Abstract
The impact of acute therapy for intracerebral hemorrhage (ICH) lags far behind that for acute ischemic stroke. Intensive blood pressure lowering is a promising therapeutic strategy for acute ICH, especially for East Asian patients whose etiological mechanism is more commonly hypertension than that of patients in the Western population. A multicenter, prospective, observational study named the Stroke Acute Management with Urgent Risk-factor Assessment and Improvement-IntraCerebral Hemorrhage (SAMURAI-ICH) study, involving 211 patients from ten Japanese stroke centers, was performed to elucidate the safety and feasibility of blood pressure lowering to 160 mmHg or less in acute ICH patients using intravenous nicardipine. When we started the study, intravenous nicardipine was not officially approved for hyperacute ICH patients in Japan. The SAMURAI-ICH study was also a pilot study to judge the feasibility of participation by many Japanese investigators in an international, randomized, controlled trial named the Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH)−2 trial. The SAMURAI-ICH study, ATACH-2 trial, and their combined individual participant data meta-analysis produced several new interesting findings on how to control blood pressure levels in acute ICH patients. Some of the findings are introduced in the present review article.
Similar content being viewed by others
Introduction: before the SAMURAI-ICH study
Hemorrhagic stroke, including intracerebral hemorrhage (ICH) and subarachnoid hemorrhage, is much more devastating than ischemic stroke. Although new cases of hemorrhagic stroke were estimated to account for 30% of the overall new stroke cases worldwide in 2016 (4, 120, 318/13, 676, 761), the total deaths (2,838,061 vs. 2,690,170) and disability-adjusted life-years lost (64.5 million vs. 51.9 million) from hemorrhagic stroke surpassed those of ischemic stroke [1]. A higher age at event onset and more severe functional outcomes for ICH patients than for ischemic stroke patients are common worldwide (Fig. 1) [2]. Nevertheless, the impact of acute therapy for ICH lags far behind that for acute ischemic stroke [3, 4]. An established therapeutic strategy for acute ICH analogous to reperfusion therapy for acute ischemic stroke has not been established. In the nationwide registry of the Japan Stroke Data Bank, functional outcomes improved for ischemic stroke patients over the past 20 years after age adjustment but did not improve for ICH patients [5]. The lack of an established strategy might be an essential reason for the difference.
Age at onset (A), initial National Institutes of Health Stroke Scale scores (B), and discharge modified Rankin Scale scores (C) of 33,178 patients with acute intracerebral hemorrhage and 125,722 patients with acute ischemic stroke from a multicenter registry between 2000 and 2018: The Japan Stroke Data Bank. Edited based on the data from Ref [2]. The National Institutes of Health Stroke Scale (NIHSS), a serial measure of neurological deficit, is a 42-point scale that quantifies neurological deficits in 11 categories, with a score of 0 indicating normal function without neurological deficit and higher scores indicating a greater severity of deficit. The modified Rankin scale grades the degree of disability or dependence in daily activities using scores ranging from 0 (no symptoms) to 6 (death). In Panel (B), the boxes represent the interquartile ranges, the lines across the boxes indicate the median values, and the whiskers represent the 10th percentile and 90th percentile values
Hypertension is a firmly established risk factor for ICH in the general population [6]. Elevated blood pressure (BP) is common after ICH and is reportedly associated with poor outcomes, presumably partly due to hematoma expansion [3, 4, 7, 8]. A theoretical risk for reducing cerebral blood flow surrounding hematomas by intensive BP lowering was also mentioned. Generally, there has been a consensus for many years that extremely high BP should be controlled during acute ICH. However, the BP goal had been set to be relatively high, with a systolic BP (SBP) of approximately 180 mmHg, due to the lack of scientific evidence for more intensive BP lowering. The recent revision to a lower goal of less than 140 mmHg required the publication of results in the mid-2010s from large, global, randomized, controlled trials, including the Second Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT2) and the Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH)−2 trial [9, 10].
