Abstract
Background/Objectives
This study aimed to compare the cost-effectiveness of prophylactic laser peripheral iridotomy (LPI) with that of observation for primary angle-closure suspect (PACS) in Japan.
Subjects/Methods
A Markov model was developed to compare the costs and utilities of prophylactic LPI with those of observation of 40-year-old patients with PACS. In the model with a yearly cycle over a 20-year time horizon, the disease was postulated to irreversibly progress from PACS to primary angle closure, followed by primary angle-closure glaucoma, unilateral blindness, and bilateral blindness. The parameters were estimated mainly based on a recent randomised controlled trial and analyses of Japanese claims data. The incremental cost-effectiveness ratio was estimated from the healthcare payer’s perspective and evaluated at the willingness-to-pay 5 million Japanese Yen per quality-adjusted life-year. The observation period and the age at entry into the cohort was changed to account for a variety of clinical courses in sensitivity analyses. We conducted one-way deterministic sensitivity analysis and probabilistic sensitivity analysis with Monte Carlo simulations with 10 000 iterations.
Results
The incremental cost-effectiveness ratio of LPI was 2,287,662 Japanese Yen (14,298 pounds sterling) per quality-adjusted life-year, which was below the willingness-to-pay threshold. The ratios were approximately 4 and 8 million in the 15-year and 10-year time horizons, respectively. Increasing the age at entry had little influence on the incremental cost-effectiveness ratio. The deterministic and probabilistic sensitivity analyses indicated that the results were robust.
Conclusions
Our results indicate that prophylactic LPI for middle-aged patients with PACS is cost-effective in Japan.
Similar content being viewed by others
Introduction
Glaucoma ranks second among the leading causes of blindness worldwide [1]. Among the several types of glaucoma, primary angle-closure glaucoma (PACG) is associated with devastating visual outcomes [2], and 77% of patients with PACG reside in Asia [3]. Primary angle-closure suspect (PACS) represents anatomically narrow angles with no other abnormalities. Some patients with PACS experience an acute angle-closure crisis, whereas a chronic course of PACG development is observed in other patients. Prophylactic laser peripheral iridotomy (LPI) is widely performed in PACS to prevent acute angle-closure crises and the development of PACG in the future. Although the exact prevalence of PACS in Japan is unknown, PACS is reported to be relatively prevalent in Asia (prevalence of 10.4% in China) [4]. Therefore, the cost of universal LPI for PACS is an economic burden in Asia.
According to two recent randomised controlled trials conducted in Asia, prophylactic LPI for PACS decreased the risk of disease progression significantly [5, 6]. However, despite the efficacy of prophylactic LPI, both randomised controlled trials questioned the cost-effectiveness of widespread prophylactic LPI for PACS as the incidence of PACG among patients with PACS was low and recommended that prophylactic LPI should be limited to patients with PACS at high risk of disease progression. Although a previous analysis of the cost-effectiveness of prophylactic LPI in the US demonstrated that prophylactic LPI was cost-effective, the model used a higher disease progression rate than the two recent trials [7]. In addition, no study has investigated the cost-effectiveness of prophylactic LPI in Asia despite the high prevalence of PACS and PACG in Asia. Therefore, the cost-effectiveness of prophylactic LPI should be investigated further, especially in Asian countries.
This study aimed to estimate the cost-effectiveness of prophylactic LPI for PACS using the data from a recent randomised controlled trial conducted in an Asian country on the incidence of PACG and efficacy of LPI [5] and cost in Japan.
Materials/subjects and methods
Model overview
This study utilised a health state transition Markov model over a 20-year time horizon from 40 to 59 years of age to evaluate the cost-effectiveness of prophylactic LPI for PACS. We set the minimum age for inclusion in the cohort as 40 years since the prevalence of PACS under the age of 40 was reported to be low [4]. We did not include patients aged ≥60 years as the frequency of cataract surgery increases over the age of 60 [8], and cataract surgery usually improves angle closure without LPI.
