Abstract
Objectives
To examine the outcome of infliximab treatment in patients with non-infectious paediatric uveitis who have previously failed biologic treatment.
Methods
A retrospective cohort study was performed at Bristol Eye Hospital, UK. Paediatric patients with chronic non-infectious uveitis who had been switched to infliximab due to inadequate uveitis control were identified. Two separate groups were evaluated: group 1 consisted of 20 children (36 eyes) who had been switched to infliximab following treatment failure with adalimumab (=in-class switching), while group 2 (5 patients; 9 eyes) included those who had been switched to infliximab from a non-TNF antagonist after failing several biologics (=across-class switching). The change in anterior chamber (AC) activity between baseline and 6- and 24-months follow-up was the primary outcome measure.
Results
A statistically significant reduction in AC activity was found between baseline and 6-months follow-up (RE: p = 0.002; LE: p < 0.001) and between baseline and 24-months follow-up (RE: p = 0.016; LE: p = 0.011) in group 1. No statistically significant difference was found for either eye in the number of steroid eye drops needed between time points or the difference in visual acuity in time. In group 2, analysis of change of AC activity, number of steroid eye drops and visual acuity failed to reach statistical significance. Treatment failure occurred in four patients (20% of group 1) and adverse events developed in six patients including three patients with acute infusion reactions.
Conclusions
This study supports the efficacy and safety of infliximab in adalimumab-refractory patients with paediatric non-infectious uveitis.
Similar content being viewed by others
Introduction
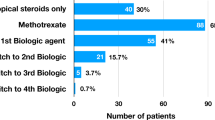
Persistent ocular inflammation in chronic, non-infectious paediatric uveitis confers a substantial risk of visual morbidity [1]. Methotrexate (MTX), a folate analogue inhibiting the enzyme dihydrofolate reductase, is usually used as an initial corticosteroid-sparing agent in childhood uveitis and is administered once a week orally or subcutaneously [1, 2]. It has been shown to be insufficiently effective in roughly one third of paediatric uveitis patients and up to 47% of children with juvenile idiopathic arthritis (JIA)-associated uveitis [2, 3]. In those with insufficient disease control, the common and licenced approach is to add adalimumab, a fully humanised monoclonal anti-TNFα antibody given subcutaneously once every two weeks. The SYCAMORE trial was a randomised controlled trial which showed the benefit of MTX combined with adalimumab for JIA-associated uveitis as compared with methotrexate alone [4]. Despite the clinical benefit, treatment failure still occurred in 27% of the group treated with MTX and adalimumab [4]. There is a scarcity of data to guide clinicians in managing this subset of children refractory to adalimumab combined with MTX. Consensus-based recommendations advise in class switching if uveitis is refractory to a first anti-TNFα although evidence to support this approach is limited [5]. Contrary to adalimumab, infliximab is an intravenously administered, chimeric human-mouse monoclonal IgG1 targeting TNFα and is not licensed in the UK for the treatment of uveitis. Previous case series have reported on the outcome of infliximab in paediatric uveitis with variable results [6]. This study aimed to assess whether paediatric patients with chronic non-infectious uveitis that have been refractory to a previous biologic therapy have a significant improvement in uveitis activity after switching to infliximab.
Methods
A retrospective chart review was conducted in the paediatric uveitis service at Bristol Eye Hospital, United Kingdom. Paediatric patients who had been switched to infliximab treatment due to inadequate control of their uveitis on another biologic between January 1, 2011, and December 31, 2021 were identified. Patients with onset of chronic non-infectious uveitis before the age of 16 years, refractory to topical and/or systemic steroid treatment, at least 1 conventional systemic immunomodulatory treatment (IMT) (MTX, mycophenolate mofetil (MMF) and/or tacrolimus), and biologic agent, were eligible for inclusion. Refractory uveitis was defined as >1 + cells on >2 steroid drops per day for >3 months on current treatment. Patients with less than 6 months of follow-up after initiation of infliximab, or in which the switch to infliximab was to control systemic disease alone or with insufficient available data were excluded.
