Abstract
Objectives
The area-under-the-curve (AUC) measures the average drug effect over time. We investigated the impact of baseline clinical and optical coherence tomography (OCT) factors on the response to fluocinolone acetonide (FAc) 0.19 mg implant in patients with diabetic macular oedema (DMO) as the AUC over 36 months.
Methods
Retrospective study of DMO eyes undergoing FAc with follow-up from 12 to 36 months. The AUC of the best-corrected visual acuity (BCVA) and the central macular thickness (CMT) were calculated with the trapezoidal rule. Demographic and clinical data at the time of FAc administration were collected, and associations with BCVA and CMT changes were investigated with linear mixed models.
Results
Eighty-nine eyes of 63 patients were enroled; median follow-up was 26 months. Mean±standard deviation (SD) AUCBCVA and AUCCMT after FAc injection were 0.24 ± 0.17 LogMAR/month and 179.6 ± 54.3 μm/month, respectively. Worse baseline BCVA (β = 0.30 LogMAR/month, p < 0.001), higher AUCCMT after FAc administration (β = 0.08 LogMAR/month, p < 0.001), diagnosis of type 1 diabetes (β = −0.04 LogMAR/month, p = 0.04), and absent ELM/EZ layers (β = 0.06 LogMAR/month, p = 0.01) were associated with worse vision over time (higher AUCBCVA). Eyes with higher CMT at baseline (β = 9.61 μm/month, p < 0.001) and those with tractional DMO (β = 24.7 μm/month, p = 0.01) had worse anatomic outcomes (higher AUCCMT). The need for additional treatments after FAc was also associated with higher AUCCMT (β = 33.9 μm/month, p = 0.001).
Conclusion
Baseline better visual acuity, lower macular thickness, and photoreceptors’ layers integrity are associated with better functional response to FAc in DMO. Eyes with severe DMO at the time of implant or tractional oedema have worse anatomic response. These findings might guide clinicians in a more informed decisional algorithm in treating DMO.
Similar content being viewed by others
Introduction
Diabetic macular oedema (DMO) pathogenesis is multifactorial and encompasses vascular, inflammatory, and neurodegenerative processes [1]. Vascular endothelial growth factor (VEGF) antagonists are the first-line treatment for most DMO patients. However, a proportion of eyes have a suboptimal response to anti-VEGF in terms of visual acuity and macular thickness [2] and may benefit from switching to intravitreal corticosteroids [3]. Sustained-release formulations of steroids allow for longer drug persistence into the vitreous cavity and provide good long-term control of disease with fewer injections [4]. The fluocinolone acetonide (FAc) 0.19 mg non-bioerodable implant (Iluvien®, Alimera Sciences, Inc., Alpharetta, GA, USA) releases a sub-microgram dose of steroid into the vitreous over 36 months. It has been approved in the USA and EU for the treatment of DMO based on the results of Fluocinolone Acetonide for Diabetic Macular Edema (FAME) randomised clinical trials (RCTs) [5, 6].
Real-world registry data have confirmed the efficacy of the FAc implant and its safety profile, with cataract progression and intraocular pressure (IOP) elevation being the most common side effects [7,8,9]. Real-world studies are a good complement to clinical trials, and they expand and generalise the results of RCTs. However, patients in the real world considerably differ from research settings and have a wider range of baseline visual acuity and macular thickness, more severe macular damage, and older age [10].
There is a lack of studies evaluating the clinical predictors of good response to FAc implant, and it is still unclear which patients are the best candidates for FAc. The identification of patients who benefit the most from FAc may allow a more individualised therapeutic approach. The present study was designed to investigate the impact of different baseline demographic, clinical, and optical coherence tomography (OCT) factors on the functional and morphologic response to FAc implant in patients with DMO, evaluated as the area-under-the-curve (AUC). As a secondary analysis, factors associated with the risk of glaucoma surgery were examined.
Materials and methods
This was a single-centre retrospective study of patients undergoing FAc implant at the Medical Retina Unit of the Department of Ophthalmology, San Raffaele Hospital (Milan, Italy) between July 2017 and April 2020. The study was conducted in agreement with the Declaration of Helsinki for research involving human subjects; all the subjects signed a written consent at the time of FAc administration, approved by the local ethics committee (CE-HSR-V1-130212, approval date 28/03/2012). The data were reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations for longitudinal studies (Supplementary Table 1).
