Abstract
Background
Botulinum toxin (BTX) is useful for inducing temporary ptosis in patients with ocular surface disease. However, transcutaneous BTX often causes inadvertent superior rectus (SR) paresis. Furthermore, Muller’s muscle is unaffected by transcutaneous BTX, resulting in lagophthalmos and incomplete ptosis.
Methods
We report a novel BTX injection technique, in which the upper lid is double everted over a Desmarres retractor, and BTX injected transconjunctivally at the superior aspect of Muller’s muscle, where it lies close to levator palpebrae superioris.
Results
In our series of 21 patients, one had inadvertent subcutaneous BTX and developed incomplete ptosis. The remaining 20 patients had complete ptosis within 48 h. No patients had SR underaction.
Conclusion
We conclude that transconjunctival BTX injection is safe, effective, and superior to transcutaneous BTX injection, because of the low risk of superior rectus underaction and incomplete ptosis.
Similar content being viewed by others
Introduction
Persistent epithelial defects and ocular surface exposure secondary to lagophthalmos can result in significant ocular morbidity [1, 2]. Treatment of these cases can be challenging and prolonged because they are often poorly responsive to medical therapy, and reversible physical corneal protection is a useful treatment strategy. The traditional method has been surgical tarsorrhaphy, which involves initial surgery followed by surgical reversal at a later date. However, this carries a risk of scarring and disfigurement, and depending on the extent of the procedure, clinical assessment of the ocular surface and application of topical medication may not be possible [2].
Temporary botulinum toxin A (BTX) chemodenervation of levator palpebrae superioris (LPS) has been shown to be effective in inducing an appropriate degree of ptosis to permit healing of the ocular surface. Superior rectus underaction leading to hypotropia and diplopia is a common side effect, occurring in approximately two-thirds of cases [3, 4]. Furthermore, this can be associated with incomplete ptosis, which may exacerbate corneal exposure due to loss of Bell’s phenomenon. Underaction of superior rectus most likely results from its proximity to the levator muscle. The administration of botulinum toxin A is commonly via a transcutaneous route into the orbit and into the levator muscle, but the inability to visualise the needle when injecting into the orbit may account for incomplete ptosis and superior rectus underaction if the needle is not inserted deep enough or too deep, respectively. Moreover, a sharp needle technique always runs the risk of inadvertent globe perforation [5].
We present results of a novel transconjunctival method of botulinum toxin A induced ptosis. We believe that it is a simple technique that produces a complete ptosis, without superior rectus underaction or the risk of inadvertent globe perforation.
Methods
Retrospective, non-comparative interventional case note analysis of patients presenting to the ophthalmology clinic between 2011–2018, requiring temporary corneal protection were included. All patients were offered the choice of either temporary lateral tarsorraphy or BTX-induced ptosis. The latter group are presented here. NT was the sole operator for all cases and this series represents all the cases treated by NT during the study period. Patient demographics, diagnosis, degree of induced ptosis, ocular motility and complications were noted. The effectiveness of BTX-induced ptosis in promoting ocular surface healing was not assessed. The main outcome measures were the degree of ptosis induction and superior rectus underaction. The study was approved as an audit by the institutional ethics review board.
Technique
After informed consent, the ocular surface was anaesthetised using proxymetacaine 0.5% minims drops (Bausch & Lomb, UK). This was further supplemented with tetracaine 0.5% minims (Bausch & Lomb, UK). Dysport® 500U (Ipsen Ltd, UK) was reconstituted with 0.9% sodium chloride for injection as per manufacturer instructions, resulting in a working concentration of 10U/0.1 ml. Dysport was drawn up into a 1 ml syringe and a 30 gauge needle attached for injection, after which air was expelled, leaving 0.25 ml (25U) for administration. The equivalent dosages when using Botox® (Allergan, UK) were 5U/0.1 ml concentration.
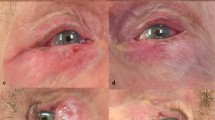
The patient was positioned supine on an examination couch. The upper lid was double everted over an appropriately sized Desmarres retractor (see Fig. 1A–D), permitting visualisation of the superior border of the tarsal plate and Muller’s muscle up to the superior fornix. A cotton tipped applicator soaked in tetracaine was then applied to the conjunctiva overlying Muller’s muscle to provide further anaesthesia. The injection needle was inserted subconjunctivally at the superior aspect of Muller’s muscle where its origin is in close proximity with the LPS muscle (Fig. 1C, D). The needle was advanced ~5 mm superiorly and slightly posteriorly where the levator muscle should be located, and 20U (in 0.2 ml) (10U in 0.2 ml of Botox®) was injected (Fig. 2). Care was taken not to advance the needle too deep in relation to the conjunctiva, thereby preventing injection of BTX subcutaneously or into the pre-aponeurotic fat pad. The remaining 10U (in 0.1 ml) (5U in 0.1 ml of Botox®) was injected subconjunctivally over Muller’s muscle to create a small bleb. Care was taken not to damage the marginal arcade, as this can result in bleeding.