Two groups were assessed separately: group 1 included children who had failed adalimumab (as the sole prior biologic) and were subsequently switched to infliximab (=in-class switching) and group 2 contained those who had insufficient response to both adalimumab and other biologics and were switched to infliximab from a non-TNF antagonist (=across-class switching). Infliximab treatment was typically administered at a dose of 6 mg/kg with a dose at baseline, week 2, week 6 and then every 4–8 weeks depending on the clinical response. A standardised proforma was used to extract demographic and clinical information from ophthalmology and rheumatology medical records at baseline (i.e. at the time of the decision to switch to infliximab from another biologic) and 3, 6, 12 and 24 months following the switch to infliximab. Data collected at baseline visit included date of birth, sex, uveitis characteristics (onset date, diagnosis date, laterality, location), ocular complications and ocular surgeries. The anatomical location and grading of anterior chamber (AC) inflammation were in accordance with the Standardization of Uveitis Nomenclature (SUN) Classification Scale [7]. Adverse events and safety outcomes were also recorded and analysed. Controlled/quiescent uveitis was defined as an AC activity <1 + cells on ≤2 steroid eye drops per day. This maximum acceptable dose of topical steroids is based on research indicating an increased risk of IOP elevation [8] and cataract development [9] on 3 drops of prednisolone or more and aligns with the 2019 American College of Rheumatology (ACR) guidelines [10].
The primary outcome measure was the change in SUN grade of AC inflammation between baseline (the clinical examination prior to starting infliximab) and 6- and 24-months follow-up. This outcome measure was deemed appropriate for both anterior uveitis and the subset of intermediate and panuveitis as a flare of AC activity was the reason for switching to IFX in all patients included. Secondary outcome measures included the change in number of steroid eye drops (prednisolone acetate 1%), change in best-corrected visual acuity, the occurrence of cystoid macular oedema and the safety of infliximab treatment. Acute infusion reactions were defined as any adverse event occurring during the infusion or within 1 hour of termination [11].
All extracted data was tabulated in Microsoft Excel (version 16.16.10, Microsoft Corp, Redmond, WA, USA) and analysed using SPSS software for Windows version 25.0 (SPSS Inc., Chicago, Illinois, United States). Spearman’s rank-order correlation test was applied to anterior chamber activity (ordinal data from the SUN grading) to investigate the degree of correlation between right and left eyes [12]. The non-parametric one-way repeated measures Friedman test was conducted to test for time differences (baseline, 6-months follow up, and 24-months follow-up) for anterior chamber activity and the number of steroid eye drops needed in groups 1 and 2 separately. A post-hoc test for multiple comparisons was applied (Wilcoxon signed-rank test) with Bonferroni correction; for a 0.05 significance level, the alpha error per comparison was set to 0.017 (0.05/3). One-way repeated measures ANOVA test was performed to ascertain whether visual acuity changed over time (baseline, 6-months follow up, and 24-months follow-up). Shapiro-Wilk test, Mauchly’s test of sphericity, and Levene’s test indicated that the assumptions of normality, sphericity and homogeneity of variances, respectively, had not been violated. The level of significance was set to 0.05 unless stated otherwise.
Results
Of the 28 eligible patients identified, 25 (89.3%) patients (45 eyes) were included: 20 patients (36 eyes) in group 1 and five (9 eyes) in group 2. In two patients, the switch to infliximab had been managed locally and insufficient data could be retrieved, and another patient had not reached 6 months of follow-up since the first infliximab dose. As part of a preliminary analysis, moderate to high correlation was found between right and left eyes in some instances under investigation (Group 1: baseline: ρ = 0.40, p = 0.12; 6-months follow-up: ρ = 0.54, p = 0.045, and 24-months follow-up: ρ = 0.68, p = 0.06 ; Group 2: baseline: ρ = −0.24, p = 0.77; 6-months follow-up: ρ = 1.0, p = n/a, and 24-months follow-up: ρ = −0.24, p = 0.76; Spearman’s rank-order correlation test). Consequently, to reduce the risk of Type 1 errors, the right and left eyes were assessed separately. A summary of the patients’ characteristics is provided in Table 1. All patients had received conventional IMT prior to and in conjunction with their previous biologic. Fourteen (56%) patients had been switched to MMF during follow-up due to intolerance to MTX. Every patient used at least one IMT in conjunction with infliximab; in five patients (20%), a combination of MTX and MMF was prescribed. Two patients, both with Blau syndrome, received frequent IV methylprednisolone treatment in conjunction with their infliximab infusions because of difficult-to-control uveitis. One of these patients with Blau syndrome was also the only patient on low-dose systemic prednisolone treatment while on infliximab treatment.