Criteria for FAc implant administration and subsequent patient management in our centre
As this study was retrospective, criteria for FAc administration were not set in advance, and the use of the FAc implant was determined by the treating physician on an individual basis, considering patients’ preferences, expected compliance, and the perceived risk of complications. Overall, FAc implant was administered in DMO with a suboptimal response to previous therapeutic strategies (i.e., focal/grid laser, anti-VEGF, or dexamethasone (DEX) sustained-release implant), such as persistence or recurrence of macular thickening despite treatment. All eyes had previously undergone cataract surgery, as per Italian reimbursement guidelines, and had no history of progressing glaucoma or IOP increase with other steroid agents not controlled by topical IOP-lowering medications [11]. In the case of non-resolving or recurrent DMO after the FAc implant injection, additional focal laser, intravitreal anti-VEGF agents, or DEX implant were administered on a pro-re-nata basis. In the case of IOP elevation judged as potentially harmful by the treating physician, IOP-lowering measures were deployed.
Study population
Patients were included in this study if they fulfilled the following inclusion criteria: (1) age ≥18 years; (2) diagnosis of diabetes mellitus (DM), type 1 or type 2; 3) at least two visits available in the chart review with a minimum follow-up of 12 months. Exclusion criteria were: 1) visual loss or macular oedema secondary to other causes than DMO; 2) media opacity; 3) history of ocular surgery ≤6 months before FAc injection; 4) IV anti-VEGF injection <1 month or DEX implant injection <4 months before FAc administration. Both eyes of each patient were included, if eligible; for each participant, the electronic clinical charts of all available visits were reviewed. A smaller portion of this population has served for previous publications [12,13,14].
Baseline variables collected included: age, gender, ethnicity, duration and type of DM, glycated haemoglobin (HbA1c), diabetic retinopathy grade (non-proliferative DR [NPDR] or proliferative DR [PDR]), duration of DMO (expressed as months from the first DMO reporting in the medical charts), type and number of previous DMO treatments, history of vitrectomy, number of IOP-lowering medications. The following variables were collected visit-by-visit: best-corrected visual acuity (BCVA) measured on decimal charts, IOP measured by applanation tonometry, central macular thickness (CMT) measured on spectral-domain OCT (SD-OCT; Spectralis HRA, Heidelberg Engineering; Heidelberg, Germany).
Baseline SD-OCT parameters were evaluated by a trained reader (L.C.) in a horizontal scan passing through the fovea for the presence of (1) subfoveal disruption of the ellipsoid zone (EZ) or external limiting membrane (ELM); (2) disorganisation of the inner retinal layers (DRIL); (3) epiretinal membrane (ERM); (4) hyperreflective intraretinal spots (HRS), labelled present if >20; and (5) subfoveal fluid (SF).
The additional treatments given after FAc injection, adverse events related to FAc injection, and IOP-lowering medications/procedures were recorded.
Statistical analysis
Statistical calculations were performed with the open-source programming language R.
The BCVA and CMT changes over time were investigated with linear mixed models with a repeated-measures design: the BCVA and CMT, separately, were the dependent variable; the follow-up visit was included as a fixed factor; the patients’ and eyes’ identification numbers the random effect terms with a nested structure to account for within-subject and within-eye correlations. Pairwise differences between the various time points were assessed with Tukey’s correction for multiple comparisons.
The primary study outcome was to identify baseline factors associated with BCVA and CMT changes, defined as the AUCBCVA (expressed as LogMAR) and the AUCCMT (expressed as μm) from baseline to month 36. The AUC was measured with the trapezoidal rule, including all the BCVA and CMT observations available for each patient, respectively (Fig. 1A, B). For each eye, the AUC values were normalised for the follow-up months to account for the heterogeneous follow-up time [15, 16]. As not all the eyes had complete information due to suboptimal compliance to doctors’ visits, missing longitudinal data (Supplementary Fig. 1A) were imputed with the nearest available observation [17]. As the BCVA was expressed in LogMAR, higher AUC indicated worse visual acuity over the follow-up and vice versa.
For each eye, the BCVA and CMT values were plotted as a function of follow-up time. Each visit corresponded to a different observation (x axis). Adjacent time points (showed in magenta) were joined by a straight line, forming multiple sub-intervals with a trapezoidal shape. The areas of each trapezium were summed together, giving the total AUC for each eye. Panels A and B show the curve of the same eye.