The upper eyelid is everted (A) and then double everted over a Desmarres retractor (B). The extent of Mullers muscle can also be easily visualised, and is shown by the white arrow, and the site of injection is indicated by * (C). A 30G needle is used to inject at the superior border of Muller’s muscle (D).
Results
Twenty-one patients met the criteria for inclusion into the study. The mean age was 65.7 years (range 34–89). Seven patients had microbial keratitis, and two patients had paralytic lagophthalmos secondary to seventh nerve palsy or cerebrovascular disease. The remaining patients had neurotrophic ulcers (two patients), herpes simplex keratitis (six patients), chemical injury (one patient), atopic eye disease (one patient) or post-penetrating keratoplasty defects (two patients). One patient had inadvertent subcutaneous BTX and as a result did not develop a complete ptosis. The remaining 20 patients had complete ptosis induced within 48 h of the BTX administration. None of the 21 patients developed superior rectus underaction (Fig. 3). In two patients, the ptosis had not resolved 6 months after administration. Our results can be seen in Table 1.
Discussion
The transcutaneous administration of BTX to induce protective ptosis was first reported by Adams et al., who demonstrated it to be an effective method of aiding healing of corneal epithelial defects and a suitable alternative to surgical tarsorraphy [1, 4]. Since then it has gained increasing acceptance as a rapid minimally invasive outpatient procedure. However, it is not without complications, the main unwanted effect being underaction of the superior rectus muscle (SRM), with resultant diplopia [1, 3, 4, 6].
The LPS is the main elevator of the upper eyelid and has its origins on the lesser wing of sphenoid just above the optic foramen. It courses anteriorly where it becomes the aponeurosis before finally inserting on to the superior tarsal plate and the skin of the eyelid. The second elevator is the involuntary superior tarsal muscle (Muller’s muscle), which arises from the undersurface of the LPS and inserts on to the superior aspect of the superior tarsal plate. This muscle is responsible for ~2 mm of the upper eyelid height. The LPS is in close relationship with the SRM though it is separated by fibrous connective tissue. This is particularly so at the mid and posterior portions [7,8,9]. The SRM inserts on to the globe at 7.7 mm from the limbus and is surrounded by its own fascial sheath, while the LPS courses anteriorly into the upper lid and inserts on to the skin and superior tarsus [7, 9]. It is logical that complete ptosis can only be induced if both Muller’s muscle and the levator are chemodenervated.
In all previous studies, the approach for chemodenervation is via a transcutaneous approach to deliver BTX to the LPS muscle belly. These techniques utilise a 25 mm needle into the orbit in an attempt to locate the LPS [1, 3, 4, 10]. Given that the needle cannot be visualised during the procedure, the successful location of the LPS cannot be guaranteed. Given the close proximity of the SRM to the LPS it is likely that the deep, posterior placement of the needle results in combined LPS and SRM paresis with resultant hypotropia, loss of Bell’s phenomenon and diplopia. In a prospective study by Adams et al. [4] and later by Kirkness et al. [3], SRM underaction was seen in 67% and 68%, respectively. Moreover, ocular misalignment with resultant diplopia was seen in 13 and 16% of patient after recovery of ptosis [3, 4]. In a later study by Ellis and Daniell, where a similar method of BTX administration was undertaken, diplopia occurred in 24% of cases [1]. This would suggest that SRM underaction persisted after the resolution of the ptosis. They however do not report the extent of SRM underaction with ptosis present, which may be higher.
Given the high incidence of SRM underaction, Naik et al. proposed a modified technique involving the placement of a half-inch 26G or 30G needle placed near the orbital roof just behind the superior orbital rim before injecting BTX. They present ten cases and report no SRM underaction in any of the patients. They also report a longer duration of ptosis and postulate that this may be the result of using a larger dosage of BTX compared to previous studies. However, partial recovery of ptosis was seen in the majority at 4 weeks post-injection [2]. The lack of SRM underaction can be explained by the anterior placement of the needle and the barrier provided by the LPS in preventing the BTX affecting the SRM.
An important feature to note in these studies is that, although significant ptosis was achieved in the majority, complete ptosis was seen in very few patients [2,3,4]. Incomplete ptosis was seen in seven patients (46%) [4] whilst complete ptosis was not achieved in any of the patients reported by Naik et al. [2]. Five patients reported by Naik et al. had significant lagophthalmos after BTX injection, but this may be the result of paralytic ectropia commonly seen in longstanding Bell’s palsy. Nonetheless, complete ptosis was not seen in the other cases either [2]. This is most likely the result of a functioning Muller’s muscle, as there were no attempts to chemodenervate this muscle.
The BTX effect on the autonomic nervous system is no different from its effect on the striate neuromuscular synapse [10]. BTX has been used to treat many types of hyperactive smooth muscle including the distal oesophageal sphincter in achalasia, the internal anal sphincter in anal fissures and anismus, the detrusor muscle and the pylorus in gastroparesis [10].