An overview of the treatment response to infliximab is provided in Tables 2 (Group 1) and 3 (Group 2).
Group 1 (In-class switching)
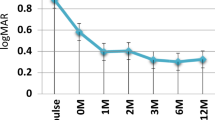
A statistically significant difference in AC activity between time points (baseline, 6-months follow up and 24-months follow up) was found for both eyes (RE: χ2 = 11.08, p = 0.004; LE: χ2 = 15.93, p < 0.001). The corresponding post-hoc test revealed that AC activity was statistically significant different between baseline and 6-months follow-up (RE: p = 0.002; LE: p < 0.001) and between baseline and 24-months follow-up (RE: p = 0.016; LE: p = 0.011). There was no statistically significant difference in anterior chamber activity between 6-months and 24-months follow-up (RE: p = 0.26; LE: p = 0.18). No statistically significant difference in the number of steroid eye drops needed between time points (baseline, 6-months follow-up, and 24-months follow-up) was found for either eye in group 1 (RE: χ2 = 3.00, p = 0.22; LE: χ2 = 5.87, p = 0.053). No statistically significant difference in visual acuity in time (baseline, 6-months follow up and 24-months follow-up) was found for either eye in group 1 (RE: F(2,18) = 1.52, p = 0.23, ƞp2 = 0.42; LE: F(2,18) = 0.46, p = 0.64, ƞp2 = 0.36).
Group 2 (across-class switching)
Differences in AC activity between time points (RE: χ2 = 8.82, p = 0.012; LE: χ2 = 6.53, p = 0.038) were statistically significant, however, when taking Bonferroni correction into account in post-hoc testing, most differences lost their statistical significance: AC activity between baseline and 6-months follow-up (RE: p = 0.041; LE: p = 0.066), between baseline and 24-months follow-up (RE: p = 0.043; LE: p = 0.063), and between 6-months and 24-months follow-up (RE: p = 1.00; LE: p = 0.56). The difference in number of steroid eye drops needed between time points was not statistically significant (RE: χ2 = 4.77, p = 0.092; LE: χ2 = 3.80, p = 0.15), and neither was the difference in visual acuity for either eye (RE: F(2,8) = 0.40, p = 0.70, ƞp2 = 0.37; LE: F(2,8) = 1.38, p = 0.30, ƞp2 = 0.27).
Ocular complications were present at baseline (pre-infliximab) in most patients (Table 4). In 7 eyes in which cataracts had developed prior to infliximab treatment, quiescence of uveitis on infliximab (<1 + cells for >3 months) allowed for surgical intervention with a short course of oral corticosteroids of 4-6 weeks as perioperative cover. No patients developed glaucoma or ocular hypertension while on infliximab. Glaucoma surgery was performed in two eyes of two patients with pre-existing glaucoma while on infliximab. During infliximab treatment, two patients (1 intermediate uveitis and 1 Blau syndrome) developed a unilateral rhegmatogenous retinal detachment which required both scleral buckling and pars plana vitrectomy with oil tamponade.