Linear mixed models were used to evaluate factors associated with BCVA and CMT changes. In all the models, either AUCBCVA or AUCCMT values were the dependent variable, while the patient identification number was the random effect to account for within-subject correlations. Demographic data, clinical data, and OCT biomarkers were candidate covariates. Variables were selected with a parsimonious approach, using a least absolute shrinkage and selection operator regression (LASSO) [18]. For each model, the regression coefficients (β), the 95% confidence intervals (CI), and the p values were reported, after inspection of the residuals. The total variance explained by each model was expressed as R2, according to Snijders and Bosker [19]. Missing values within the predictors were handled with multiple imputations through a chain equation with the mice R package [20]. Fifteen randomly generated datasets were imputed, and missing data were estimated in each of the imputed datasets and combined using Rubin’s rules.
Adverse events and additional treatments were reported for the entire cohort receiving FAc implant. Worse BCVA was expected in eyes experiencing IOP-related complications; therefore, for the AUCBCVA calculation and its associated factors, the BCVA values were censored from the glaucoma surgery day onwards. The potential associations with trabeculectomy were explored using a Cox regression model, where the patient identification number was the random effect. The variables were selected based on literature review and data availability on retrospective data collection. The survival time was measured from FAc implantation to the date of trabeculectomy. The effect of covariates was evaluated with univariable models; for each variable, the hazard ratio (HR) and the 95% confidence interval (CI) are reported.
Statistical significance was set at p < 0.05.
Results
Demographics and baseline clinical features
A total of 112 eyes of 67 subjects, all Caucasian, underwent FAc implant. Of these, 89 eyes (79%) of 63 patients completed a minimum 12-month follow-up and were included in the study. Median (interquartile range [IQR]) available follow-up was 26 (20–32) months (Supplementary Fig. 1B).
Overall, most patients were males (62%), with type 2 DM patients (68%), NPDR eyes (65%), and long-standing DMO (mean duration 4.2 ± 2 years). Seventy eyes (80%) had DMO for >3 years. All eyes had previously undergone a variable combination of intravitreal treatments and macular laser (Table 1).
Visual acuity changes over time and associated factors
Overall, BCVA improved following FAc implant (p = 0.01), with a progressive increase from baseline (0.57 ± 0.40 LogMAR, equal to 20/70) to month 12 (0.46 ± 0.37 LogMAR, equal to 20/60) (p = 0.004) (Supplementary Table 2, Fig. 2A). The visual acuity remained unchanged or slightly worsened thereafter. Worse baseline BCVA was associated with greater visual gain up to 24 months; on the other hand, better visual acuity at baseline was associated with higher BCVA for the entire follow-up (Fig. 2B). Patients undergoing trabeculectomy had worse visual acuity over time than eyes with no history of IOP-lowering surgery (Fig. 2C).
A Boxplots illustrating the overall changes in BCVA. Straight bold lines within the boxplot indicate the median values, diamonds the mean values, and single points the outliers. B BCVA changes with eyes stratified as a function baseline visual acuity. Eyes with worse baseline BCVA had the greatest improvement after FAc implant, but the worst BCVA at the end of follow-up. C Boxplots of the cohort stratifying eyes based on the history of trabeculectomy. Eyes undergoing trabeculectomy had a worse vision over time. D Linear correlation between the mean macular thickness over time (AUCCMT) and the mean visual acuity (AUCBCVA) over time. The regression coefficient was calculated with a Pearson’s correlation.
The mean AUCBCVA was 0.24 ± 0.17 LogMAR/month, ranging from 0.01 to 0.75 LogMAR/month. The visual acuity and the macular thickness over time followed a linear relationship (R2 = 0.44, p < 0.001) (Fig. 2D). Higher baseline BCVA (β = 0.30 LogMAR/month; 95% CI = 0.24–0.36, p < 0.001), less severe macular oedema after FAc administration (expressed as AUCCMT, β = 0.08 LogMAR/month; 95% CI = 0.03–0.12, p < 0.001), type 2 diabetes (β = −0.04 LogMAR/month; 95% CI = −0.08 to −0.02, p = 0.04), and higher number of DEX implants administered before FAc (β = −0.01 LogMAR/month; 95% CI = −0.02–0.00, p = 0.003) were associated with higher BCVA after FAc (lower AUCBCVA). On the other hand, eyes with subfoveal ELM/EZ absence on OCT had worse vision during the follow-up (higher AUCBCVA) (β = 0.06 LogMAR/month; 95% CI = 0.01–0.11, p = 0.01).