In a series of three cases, Reddy and Woodard demonstrate partial ptosis induction in thyroid eye disease associated lid retraction after BTX injection into Muller’s muscle via a subconjunctival approach. They also combine transcutaneous and transconjunctival to successfully induce ptosis in another case [11].
Our study demonstrates the efficacy of transconjunctival administered BTX after double eversion of the upper eyelid using a Desmarres retractor. Complete ptosis was achieved in 20 cases within 48 h. Moreover, the complete ptosis was achieved as a result of chemodenervating both the LPS and Muller’s muscle. The single failure was the result of a poorly placed needle which we suspect penetrated through the aponeurosis resulting in subcutaneously placed BTX—in this case there would have been some BTX effect on the LPS but not on Muller’s muscle, hence the incomplete ptosis. Two patients had some residual ptosis 6 months after BTX administration leading to lid asymmetry. The pathogenesis in these two patients is unclear but it is possible that the double eversion technique has caused some iatrogenic attenuation of the aponeurosis.
There were no cases of SRM underaction in our series, which we attribute to clear visualisation of Muller’s muscle and the greater likelihood of localising LPS without affecting SRM. Furthermore, we believe this technique to be safer as the needle is at no time directed towards the globe or into the orbit. Though not formally assessed, we believe also that this technique is less uncomfortable than the transcutaneous approach, because topical anaesthesia is highly effective in providing excellent conjunctival anaesthesia prior to injection. It should be noted that care must be taken when injecting Muller’s muscle to avoid damage to the vascular arcade with resultant subconjunctival haematoma. In addition, care must be undertaken not to insert the needle too deep (from conjunctival approach) as not to perforate through the LPS and its aponeurosis and deliver the BTX into the subcutaneous space.
We propose that the transconjunctival approach for injecting botulinum toxin A is an effective method for inducing complete ptosis and avoids the risk of associated superior rectus underaction or inadvertent globe perforation associated with the transcutaneous injection reported in previous studies.
Summary
What was known before
-
Botulinum toxin can be used to perform chemodenervation ptosis side effects, e.g. superior rectus weakness, are not uncommon.
-
Transcutaneous and transconjunctival routes have been previously described.
What this study adds
-
A novel transconjunctival technique to perform botulinum ptosis, using double lid eversion reduced risk of superior rectus under action.
-
No reports of globe rupture with novel technique.
-
Double lid eversion allows precise and safe administration of the botulinum toxin.
References
Ellis MF, Daniell M. An evaluation of the safety and efficacy of botulinum toxin type A (BOTOX) when used to produce a protective ptosis. Clin Exp Ophthalmol. 2001;29:394–9.
Naik MN, Gangopadhyay N, Fernandes M, Murthy R, Honavar SG. Anterior chemodenervation of levator palpebrae superioris with botulinum toxin type-A (Botox) to induce temporary ptosis for corneal protection. Eye. 2008;22:1132–6.
Kirkness CM, Adams GG, Dilly PN, Lee JP. Botulinum toxin A-induced protective ptosis in corneal disease. Ophthalmology. 1988;95:473–80.
Adams GG, Kirkness CM, Lee JP. Botulinum toxin A induced protective ptosis. Eye. 1987;1:603–8.
Duker JS, Belmont JB, Benson WE, Brooks Jr HL, Brown GC, Federman JL, et al. Inadvertent globe perforation during retrobulbar and peribulbar anesthesia. Patient characteristics, surgical management, and visual outcome. Ophthalmology. 1991;98:519–26.
Heyworth PL, Lee JP. Persisting hypotropias following protective ptosis induced by botulinum neurotoxin. Eye. 1994;8:511–5.
Ettl A, Kramer J, Daxer A, Koornneef L. High-resolution magnetic resonance imaging of the normal extraocular musculature. Eye. 1997;11:793–7.
Ettl A, Priglinger S, Kramer J, Koornneef L. Functional anatomy of the levator palpebrae superioris muscle and its connective tissue system. Br J Ophthalmol. 1996;80:702–7.
Kakizaki H, Malhotra R, Selva D. Upper eyelid anatomy: an update. Ann Plast Surg. 2009;63:336–43.
Dressler D, Adib Saberi F. Botulinum toxin: mechanisms of action. Eur Neurol. 2005;53:3–9.
Reddy UP, Woodward JA. Abobotulinum toxin A (Dysport) and botulinum toxin type A (Botox) for purposeful induction of eyelid ptosis. Ophthal Plast Reconstr Surg. 2010;26:489–91.
Author information
Authors and Affiliations
Contributions
All named authors have contributed sufficiently to the intellectual content of the submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Informed consent
Written informed consent was obtained for the publication of all images.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tint, N.L., Saxby, E.P.I., Young, SL. et al. A modified transconjunctival technique for botulinum toxin chemodenervation of levator palpebrae superioris for corneal protection. Eye 36, 1217–1221 (2022). https://doi.org/10.1038/s41433-021-01587-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01587-x