunctival and suprachoroidal pathways may be the main mechanisms by which CLASS reduces the IOP, while internal drainage pathways such as the intrascleral, trabecular-meshwork, and suprachoroidal pathways play greater roles over time.
Summary
What was known before
-
CLASS is a recent modification of nonpenetrating glaucoma filtration surgery that has the same effect as traditional nonpenetrating deep sclerectomy (NPDS) in the lowering IOP.
What this study adds
-
We combined IBAGS and UBM to image the filtration in the CLASS surgical area and evaluate the drainage mechanism of aqueous humour in CLASS. We also studied the correlations between scleral reservoir measurement parameters and intraocular pressure (IOP).
References
Greifner G, Roy S, Mermoud A. Results of CO2 laser-assisted deep sclerectomy as compared with conventional deep sclerectomy. J Glaucoma. 2016;25:e630–638.
Eldaly MA, Bunce C, Elsheikha OZ, Wormald R. Non-penetrating filtration surgery versus trabeculectomy for open-angle glaucoma. Cochrane Database Syst Rev. 2014;15:CD007059.
Ton Y, Geffen N, Kidron D, Degani J, Assia EI. CO2 laser-assisted sclerectomy surgery part I:concept and experimental models. J Glaucoma. 2012;21:135–140.
Kazakova D, Roters S, Schnyder CC, Achache F, Jonescu-Cuypers C, Mermound A, et al. Ultrasound biomicroscopy images: long-term results after deep sclerectomy with collagen implant. Graefes Arch Clin Exp Ophthalmol. 2002;240:918–923.
Wells AP, Ashraff NN, Hall RC, Purdie G. Comparison of two clinical Bleb grading systems. Ophthalmology. 2006;113:77–83.
Aptel F, Dumas S, Denis P. Ultrasound biomicroscopy and optical coherence tomography imaging of filtering blebs after deep sclerectomy with new collagen implant. Eur J Ophthalmol. 2009;19:223–230.
Geffen N, Mimouni M, Sherwood M, Assia EI. Mid-term clinical results of CO2 laser-assisted sclerectomy surgery (CLASS) for open-angle glaucoma treatment. J Glaucoma. 2016;25:946–951.
Yamamoto T, Sakuma T, Kitazawa Y. An ultrasound biomicroscopic study of filtering blebs after Mitomycin C trabeculectomy. Ophthalmology. 1995;102:1770–1776.
Cantor LB, Mantravadi A, WuDunn D, Swamynathan K, Cortes A. Morphologic classification of filtering blebs after glaucoma filtration surgery: the Indiana bleb appearance grading scale. J Glaucoma. 2003;12:266–271.
Yick DW, Lee JW, Tsang S, Yeung BY, Yuen CY. Preliminary results of CO2 laser-assistedsclerectomy surgery (CLASS) in the treatmentof advanced glaucoma in a Chinese population. Medicine. 2016;95:e5294.
Skaat A, Goldenfeld M, Cotlear D, Melamed S. CO2 laser-assisted deep sclerectomy in glaucoma patients. J Glaucoma. 2014;23:179–184.
Cutolo CA, Bagnis A, Scotto R, Bonzano C, Traverso CE. Prospective evaluation CO2 laser-assisted sclerectomy surgery (CLASS) Mitomycin C. Graefes Arch Clin Exp Ophthalmol. 2018;256:181–186.
Cheng JW, Cheng SW, Cai JP, Li Y, Wei RL. Systematic overview of the efficacy of nonpenetrating glaucoma surgery in the treatment of open angle glaucoma. Med Sci Monit. 2011;17:RA155–163.
Jankowskaszmul J, Dobrowolski D, Wylegala E. CO2 laser-assisted sclerectomy surgery compared with trabeculectomy in primary open-angle glaucoma and exfoliative glaucoma a 1-year follow-up. Acta Ophthalmol. 2018;96:e582–e591.
Chiou AG, Mermoud A, Underdahl JP, Schnyder CC. An ultrasound biomicroscopic study of study of eyes after deep sclerectomy with collagen implant. Ophthalmology. 1998;105:746–750.