Anatomical changes over time and associated factors
CMT decreased after FAc from 507.6 ± 187.5 to 317.8 ± 120.2 μm at 36 months (p < 0.001) (Supplementary Table 2). The reduction in CMT was sustained over time.
The mean AUCCMT was 179.6 ± 54.3 μm/month, ranging from 85.71 to 343.7 μm/month. A history of anti-VEGF injections administered before receiving FAc (β = −35.5 μm/month; 95% CI = 0.03–0.12, p < 0.001) was associated with a thinner macula during the follow-up (lower AUCCMT). Eyes with higher CMT at baseline (β = 9.61 μm/month; 95% CI = 3.97–15.2, p < 0.001) and those with tractional DMO (β = 24.7 μm/month; 95% CI = 5.59–43.8, p = 0.01) had a worse morphologic response (higher AUCCMT) to FAc implant. The need for additional treatments after FAc was associated with higher macular thickness over 36 months (β = 33.9 μm/month; 95% CI = 15.1–52.7, p = 0.001) (Table 2).
Additional treatments and safety
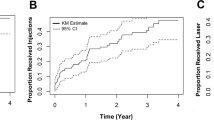
Thirty-six eyes (32%) underwent additional anti-VEGF injections after FAc for recurrent or persisting DMO, with a mean of 2.1 ± 1.34 (range 1–7) per eye. Forty-two eyes (37%) were treated with one or more IOP-lowering drugs after FAc administration. Thirteen eyes (12%) underwent trabeculectomy after a median (IQR) of 18 (16–23) months. Glaucoma surgery was performed as early as seven months until 33 months after FAc implant. The survival curve is shown in Fig. 3; none of the inspected factors significantly predicted the risk of trabeculectomy (Supplementary Table 3), but having PDR and the history of IOP rise after previous DEX carried the highest risk magnitude (HR = 4.48 and HR = 3.11, respectively).
No sight-threatening complications, such as endophthalmitis, choroidal haemorrhage, or retinal detachment, occurred. Recorded adverse events included vitreous haemorrhage secondary to the intravitreal injection (one eye), and anterior chamber migration of FAc insert (vitrectomized eye with open posterior capsule). One patient had a sterile hypopyon, managed with topical antibiotics and topical steroids, which subsided within a few days.
Discussion
In our cohort, continuous low-dose corticosteroid release yielded a significant improvement in visual acuity and macular thickness over a median follow-up of two years. Patients with type 2 DM, eyes with better baseline vision, those with a lower macular thickness over follow-up, and intact subfoveal photoreceptors OCT bands had better visual outcomes up to 3 years. Eyes with lower CMT at baseline retina and non-tractional DMO had the best morphologic response. None of the inspected factors predicted the risk of glaucoma surgery.
We calculated the BCVA and CMT changes over time using the AUC method. AUC is an informative approach for the longitudinal evaluation of treatment efficacy, especially when dealing with sustained-release medications. While time-point measurements give only a single snapshot of the visual function or the macular thickness at each visit, the AUC provides a clearer view of the average functional/anatomical drug effect over time. The AUC method has been used for comparing the FAME results with other RCTs [15, 16]. Only one study assessed the BCVA changes after FAc administration in a real-life setting using the AUC method [8]. Our study provides new clinical information and may serve as a reference for other cohorts.
Patients with the highest starting vision had the best functional outcomes after FAc administration; contrarily, the relative change in BCVA progressively increased as baseline BCVA declined. This finding is not surprising, as a similar relationship has been previously observed in patients undergoing anti-VEGF treatment [21]. Our data are in accordance with previous cohorts receiving the FAc implant. The USER study, a retrospective cohort study in four U.S. centres, reported that patients with low starting BCVA (i.e., <20/100) had limited BCVA potential as opposed to those starting with good BCVA (i.e., >20/40) [9]. In the Medisoft audit study from the UK, a considerable number of eyes with poor baseline BCVA (~40%) remained in the 0–33 EDTRS letters category at 24 months [7]. In a UK-based real-world study, Alfaqawi et al. showed that eyes with poor baseline BCVA (ETDRS letter score of ≤35) achieved the greatest improvement at 12 months (+16 letters), while the “relatively good visual acuity” group at baseline achieved the least letters’ gain [22].