We developed a yearly cycle Markov model that tracks the transitions of the patients across mutually exclusive health states (Fig. 1). Patients with PACS who underwent prophylactic LPI during the first year were assigned to the LPI group, whereas patients who did not undergo prophylactic LPI were assigned to the observation group. The patients in both groups were at risk of irreversible progression from PACS to primary angle closure (PAC), followed by PACG, unilateral blindness, and bilateral blindness [9,10,11]. All patients with progression to PAC underwent lens extraction in accordance with the treatment guidelines in Japan [12]. Patients with PACG, whose intraocular pressure could not be controlled with medication, underwent trabeculectomy [13]. In the observation group, patients with PACS were at risk of acute angle-closure crisis and underwent emergency LPI with an intravenous drip of D-mannitol upon the incidence of acute angle-closure crisis [12]. A dead state was not included in the model as the mortality rates for individuals between the ages of 40 and 59 years is very low in Japan [14]. The patients accumulated quality-adjusted life-years (QALYs) and costs during each state, and the transitions occurred according to the input probabilities (Table 1).
(a) LPI group. (b) observation group. The patients in the LPI group (a) underwent prophylactic LPI during the first year. The patients in the observation group (b) who experienced AACC underwent LPI under emergency settings. LPI laser peripheral iridotomy, PACS primary angle-closure suspect, PAC primary angle-closure, PACG primary angle-closure glaucoma, AACC acute angle-closure crisis.
Input parameters
The transition probabilities of PACS to PAC and PACG for each group were obtained from a recent randomised controlled trial [5]. The data regarding the progression rate from PACG to unilateral and bilateral blindness were obtained from a previous study that performed a cost-effectiveness analysis of population-based glaucoma screening in China [10]. The transition probability of PACG to trabeculectomy was obtained from a previous study that analysed the Japanese claims database [15].
All costs were recorded from the healthcare payer’s perspective in Japanese Yen (JPY), in accordance with the Japanese guideline for evaluation of cost-effectiveness [16]. The costs were based on the medical fees provided by the government as of May 2022. Supplementary Table 1 presents the procedure codes and costs included in the calculations. The annual cost of the drugs used in the treatment of PACG was obtained from a cost analysis study conducted in Japan [15]. The costs of trabeculectomy and 1-year postoperative treatment were obtained from another cost analysis study conducted in Japan [17]. The one-year treatment cost for each health state was inferred from clinical practice based on the guidelines for glaucoma treatment in Japan (Supplementary Table 2) [12].
The utility values for each health state were obtained from previous studies [9, 18, 19]. A utility loss of 0.007 was applied in the case of glaucoma surgery [20].
Analysis
The incremental cost-effectiveness ratio (ICER) was calculated as the incremental cost per QALY of prophylactic LPI from the healthcare payer’s perspective using a Markov model. ICER was evaluated at a commonly used willingness-to-pay threshold in Japan (5 million JPY per QALY) [21]. The exchange rate as of 20 January 2023 was used in this study (160 JPY = 1 pound sterling, £). An annual discount rate of 2% was applied to the costs and outcomes [16]. All analyses were conducted using TreeAge Pro 2023 software (TreeAge Software, Williamstown, MA, USA).
Six scenarios with different ages at entry and exit from the cohort (40–59 years in the base-case analysis) were used for the sensitivity analysis: 40–54 years (15-year horizon), 40–49 years (10-year horizon), 50–69 years (20-year horizon), 50–64 years (15-year horizon), 50–59 years (10-year horizon), and 40–99 years (60-year horizon). The age at entry was changed to simulate cases in which PACS was detected at the age of 50 years. The age at exit was changed to account for the variations in the timing of cataract surgery in the first five scenarios and to estimate the lifetime cost-effectiveness in the last scenario.