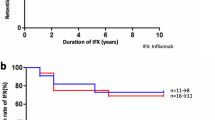
Treatment failure that resulted in cessation of IFX occurred in four patients (all in group 1 (20%); 3 JIA-associated uveitis and 1 Blau syndrome), after 4 months (n = 2), 18 months (n = 1) and 42 months (n = 1) respectively. Adverse events developed in six patients of group 1 (30%) and no patients of group 2. Three patients had acute infusion reactions: breathlessness and wheezing (n = 2; after 9 and 15 months of treatment) and mild chest pain during infusion (n = 1). In the former two patients, infliximab treatment was stopped. In the latter, work-up including anti-infliximab antibody analysis was negative and no new issues arose upon continuation of treatment. Two patients had frequent infections, often requiring antibiotics (urinary tract infections in one, and upper respiratory tract infections in the second patient) and one patient had a transient elevation of liver transaminases which settled upon withholding methotrexate for 3 weeks.
Discussion
In this study, infliximab was effective at achieving disease control in paediatric patients with non-infectious uveitis who were refractory to prior biologic treatment including adalimumab. Statistical significance was reached for the primary endpoint (AC activity) at the 6- and 24-month timepoints for the group of 20 patients switched from adalimumab, but not for the small group of five patients. Due to the low number of patients in this second group, however, this result should be interpreted with caution. The secondary treatment endpoints (steroid drops and visual acuity) also showed improvement but did not attain statistical significance, neither did analysis of the small group of five patients who switched from non-TNFα biologic. At a patient level, bilateral uveitic control (AC activity <1 + cells on <2 steroid eye drops per day for all eyes involved) was achieved in 72.2% after 6 months of infliximab treatment following adalimumab failure, which was maintained at the 24-month time point. A previous study also reported excellent treatment response to infliximab, with steroid-remission on infliximab treatment (5–10 mg/kg/dose 4–6 weekly) in 69% of 13 adalimumab-refractory patients with chronic non-infectious uveitis [13]. These data support the efficacy of infliximab – despite targeting the same TNFα cytokine—in adalimumab-refractory patients. In biologic treatment-naïve patients with childhood uveitis, a similar average response rate of infliximab of 72% was calculated by Simonini et al. in their meta-analysis [6]. As compared to adalimumab, infliximab has wider dosing customisation in both dose and frequency of administration, which may contribute to the efficacy in adalimumab-refractory patients. In the overall cohort of 25 patients in this study, 52% required 4-weekly administration of infliximab at a dose of 6 mg/kg/dose, with an additional eight patients (32%) receiving 6-weekly infusions. Studies on the outcome of infliximab in paediatric uveitis in which less favourable results were reported, have frequently utilised doses of 3–5 mg/kg/dose every 6–8 weeks [14,15,16,17]. Clinicians need to take this issue into account when assessing treatment response to infliximab, in differentiating insufficient dosing and/or premature extension of infliximab from true infliximab failure.
No patients developed new-onset cataract and glaucoma whilst on infliximab treatment and quiescence of uveitis allowed for surgical intervention of pre-existent cataract. Peripheral vitreous traction in intermediate or panuveitis can seldomly result in rhegmatogenous retinal detachment [18, 19]. Both patients who developed a unilateral retinal detachment in this study initially had scleral buckle surgery but required pars plana vitrectomy with oil tamponade 1-2 weeks following the first surgery. Large series of paediatric retinal detachments similarly indicate that most patients require scleral buckling and pars plana vitrectomy, with oil tamponade being the most frequently used in the paediatric population [20, 21]. Three patients (12%) experienced acute infusion reactions (with full recovery), which led to cessation of infliximab treatment in two patients. This rate aligns with other series in which a dose of 6 mg/kg/dose 4–8 weekly and co-medication with conventional immunomodulatory treatment (mainly MTX) was used [22, 23]. Anti-infliximab antibodies are associated with both secondary loss of clinical response and a significant increased risk of drug reactions [23, 24]. Both higher and more frequent dosing—such as appears to be indicated in uveitis—as well as routine co-medication with conventional IMT have been shown to be protective against antibody formation [11, 23, 24].