Higher CMT was associated with less pronounced anatomic changes after FAc administration. The relationship between baseline central subfield thickness (CST) and the anatomical response to treatment seems to contradict the retrospective analysis of the Iluvien Clinical Evidence study (ICE-UK), where eyes with a CST of ≥400 μm at the time of injection were more likely to achieve a reduction in CST of ≥25% at 12 months [23]. However, we previously reported that patients with higher CMT at the time of FAc injection maintained persistent oedema throughout the follow-up and underwent more additional treatments [12]. Proper management of eyes with worse CMT at the time and after FAc implant injection has relevant functional consequences, as the visual acuity and the macular thickness over time were linearly correlated.
While many groups have investigated predictors and biomarkers of the response to anti-VEGF agents [24] or DEX implant [25], no such analysis has been conducted on patients receiving FAc. The EZ and the ELM layers on OCT correspond to the outer segments of the photoreceptors; in our study, the absence of these layers was identified as an independent factor associated with a poorer visual acuity in response to FAc implant, consistently with previous studies [26]. The presence of ERM was associated with a worse morphologic response. We think that the epiretinal tissue constituting ERM exerts tractional forces non-responsive to medical treatments; alternatively, ERM may act as a physical barrier decreasing drug penetration into the retina [27]. The presence of DRIL, HRS, and SF was not meaningfully associated with the FAc effect. As we considered eyes with a long history of DMO receiving multiple treatments, we suspect the contribution of each OCT feature on the visual and anatomic outcomes was difficult to isolate.
In our study, all the eyes undergoing FAc had received other anti-DMO treatments, like other real-life cohorts [9, 28, 29]. We observed a better visual response to FAc in eyes receiving a higher number of intravitreal steroidal injections before FAc implant. This may suggest a better outcome in eyes with tight therapeutic control before FAc. On the other hand, it may be a performance bias; as eyes with a more inflammatory DMO are more responsive to intravitreal steroids, they might have received more DEX injections before FAc implant [13]. A higher number of injections may also imply a longer DMO duration, and previous studies have suggested better BCVA response in eyes with long-standing DMO [30,31,32]. Nevertheless, we did not find a direct linear correlation between DMO duration and BCVA nor the CMT after receiving FAc. Admittedly, only 20% of eyes had DMO for less than 3 years, and the association between DMO duration and FAc response may be attenuated.
The interpretation of the lack of association between the visual acuity (AUCBCVA) and the administration of additional treatments after FAc is complicated. Being a retrospective study, the reason and the schedule for giving rescue DMO treatments was not standardised. We cannot exclude the need for anti-VEGF post-FAc was masking the effect of additional baseline factors on the visual outcomes. Moreover, we cannot state conclusively if the AUCBCVA after FAc did not vary despite or because of the need for additional DMO treatments.
Older age has been identified as a negative predictor factor for the response to intravitreal anti-VEGF agents [33] and DEX implant [34]. No relationship between age and response to FAc was found in the present study. This may be due to a true lack of association between the two variables or a selection bias. As the FAc implant is a second- or a third-line agent, an older population might have been selected in the study, non-responsive to previous therapeutic options. This study confirms the previously reported lack of association between gender and treatment response [34]. In agreement with previous studies [35, 36], we found no significant impact of prior vitrectomy on either the visual or the anatomical changes over 36 months. However, only 13% had undergone vitreoretinal surgery in our cohort, and the analysis might be underpowered.
IOP elevation was the most common complication. The proportion of eyes receiving IOP-lowering drops (31%) fell within the range of published cohorts (range 7–46%) [10]. Conversely, the rate of patients who eventually required glaucoma surgery (11%) was higher than other reports (range 0.3–9.5%). As our study is based on a single centre’s experience, these results are difficult to compare. Previous studies have shown that history IOP elevation following previous steroid treatments is a major risk factor for IOP elevation and glaucoma surgery following FAc implant [7, 13, 28]. In our cohort, the history of previous IOP elevation after steroid exposure carried a 3-fold risk of subsequent surgery, but the association was not statistically significant. However, due to uneven adherence to follow-up schedule and irregular visits’ intervals, eyes with previous IOP elevation following DEX implant could have been missed, deflating the statistical power of the analysis.