A one-way deterministic sensitivity analysis was performed to determine the impact of transition probabilities, utilities, and costs. A deviation of 30%, 8%, and 20% was assigned for transition probabilities, utilities, and cost, respectively [9, 22]. A discount rate of 0–4% was used based on the Japanese guidelines [16]. A tornado diagram was created to show the 12 factors to which ICER was sensitive. Considering the higher transition probabilities of PACS to PAC and PAC to PACG applied in a previous cost-effectiveness study [7], an additional deterministic sensitivity analysis was performed with the probabilities applied in that study.
Probabilistic sensitivity analysis was performed using Monte Carlo simulations with 10,000 iterations. Beta distributions with standardised differences of 10% and 5% were adopted for transition probabilities and utilities, respectively [22]. Gamma distributions with a standardised difference of 10% were adopted for costs. Acceptability curves depicting the probability of prophylactic LPI being cost-effective based on the basis of willingness-to-pay were created.
Lastly, an analysis was performed from a societal perspective. Disability pension, care cost, in-kind benefit, community care, and average salary based on a previous cost-effectiveness analysis regarding blindness in Japan were included as indirect costs [23]. In addition, a travel cost of 4547 JPY per visit [24] was also included in the sensitivity analysis. It was assumed that unilateral blindness incurred 30% of the indirect costs of bilateral blindness [9].
Ethics
This analysis was performed in accordance with the Consolidated Health Economic Evaluation Reporting Standards and the relevant Japanese guidelines [16, 25].
Results
Table 2 presents the results of the base-case and the six scenarios with different ages at entry and exit from the cohort. The ICER was 2,287,662 JPY (£14,298) per QALY in the base-case analysis, which is below the willingness-to-pay threshold (5 million JPY per QALY). In contrast, the ICER was approximately 7,600,000 JPY (£47,500) per QALY and above the willingness-to-pay threshold under the 10-year time horizon. The ICER showed no significant changes on increasing the age at entry into the cohort to 50 years of age. The ICER was 124,675 JPY (£779) per QALY under the 60-year time horizon, which was lower than the willingness-to-pay threshold.
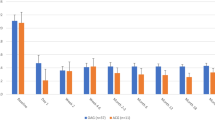
Figure 2 presents the results of the one-way deterministic sensitivity analysis as a tornado diagram. The costs of PACS follow-up had the greatest influence on ICER. However, no parameter could increase the ICER above a threshold of 5 million JPY per QALY. The ICER in the additional deterministic sensitivity analysis, in which higher transition probabilities from a previous study were applied, was lower than that in the base-case analysis (Supplementary Table 3).
The red bar shows the variation on increasing the parameter. The blue bar shows the variation on decreasing the parameter. The upper parameters had a greater impact on the incremental cost-effectiveness ratio. LPI laser peripheral iridotomy, PACS primary angle-closure suspect, PAC primary angle-closure, PACG primary angle-closure glaucoma, AACC acute angle-closure crisis.
In the probabilistic sensitivity analysis, 89.4% of the ICER estimates were below the willingness-to-pay threshold of 5 million JPY per QALY, and approximately 10% were located in the dominant area (Fig. 3). Supplementary Fig. 1 presents the acceptability curve showing the probability of being cost-effective according to the willingness-to-pay threshold. Prophylactic LPI was more likely to be cost-effective than observation when the willingness-to-pay threshold exceeded approximately 2.3 million JPY (approximately £14,375).
The ICER was 2,134,471 JPY (£13,340) per QALY according to the analysis conducted from the societal perspective.
Discussion
This study investigated the cost-effectiveness of prophylactic LPI for PACS versus that of observation using a Markov model. The ICER was 2,287,662 JPY (£14,298) per QALY, indicating that prophylactic LPI was cost-effective. These results were confirmed to be robust by several sensitivity analyses.