Chiou AG, Mermoud A, Hédiguer SE, Schnyder CC, Faggioni R. Ultrasound biomicroscopy of eyes undergoing deep sclerectomy with collagen implant. Br J Ophthalmol. 1996;80:541–544.
Johnson DH, Johnson M. How does nonpenetrating glaucoma surgery work? Aqueous outflflow resistance and glaucoma surgery. J Glaucoma. 2001;10:55–67.
Oh LJ, Wong E, Lam J, Clement CI. Comparison of bleb morphology between trabeculectomy optical coherence tomography. Clin Exp Ophthalmol. 2017;45:701–707.
Jankowska-Szmul J, Wylegala E. The CLASS surgical site characteristics in a clinical grading scale and anterior segment optical coherence tomography: a one-year follow-up. J Health Eng. 2018;15:5909827.
Cabrejas L, Rebolleda G, Muñoz-Negrete FJ, Losada D. An ultrasound biomicroscopy study of filtering blebs after deep sclerectomy with a new acrylic implant. Eur J Ophthalmol. 2011;21:391–399.
Lee RY, Chon BH, Lin SC, He M, Lin SC. Association of ocular conditions with narrow angles in different ethnicities. Am J Ophthalmol. 2015;160:506–515.
Zhang Y, Cheng GW. Modified CO2 laser-assisted sclerectomy surgery in Chinese patients with primary open-angle glaucoma and pseudoexfoliative glaucoma: a two-year follow-up study. J Glaucoma. 2020;29:367–373.
Di Matteo F, Bettin P, Fiori M, Ciampi C, Rabiolo A, Bandello F. Nd:Yag laser goniopuncture for deep sclerectomy: efficacy and outcomes. Graefes Arch Clin Exp Ophthalmol. 2016;254:535–539.
Puerto B, López-Caballero C, Sánchez-Sánchez C, Oblanca N, Blázquez V, Contreras I. Clinical outcomes after Ex-PRESS glaucoma shunt versus non-penetrating deep sclerectomy: two-year follow-up. Int Ophthalmol. 2018;38:2575–2584.
Shaarawy T, Karlen M, Schnyder C, Achache F, Sanchez E, Mermoud A. Five-year results of deep sclerectomy with collagen implant. J Cataract Refract Surg. 2001;27:1770–1778.
Cillino S, Di Pace F, Casuccio A, Calvaruso L, Morreale D, Vadalà M, et al. Deep sclerectomy versus punch trabeculectomy with or without phacoemulsification: a randomized clinical trial. J Glaucoma. 2004;13:500–506.
Emi K, Pederson JE, Toris CB. Hydrostatic pressure of the suprachoroidal space. Invest Ophthalmol Vis Sci. 1989;30:233–238.
Mavrakanas N, Mendrinos E, Shaarawy T. Postoperative IOP is related to intrascleral bleb height in eyes with clinically flflat blebs following deep sclerectomy with collagen implant and mitomycin. Br J Ophthalmol. 2010;94:410–413.
Khairy HA, Atta HR, Green FD, van der Hoek J, Azuara-Blanco A. Ultrasound biomicroscopy in deep sclerectomy. Eye. 2005;19:555–560.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yan, X., Zhang, H., Li, F. et al. Surgical site characteristics after CLASS followed by ultrasound biomicroscopy and clinical grading scale: a 2-year follow-up. Eye 35, 2283–2293 (2021). https://doi.org/10.1038/s41433-020-01235-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01235-w
This article is cited by
-
Effect of Trabeculodescemetic Window Perforation in Deep Sclerectomy on Intraocular Pressure in Primary Congenital Glaucoma
Ophthalmology and Therapy (2024)
-
CO2 Laser-Assisted Sclerectomy Surgery Alone or Combined with Phacoemulsification in Primary Open-Angle Glaucoma: Comparison of 1-Year Outcomes
Ophthalmology and Therapy (2022)