The main limitation of this study is its retrospective nature with heterogenous follow-up. We had to deal with longitudinal repeated-measures missing data; although complex solutions with multi-level imputation have been proposed, the nearest available observation has been shown to produce reliable results [17]. This study provides no information about the perfusion state of the macula and its relationship with the response to the FAc implant; other variables may be associated with the treatment outcomes not investigated in our analysis. As we do not have a control group, the reader must consider that the regression to the mean may drive longitudinal changes. The results of our models are to be interpreted as associations rather than cause-effect relationships. Some analyses could be underpowered: we invite the reader to evaluate the confidence intervals along with the regression estimates critically. When the AUC is normalised by the available follow-up, as done in previous studies [15, 16], a linear relationship between time and AUC is assumed. This approximation allows accounting for heterogenous follow-up of the included eyes. Our study is monocentric, with all patients being of European descent. The results of this study might not be generalisable to other centres with different demographic characteristics, ethnicities, disease severity, and treatment protocols. Further studies with larger sample size and multicentre design are warranted to validate our findings in a diverse clinical population.
In conclusion, this study identifies a variety of factors associated with the clinical response to FAc. Higher baseline visual acuity, lower central macular thickness over follow-up, and photoreceptors’ integrity as measured with OCT were associated with better functional response to FAc implant. Worse macular thickening and presence of ERM were associated with poor response to FAc implant. In our cohort of eyes with long-standing DMO, the duration of the disease did not help to predict the treatment outcomes. The results of this study may guide clinicians in a more informed decisional algorithm in treating DMO and better patients counselling.
Summary
What was known before
-
Patients with diabetic macular oedema (DMO) undergoing intravitreal fluocinolone acetonide (FAc) 0.19 mg-implant experience significant visual improvement over 3 years.
-
There is a lack of studies evaluating the clinical predictors of good response to FAc implant, and it is still unclear which patients are the best candidates for FAc.
What this study adds
-
Higher baseline visual acuity, lower central macular thickness over follow-up, and photoreceptors integrity as measured with OCT are associated with better functional response to FAc implant.
-
Worse macular thickening and presence of tractional oedema are associated with poor response to FAc implant.
-
In our cohort of eyes with long-standing DMO, the duration of the disease does not predict the treatment outcomes.
References
Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology. 2015;122:1375–94.
Gonzalez VH, Campbell J, Holekamp NM, Kiss S, Loewenstein A, Augustin AJ, et al. Early and long-term responses to anti-vascular endothelial growth factor therapy in diabetic macular edema: analysis of protocol i data. Am J Ophthalmol. 2016;172:72–79.
Busch C, Fraser-Bell S, Iglicki M, Lupidi M, Couturier A, Chaikitmongkol V, et al. Real-world outcomes of non-responding diabetic macular edema treated with continued anti-VEGF therapy versus early switch to dexamethasone implant: 2-year results. Acta Diabetol. 2019;56:1341–50.
Lattanzio R, Cicinelli MV, Bandello F. Intravitreal steroids in diabetic macular edema. Dev Ophthalmol. 2017;60:78–90.
Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118:626–35 e622.
Campochiaro PA, Brown DM, Pearson A, Chen S, Boyer D, Ruiz-Moreno J, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119:2125–32.
Bailey C, Chakravarthy U, Lotery A, Menon G, Talks J, Medisoft Audit G. Real-world experience with 0.2 mug/day fluocinolone acetonide intravitreal implant (ILUVIEN) in the United Kingdom. Eye (Lond). 2017;31:1707–15.
Chakravarthy U, Taylor SR, Koch FHJ, Castro de Sousa JP, Bailey C, Group IRSSI. Changes in intraocular pressure after intravitreal fluocinolone acetonide (ILUVIEN): real-world experience in three European countries. Br J Ophthalmol. 2019;103:1072–7.
Eaton A, Koh SS, Jimenez J, Riemann CD. The USER study: a chart review of patients receiving a 0.2 microg/day fluocinolone acetonide implant for diabetic macular edema. Ophthalmol Ther. 2019;8:51–62.