The prevalence of PACG in Asia is estimated to be 1.09%, which is more than double the global prevalence, indicating that Asia has the highest prevalence of PACG [3]. In addition, Asia accounts for approximately 60% of the world’s total population [26]. Therefore, it is important to assess the cost-effectiveness of prophylactic treatment for PACG in Asia. Two randomised controlled trials conducted in China and Singapore reported that prophylactic LPI for PACS was effective in preventing disease progression [5, 6]. Nevertheless, the cost-effectiveness of widespread prophylactic LPI has been questioned since the incidence of PAC and PACG was very rare. Our cost-effectiveness analysis indicates that prophylactic LPI for middle-aged patients with PACS is likely to be cost-effective in Japan, and as the incidence of adverse events after LPI was reportedly very low [5, 6], prophylactic LPI for middle-aged patients with PACS can be recommended.
Cataracts are a trigger for narrow angles, and cataract surgery can improve angle closure [27] and reduce the intraocular pressure; [28] however, some patients experience glaucomatous progression of PACS after cataract surgery [29]. The influence of cataract and cataract surgery on the disease progression remains unclear; therefore, the cost-effectiveness of prophylactic LPI was evaluated in a population that was unlikely to be affected by cataracts, that is, a population aged 40–59 years. Thus, the main analysis demonstrates the cost-effectiveness of LPI irrespective of cataract. Moreover, the scenarios with different ages at entry demonstrated that ICERs under the 10-year horizon exceeded the willingness-to-pay threshold, whereas those under the 15- and 20-year horizons were below the threshold. These results indicate that prophylactic LPI is unlikely to be cost-effective for eyes with progressive cataracts that would require cataract surgery within 15 years.
Since glaucoma is a chronic disease, the lifetime cost-effectiveness plays an important role in treatment selection. It was estimated that the lifetime cost-effectiveness of prophylactic LPI would be significantly lower than the willingness-to-pay threshold (124,675 JPY per QALY under the 60-year time horizon), although the impact of cataract surgery on the disease progression was not incorporated owing to the lack of data. Cataract surgery would impact the transition probabilities by slowing the disease progression. As the one-way sensitivity analysis indicated that the changes in transition probabilities would have a small effect on ICER, it was speculated that prophylactic LPI would remain cost-effective in the lifetime horizon even if the impact of cataract surgery was considered.
Lens extraction has been recommended as a treatment for PACG based on its efficacy and cost-effectiveness compared with therapeutic LPI [30]. Some ophthalmologists suggest that lens extraction is also more effective than LPI as a prophylactic procedure for PACS; however, there has been little evidence to support this assumption [12], thereby warranting further analyses comparing the efficacy and cost-effectiveness of prophylactic LPI and lens extraction for PACS.
A cost-effectiveness analysis of prophylactic LPI in the US reported that the ICER of LPI versus that of observation was $2,915,165 per QALY under the 2-year horizon and that LPI overshadowed observation after 3 years up to the age of 50 years. Our results provided less cost-effective estimates of LPI than theirs. Two possible reasons could explain this discrepancy. First, the data source for the transition probability of PACS to PAC was different. The previous study used a transition probability based on a previous cost-effectiveness model of glaucoma screening [9], which referred to three observational studies published in 1992 and 2003 [31,32,33]. In contrast, the present study used the progression rate reported in a randomised controlled trial that was published in 2022 [5]. As a result, the transition probabilities that were applied in our model were less than half of those applied in their study. However, the main findings of the sensitivity analysis in the present study did not vary on using the transition probabilities used in their study. Second, the cost parameters were generally lower in our study compared with those in their study, possibly due to the differences between the two countries in terms of the status of medical care and the different perspectives used in the analysis (healthcare payer’s perspective in our study versus societal perspective in their study). As the cost parameters in the current study were based on our previous study using claims databases in Japan, we believe that our analysis accurately reflected the real-world clinical practice. Although the one-way sensitivity analysis revealed that the costs for the PACS follow-ups had the greatest influence on ICER, the ICER did not exceed the willingness-to-pay threshold in the worst situations. The ICER estimated from a societal perspective was also lower than the willingness-to-pay threshold in the present study, indicating that prophylactic LPI would be cost-effective both from the societal and healthcare payer’s perspectives.