Several limitations of this study need to be considered. The design as a retrospective, single-centre uncontrolled study with heterogeneity of disease and duration of follow-up renders it at risk of bias and confounding. During follow-up, 56% of patients were also switched to MMF due to MTX intolerance, which may have impacted disease control as well. Anti-infliximab antibodies and drug levels were not routinely measured. Therapeutic options not requiring IV administration, including weekly adalimumab and/or subcutaneous infliximab, were not assessed in this study. Treatment adherence, which has previously been shown to impact the odds of having active disease on infliximab, could not be assessed due to the retrospective nature of the study [25].
The data presented in this study indicates that, in cases where a child’s uveitis remains active despite systemic IMT in combination with a biologic agent, switching to infliximab is effective and well-tolerated. A larger, multicentre, prospective study, with measurement of trough infliximab levels and anti-infliximab antibodies, is warranted to address this question more robustly and help determine patient-specific dosing of infliximab for the indication of uveitis.
Summary
What was known before
-
Adalimumab is the only biologic licenced for use in non-infectious childhood uveitis in the UK. Little data is available on the efficacy of infliximab in adalimumab-refractory patients.
What this study adds
-
Infliximab is an effective and well-tolerated treatment for adalimumab-refractory paediatric uveitis.
Data availability
All data generated or analysed during this study are included in this published article.
References
Maleki A, Anesi SD, Look-Why S, Manhapra A, Foster CS. Pediatric uveitis: a comprehensive review. Surv Ophthalmol. 2022;67:510–29.
Simonini G, Paudyal P, Jones GT, Cimaz R, Macfarlane GJ. Current evidence of methotrexate efficacy in childhood chronic uveitis: a systematic review and meta-analysis approach. Rheumatology. 2013;52:825–31.
Cann M, Ramanan AV, Crawford A, Dick AD, Clarke SLN, Rashed F, et al. Outcomes of non-infectious paediatric uveitis in the era of biologic therapy. Pediatr Rheumatol Online J. 2018;16:51.
Ramanan AV, Dick AD, Jones AP, McKay A, Williamson PR, Compeyrot-Lacassagne S, et al. Adalimumab plus methotrexate for uveitis in juvenile idiopathic arthritis. N. Engl J Med. 2017;376:1637–46.
Constantin T, Foeldvari I, Anton J, de Boer J, Czitrom-Guillaume S, Edelsten C, et al. Consensus-based recommendations for the management of uveitis associated with juvenile idiopathic arthritis: the SHARE initiative. Ann Rheum Dis. 2018;77:1107–17.
Simonini G, Druce K, Cimaz R, Macfarlane GJ, Jones GT. Current evidence of anti-tumor necrosis factor α treatment efficacy in childhood chronic uveitis: a systematic review and meta-analysis approach of individual drugs. Arthritis Care Res. 2014;66:1073–84.
Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) working group. standardization of uveitis nomenclature for reporting clinical data. results of the first international workshop. Am J Ophthalmol. 2005;140:509–16.
Kothari S, Foster CS, Pistilli M, Liesegang TL, Daniel E, Sen HN, et al. The risk of intraocular pressure elevation in pediatric noninfectious uveitis. Ophthalmology. 2015;122:1987–2001.
Thorne JE, Woreta FA, Dunn JP, Jabs DA. Risk of cataract development among children with juvenile idiopathic arthritis-related uveitis treated with topical corticosteroids. Ophthalmology. 2020;127:S21–6.
Angeles-Han ST, Ringold S, Beukelman T, Lovell D, Cuello CA, Becker ML, et al. American college of rheumatology/arthritis foundation guideline for the screening, monitoring, and treatment of juvenile idiopathic arthritis-associated uveitis. Arthritis Care Res. 2019;71:703–16.
Ruperto N, Lovell DJ, Cuttica R, Wilkinson N, Woo P, Espada G, et al. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2007;56:3096–106.
Armstrong RA. Statistical guidelines for the analysis of data obtained from one or both eyes. Ophthalmic Physiol Opt. 2013;33:7–14.