Fallico M, Maugeri A, Lotery A, Longo A, Bonfiglio V, Russo A, et al. Fluocinolone acetonide vitreous insert for chronic diabetic macular oedema: a systematic review with meta-analysis of real-world experience. Sci Rep. 2021;11:4800.
Riclassificazione del medicinale per uso umano “Iluvien”, ai sensi dell’articolo 8, comma 10, della legge 24 dicembre 1993, n. 537. (Determina n. 33/2017). [Available from: https://www.federfarmalombardia.it/cat_authority/normativa/page/34/.
Cicinelli MV, Rabiolo A, Zollet P, Capone L, Lattanzio R, Bandello F. Persistent or recurrent diabetic macular edema after fluocinolone acetonide 0.19 mg implant: risk factors and management. Am J Ophthalmol. 2020;215:14–24.
Cicinelli MV, Rosenblatt A, Grosso D, Zollet P, Capone L, Rabiolo A, et al. The outcome of fluocinolone acetonide intravitreal implant is predicted by the response to dexamethasone implant in diabetic macular oedema. Eye (Lond). 2021;35:3232–42.
Arrigo A, Capone L, Lattanzio R, Aragona E, Zollet P, Bandello F. Optical coherence tomography biomarkers of inflammation in diabetic macular edema treated by fluocinolone acetonide intravitreal drug-delivery system implant. Ophthalmol Ther. 2020;9:971–80.
Singer MA, Miller DM, Gross JG, Greven CM, Kapik B, Bailey C, et al. Visual acuity outcomes in diabetic macular edema with fluocinolone acetonide 0.2 mug/day versus ranibizumab plus deferred laser (DRCR Protocol I). Ophthalmic Surg Lasers Imaging Retin. 2018;49:698–706.
Zarranz-Ventura J, Mali JO. Effectiveness of 190 microg fluocinolone acetonide and 700 microg dexamethasone intravitreal implants in diabetic macular edema using the area-under-the-curve method: the CONSTANT analysis. Clin Ophthalmol. 2020;14:1697–704.
Mongin D, Lauper K, Turesson C, Hetland ML, Klami Kristianslund E, Kvien TK, et al. Imputing missing data of function and disease activity in rheumatoid arthritis registers: what is the best technique? RMD Open. 2019;5:e000994.
Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Ser B (Methodol). 1996;58(1):267–88.
Snijders T & Bosker RJ. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling. London, UK: SAGE Publications Ltd; 2012.
van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. 2011. 2011;45:67.
Ciulla TA, Bracha P, Pollack J, Williams DF. Real-world outcomes of anti-vascular endothelial growth factor therapy in diabetic macular edema in the United States. Ophthalmol Retin. 2018;2:1179–87.
Alfaqawi F, Lip PL, Elsherbiny S, Chavan R, Mitra A, Mushtaq B. Report of 12-months efficacy and safety of intravitreal fluocinolone acetonide implant for the treatment of chronic diabetic macular oedema: a real-world result in the United Kingdom. Eye (Lond). 2017;31:650–6.
Currie CJ, Holden SE, Owens DR. Patterns of retinal thickness prior to and following treatment with fluocinolone acetonide 190 microg intravitreal implant for diabetic macular edema. Curr Med Res Opin. 2017;33:33–43.
Vujosevic S, Toma C, Villani E, Muraca A, Torti E, Florimbi G, et al. Diabetic macular edema with neuroretinal detachment: OCT and OCT-angiography biomarkers of treatment response to anti-VEGF and steroids. Acta Diabetol. 2020;57:287–96.
Zur D, Iglicki M, Busch C, Invernizzi A, Mariussi M, Loewenstein A, et al. OCT biomarkers as functional outcome predictors in diabetic macular edema treated with dexamethasone implant. Ophthalmology. 2018;125:267–75.
Ito S, Miyamoto N, Ishida K, Kurimoto Y. Association between external limiting membrane status and visual acuity in diabetic macular oedema. Br J Ophthalmol. 2013;97:228–32.
Karaca EE, Kepez Yldz B, Cubuk MO, Ozdek S. Epiretinal membranes in neovascular age-related macular degeneration: effect on outcomes of anti-vascular endothelial growth factor therapy. Retina. 2015;35:1540–6.
Mansour SE, Kiernan DF, Roth DB, Eichenbaum D, Holekamp NM, Kaba S, et al. Two-year interim safety results of the 0.2 microg/day fluocinolone acetonide intravitreal implant for the treatment of diabetic macular oedema: the observational PALADIN study. Br J Ophthalmol. 2021;105:414–19.