There are several mechanisms underlying narrow angles, such as relative pupillary block, lens thickness and position, and plateau iris. Prophylactic LPI is effective in cases with relative pupillary block; however, the efficacy of LPI in cases with plateau iris is limited. A previous study demonstrated the persistence of plateau iris after peripheral iridotomy in 26% of preoperative plateau iris configurations [34]. Since the data source for the efficacy of prophylactic LPI in our analysis did not distinguish between narrow-angle mechanisms, the analysis in the present study estimated the cost-effectiveness of LPI for PACS as a whole. The efficacy of prophylactic LPI for PACS of each mechanism should be clarified in the future to improve treatment strategies.
The results of the present study regarding the cost-effectiveness of prophylactic LPI can be generalised to some countries. For example, in Korea, the cost of glaucoma treatment is comparable with that in Japan, and the willingness-to-pay threshold is estimated to be approximately 3 million JPY (30,000,000 South Korean Won) per QALY [35]. Consequently, prophylactic LPI for PACS in Korea would be cost-effective as the probability of LPI being cost-effective was approximately 65% at the Korean threshold. Several costs in China were beyond the ranges in the current analysis; for example, the estimated treatment costs of PACS, PAC, and PACG were lower, whereas those of unilateral and bilateral blindness were higher than their counterparts in Japan [9]. The one-way deterministic sensitivity analysis showed that the costs of blindness hardly influenced the ICER; in contrast, the decreased first-year cost of PACS in the LPI group reduced the ICER. Therefore, as the willingness-to-pay threshold in China was estimated as 23,850 US dollars (approximately 3 million JPY) per QALY [9], prophylactic LPI would also be cost-effective.
Our study had some limitations. First, although we attempted to estimate the parameters using real-world data in Japan, we were unable to obtain some parameters, such as utilities and the transition probabilities. We referred to studies conducted in Asian countries in such situations. Second, we were unable to consider the influence of cataracts and cataract surgery. Cataract surgery can be performed before the age of 60 years in some cases showing early progress. The cost-effectiveness of prophylactic LPI in such patients remains unknown. Third, the timings of the transitions are not necessarily at the end of each cycle. However, half-cycle correction was not performed as the correction reportedly changes the ICER by <1% [36]. Lastly, although the results are possibly generalisable to Korean and Chinese populations, they cannot be generalised to populations across the world.
In conclusion, compared with observation, prophylactic LPI for middle-aged patients with PACS was estimated to be cost-effective in Japan.
Summary
What was known before
-
Prophylactic laser peripheral iridotomy for primary angle-closure suspect was effective in reducing the progression to primary angle closure and primary angle-closure glaucoma.
-
Although the prevalence of primary angle-closure suspect is high in Asia, the cost-effectiveness of prophylactic laser peripheral iridotomy for primary angle-closure suspect remains unknown.
What this study adds
-
Our results indicate that prophylactic laser peripheral iridotomy for middle-aged patients with primary angle-closure suspects is cost-effective in Japan.
-
Prophylactic laser peripheral iridotomy can be recommended for middle-aged patients with primary angle-closure suspect.
References
Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7.
Wright C, Tawfik MA, Waisbourd M, Katz LJ. Primary angle-closure glaucoma: an update. Acta Ophthalmol. 2016;94:217–25.
Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–90.
Liang Y, Friedman DS, Zhou Q, Yang XH, Sun LP, Guo L, et al. Prevalence and characteristics of primary angle-closure diseases in a rural adult Chinese population: the Handan Eye Study. Invest Ophthalmol Vis Sci. 2011;52:8672–9.
Baskaran M, Kumar RS, Friedman DS, Lu QS, Wong HT, Chew PTK, et al. The Singapore asymptomatic narrow angles laser iridotomy study: five-year results of a randomized controlled trial. Ophthalmology. 2022;129:147–58.