Ashkenazy N, Saboo US, Abraham A, Ronconi C, Cao JH. Successful treatment with infliximab after adalimumab failure in pediatric noninfectious uveitis. J AAPOS. 2019;23:151.e1–5.
Deitch I, Amer R, Tomkins-Netzer O, Habot-Wilner Z, Friling R, Neumann R, et al. The effect of anti-tumor necrosis factor alpha agents on the outcome in pediatric uveitis of diverse etiologies. Graefes Arch Clin Exp Ophthalmol. 2018;256:801–8.
Cecchin V, Zannin ME, Ferrari D, Pontikaki I, Miserocchi E, Paroli MP, et al. Longterm safety and efficacy of adalimumab and infliximab for uveitis associated with juvenile idiopathic arthritis. J Rheumatol. 2018;45:1167–72.
Simonini G, Taddio A, Cattalini M, Caputo R, De Libero C, Naviglio S, et al. Prevention of flare recurrences in childhood-refractory chronic uveitis: an open-label comparative study of adalimumab versus infliximab. Arthritis Care Res. 2011;63:612–8.
Simonini G, Zannin ME, Caputo R, Falcini F, de Martino M, Zulian F, et al. Loss of efficacy during long-term infliximab therapy for sight-threatening childhood uveitis. Rheumatology. 2008;47:1510–4.
Heinz C, Schoonbrood S, Heiligenhaus A. Intermediate uveitis in children and young adults: differences in clinical course, associations and visual outcome. Br J Ophthalmol. 2014;98:1107–11.
Rosenberg KD, Feuer WJ, Davis JL. Ocular complications of pediatric uveitis. Ophthalmology. 2004;111:2299–306.
Read SP, Aziz HA, Kuriyan A, Kothari N, Davis JL, Smiddy WE, et al. Retinal detachment surgery in a pediatric population: visual and anatomic outcomes. Retina. 2018;38:1393–402.
Eibenberger K, Sacu S, Rezar-Dreindl S, Schmidt-Erfurth U, Stifter E, Georgopoulos M. Clinical characteristics and surgical outcome of pediatric and early adulthood retinal detachment. Eur J Ophthalmol. 2021;31:1367–74.
Aeschlimann FA, Hofer KD, Cannizzaro Schneider E, Schroeder S, Lauener R, Saurenmann RK. Infliximab in pediatric rheumatology patients: a retrospective analysis of infusion reactions and severe adverse events during 2246 infusions over 12 years. J Rheumatol. 2014;41:1409–15.
Aeschlimann FA, Angst F, Hofer KD, Cannizzaro Schneider E, Schroeder-Kohler S, Lauener R, et al. Prevalence of anti-infliximab antibodies and their associated co-factors in children with refractory arthritis and/or uveitis: a retrospective longitudinal Cohort study. J Rheumatol. 2017;44:334–41.
Colman RJ, Xiong Y, Mizuno T, Hyams JS, Noe JD, Boyle B, et al. Antibodies-to-infliximab accelerate clearance while dose intensification reverses immunogenicity and recaptures clinical response in paediatric Crohn’s disease. Aliment Pharm Ther. 2022;55:593–603.
Miraldi Utz V, Bulas S, Lopper S, Fenchel M, Sa T, Mehta M, et al. Effectiveness of long-term infliximab use and impact of treatment adherence on disease control in refractory, non-infectious pediatric uveitis. Pediatr Rheumatol Online J. 2019;17:79.
Acknowledgements
The authors would like to thank Ms Paula Toner and Mr Andrew Crawford for their input and statistical assistance in the first draft of the paper.
Author information
Authors and Affiliations
Contributions
AVR, CMG, ADD: conception, design, data analysis, report and manuscript writing. EOK, SJE: data acquisition, data analysis, report and manuscript writing. AC: statistical analysis, report writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kreps, E.O., Epps, S.J., Consejo, A. et al. Infliximab in chronic non-infectious paediatric uveitis refractory to previous biologic therapy. Eye 38, 871–876 (2024). https://doi.org/10.1038/s41433-023-02795-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02795-3