Augustin AJ, Bopp S, Fechner M, Holz F, Sandner D, Winkgen AM, et al. Three-year results from the Retro-IDEAL study: Real-world data from diabetic macular edema (DME) patients treated with ILUVIEN((R)) (0.19 mg fluocinolone acetonide implant). Eur J Ophthalmol. 2020;30:382–91.
Chakravarthy U, Yang Y, Lotery A, Ghanchi F, Bailey C, Holz FG, et al. Clinical evidence of the multifactorial nature of diabetic macular edema. Retina. 2018;38:343–51.
Yang Y, Bailey C, Holz FG, Eter N, Weber M, Baker C, et al. Long-term outcomes of phakic patients with diabetic macular oedema treated with intravitreal fluocinolone acetonide (FAc) implants. Eye (Lond). 2015;29:1173–80.
Cunha-Vaz J, Ashton P, Iezzi R, Campochiaro P, Dugel PU, Holz FG, et al. Sustained delivery fluocinolone acetonide vitreous implants: long-term benefit in patients with chronic diabetic macular edema. Ophthalmology. 2014;121:1892–903.
Sophie R, Lu N, Campochiaro PA. Predictors of functional and anatomic outcomes in patients with diabetic macular edema treated with ranibizumab. Ophthalmology. 2015;122:1395–401.
Yoon YH, Boyer DS, Maturi RK, Bandello F, Belfort R Jr., Augustin AJ, et al. Natural history of diabetic macular edema and factors predicting outcomes in sham-treated patients (MEAD study). Graefes Arch Clin Exp Ophthalmol. 2019;257:2639–53.
Meireles A, Goldsmith C, El-Ghrably I, Erginay A, Habib M, Pessoa B, et al. Efficacy of 0.2 mug/day fluocinolone acetonide implant (ILUVIEN) in eyes with diabetic macular edema and prior vitrectomy. Eye (Lond). 2017;31:684–90.
Pessoa B, Coelho J, Correia N, Ferreira N, Beirao M, Meireles A. Fluocinolone Acetonide Intravitreal Implant 190 mug (ILUVIEN(R)) in vitrectomized versus nonvitrectomized eyes for the treatment of chronic diabetic macular edema. Ophthalmic Res. 2018;59:68–75.
Acknowledgements
The Authors thank Alimera Science for English revision. The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Funding
a. Funding/Support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. b. Financial disclosures: Maria Vittoria Cicinelli, Alessandro Rabiolo, Luigi Capone, Carlo di Biase: none Rosangela Lattanzio: Allergan Inc (Irvine, California, USA), Novartis (Basel, Switzerland), Bayer Schering-Pharma (Berlin, Germany), Sifi (Aci Sant’Antonio, Italy) Francesco Bandello consultant for Allergan Inc (Irvine, California, USA), Bayer Schering-Pharma (Berlin, Germany), Hoffmann-La-Roche (Basel, Switzerland), Novartis (Basel, Switzerland), Sanofi-Aventis (Paris, France), Thrombogenics (Heverlee, Belgium), Zeiss (Dublin, USA), Boehringer-Ingelheim, Fidia Sooft, Ntc Pharma, Sifi (Aci Sant’Antonio, Italy).
Author information
Authors and Affiliations
Contributions
MVC, AR: Conceptualisation, Writing - Original Draft. RL: Methodology, Writing - Review & Editing. LC, CdiB: Data collection, Visualisation, Data Curation. FB: Project administration, Supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41433_2021_1921_MOESM2_ESM.pdf
Best-corrected visual acuity (BCVA) and central macular thickness (CMT) at all follow-up time points after receiving the fluocinolone acetonide implant.
41433_2021_1921_MOESM3_ESM.pdf
Demographic and clinical characteristics of patients divided by the risk of undergoing trabeculectomy after fluocinolone acetonide (FAc) implant.
Rights and permissions
About this article
Cite this article
Cicinelli, M.V., Rabiolo, A., Capone, L. et al. Factors associated with the response to fluocinolone acetonide 0.19 mg in diabetic macular oedema evaluated as the area-under-the-curve. Eye 37, 242–248 (2023). https://doi.org/10.1038/s41433-021-01921-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01921-3