He M, Jiang Y, Huang S, Chang DS, Munoz B, Aung T, et al. Laser peripheral iridotomy for the prevention of angle closure: a single-centre, randomised controlled trial. Lancet. 2019;393:1609–18.
Sood S, Sanchez V, Heilenbach N, Al-Aswad LA. Cost-effectiveness of prophylactic laser peripheral iridotomy in primary angle-closure suspects. Ophthalmol Glaucoma. 2023;6:332–41.
Owen JP, Blazes M, Lacy M, Yanagihara RT, Van Gelder RN, Lee AY, et al. Refractive outcomes after immediate sequential vs delayed sequential bilateral cataract surgery. JAMA Ophthalmol. 2021;139:876–85.
Tang J, Liang Y, O’Neill C, Kee F, Jiang J, Congdon N. Cost-effectiveness and cost-utility of population-based glaucoma screening in China: a decision-analytic Markov model. Lancet Glob Health. 2019;7:e968–978.
Quek DTL, Koh VT, Tan GS, Perera SA, Wong TT, Aung T. Blindness and long-term progression of visual field defects in Chinese patients with primary angle-closure glaucoma. Am J Ophthalmol. 2011;152:463–9.
Rossetti L, Digiuni M, Montesano G, Centofanti M, Fea AM, Iester M, et al. Blindness and glaucoma: a multicenter data review from 7 academic eye clinics. PLOS One. 2015;10:e0136632.
Kiuchi Y, Inoue T, Shoji N, Nakamura M, Tanito M, Glaucoma Guideline Preparation Committee, Japan Glaucoma Society. The Japan Glaucoma Society guidelines for glaucoma 5th edition. Jpn J Ophthalmol. 2023;67:189–254.
Newman-Casey PA, Salman M, Lee PP, Gatwood JD. Cost-utility analysis of glaucoma medication adherence. Ophthalmology. 2020;127:589–98.
Ministry of Health, Labour and Welfare. https://www.mhlw.go.jp/toukei/saikin/hw/jinkou/geppo/nengai20/dl/h5.pdf. Accessed March 6, 2023.
Fujita A, Hashimoto Y, Matsui H, Yasunaga H, Aihara M. Recent trends in treatment and associated costs of primary angle-closure glaucoma: a retrospective cohort study. Ophthalmol Glaucoma. 2023;6:308–15.
Shiroiwa T, Fukuda T, Ikeda S, Takura T, Moriwaki K. Development of an official guideline for the economic evaluation of drugs/medical devices in Japan. Value Health. 2017;20:372–8.
Fujita A, Sakata R, Hashimoto Y, Matsui H, Fushimi K, Yasunaga H, et al. One-year costs of incisional glaucoma surgery and laser therapy. ACE. 2023;5:48–57.
Sun X, Zhang S, Wang N, Liang Y, Wang L, Fan S, et al. Utility assessment among patients of primary angle closure/glaucoma in China: a preliminary study. Br J Ophthalmol. 2009;93:871–4.
Brown MM, Brown GC, Sharma S, Kistler J, Brown H. Utility values associated with blindness in an adult population. Br J Ophthalmol. 2001;85:327–31.
van Gestel A, Webers CA, Beckers HJ, van Dongen MC, Severens JL, Hendrikse F, et al. The relationship between visual field loss in glaucoma and health-related quality-of-life. Eye. 2010;24:1759–69.
Shiroiwa T, Sung YK, Fukuda T, Lang H-C, Bae S-C, Tsutani K. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ. 2010;19:422–37.
Konishi T, Fujiogi M, Michihata N, Ohbe H, Matsui H, Fushimi K, et al. Cost-effectiveness analysis of trastuzumab monotherapy versus adjuvant chemotherapy plus trastuzumab in elderly patients with HER2-positive early breast cancer. Jpn J Clin Oncol. 2022;52:1115–23.
Yamada M, Nakano T, Matsuda H, Kim SW, Takagi Y. Cost-effectiveness and budget impact analysis of a patient visit support system for blindness reduction in Japanese patients with glaucoma. J Med Econ. 2020;23:1293–301.
Hanemoto T, Hikichi Y, Kikuchi N, Kozawa T. The impact of different anti-vascular endothelial growth factor treatment regimens on reducing burden for caregivers and patients with wet age-related macular degeneration in a single-center real-world Japanese setting. PLoS One. 2017;12:e0189035.
Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell C, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health. 2022;25:3–9.
United Nations. World population prospects 2019: demographic profiles. UN; New York; 2020.
Shin HC, Subrayan V, Tajunisah I. Changes in anterior chamber depth and intraocular pressure after phacoemulsification in eyes with occludable angles. J Cataract Refract Surg. 2010;36:1289–95.
Sakai D, Yamamoto S, Yoshimizu S, Hirose F, Fujihara M, Nakamura M, et al. Ten-year outcomes of cataract surgery for glaucoma management in patients with primary angle-closure disease. Jpn J Ophthalmol. 2023;67:129–37.
Song MK, Sung KR, Shin JW, Jo YH, Won HJ. Glaucomatous progression after lens extraction in primary angle closure disease spectrum. J Glaucoma. 2020;29:711–7.
Azuara-Blanco A, Burr J, Ramsay C, Cooper D, Foster PJ, Friedman DS, et al. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomised controlled trial. Lancet. 2016;388:1389–97.
Wishart PK, Batterbury M. Ocular hypertension: correlation of anterior chamber angle width and risk of progression to glaucoma. Eye. 1992;6:248–56.
Alsbirk PH. Anatomical risk factors in primary angle-closure glaucoma. A ten year follow up survey based on limbal and axial anterior chamber depths in a high risk population. Int Ophthalmol. 1992;16:265–72.
Thomas R, George R, Parikh R, Muliyil J, Jacob A. Five year risk of progression of primary angle closure suspects to primary angle closure: a population based study. Br J Ophthalmol. 2003;87:450–4.
Jain N, Zia R. The prevalence and break down of narrow anterior chamber angle pathology presenting to a general ophthalmology clinic. Medicine. 2021;100:e26195.
Choi JA, Song LD, Choi S, Park SM, Kwon JW, Jee D. The cost-effectiveness of medication, laser trabeculoplasty, and trabeculectomy for treatment of open-angle glaucoma in South Korea. Medicine. 2019;98:e14026.
Nemeth B, Vincziczki Á. The role of half-cycle correction in the models used for health technology assessment. Value Health. 2013;16:A592–A593.
Acknowledgements
The authors thank So Sato (Department of Clinical Epidemiology and Health Economics, The University of Tokyo) for his support with the analysis.
Funding
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (23AA2003 and 22AA2003), The Health Care Science Institute Research Grant, and The Institute for Health Economics and Policy. Open access funding provided by The University of Tokyo.
Author information
Authors and Affiliations
Contributions
Study concept and design: AF, TK, RS, YH, HY, and MA. Analysis: AF, TK, YH, and HY. Interpretation of the results: AF, TK, RS, YH, HY, and MA. Drafting of the manuscript: AF, YH, HY, and MA. Obtained funding: AF and HY. Administrative, technical, or material support: None. Study supervision: HY and MA.
Corresponding author
Ethics declarations
Competing interests
TK received grants from Pfizer Co. Ltd., Kanzawa Medical Research Foundation, and Japan Kampo Medicines Manufacturers Association outside the submitted work. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fujita, A., Konishi, T., Sakata, R. et al. Cost-effectiveness analysis of prophylactic laser peripheral iridotomy for primary angle-closure suspect in Japan. Eye 38, 930–936 (2024). https://doi.org/10.1038/s41433-023-02806-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02806-3