Stroke Acute Management with Urgent Risk-factor Assessment and Improvement (SAMURAI) was the original name for the study group of multiple stroke registries funded by the Japanese government. The group completed a series of studies on three different themes: acute stroke thrombolysis [11, 12], anticoagulants for atrial fibrillation-associated stroke [13, 14], and BP management for acute ICH. The studies on the last theme were named the SAMURAI-ICH study. In the present review, changes in the strategy for BP lowering in acute ICH patients worldwide and in Japan over the last decade are presented based on the achievements of and evolved or derivative projects from the SAMURAI-ICH study.
Trigger for the SAMURAI-ICH study
Of the etiological mechanisms for ICH, hypertensive arteriopathy seems to be relatively predominant in the Asian population, and cerebral amyloid angiopathy seems to be relatively predominant in the Western population [15]. Thus, intensive BP management would be more effective for preventing acute exacerbation after ICH onset in the Asian population. However, the Japanese Guidelines in the 2000s recommended acute BP lowering only when the BP was extremely high, i.e., an SBP >180 mmHg or a mean arterial pressure >130 mmHg, without indicating definite target BP levels [16], following the recommendation of the American Heart Association/American Stroke Association guidelines [17]. This recommendation was based on limited information without scientific evidence. To make matters worse, optimal intravenous antihypertensive agents for acute ICH patients were not established in Japan at that time. Of the representative intravenous antihypertensives recommended in Western countries [17], labetalol was not approved for commercial use, esmolol was used only as an antiarrhythmic drug, and the administration of nicardipine for hyperacute ICH patients was limited by the following description on the official label without scientific evidence: “nicardipine is contraindicated for (1) ICH patients with a suspicion of ongoing intracranial bleeding not to enhance bleeding and for (2) acute stroke patients with elevated intracranial pressure not to accelerate intracranial pressure elevation” [18]. One of the few remaining alternatives was diltiazem, although it often causes bradycardia or atrioventricular block. However, in our nationwide web survey in 2008, 57% of the respondents chose nicardipine as the first choice agent despite the official limitation [18]. To resolve the divergence between the official recommendation and actual clinical practice, the safety of nicardipine for Japanese ICH patients needs to be ascertained.
We had another reason for planning a clinical study on nicardipine use for acute ICH patients. In 2007, Dr. Hisatomi Arima (currently a Professor at the Department of Preventive Medicine and Public Health, Fukuoka University), a core member of the INTERACT trial, gave us an invitation from Sydney to join the INTERACT2 trial together with several Japanese sites that would soon be started. However, we had no experience and little capacity to direct multiple sites participating in international trials at that time. The refusal of this invitation was regrettable for us. The following year, Professor Yuko Palesch (Department of Public Health Sciences, Medical University of South Carolina), the coprincipal investigator of the ATACH-2 trial, visited our workplace, the National Cerebral and Cardiovascular Center, Suita, and invited us to join the trial. We did not want to miss this second chance. Since intravenous nicardipine was the only trial drug for the ATACH-2 trial, the official limitation of its use in Japan would need to be removed for trial participation.
We formed the SAMURAI study group with ten stroke centers in 2008 (Table 1). Acute BP lowering for ICH patients met our goal of “acute stroke management with an urgent risk factor assessment and improvement.” We immediately developed a study protocol.
Messages from the SAMURAI-ICH study
In the nationwide survey described above, 82% of the respondents chose the SBP goal to be ≤160 mmHg [18]. Thus, we planned a prospective, observational study, the SAMURAI-ICH study, to elucidate the safety and feasibility of the major choices from the survey of acute ICH patients to maintain SBP levels between 120 and 160 mmHg for 24 h using intravenous nicardipine [19]. We enrolled 211 patients (81 women, aged 65.6 ± 12.0 years, baseline SBP 201.8 ± 15.7 mmHg) with acute supratentorial ICH from July 2009 through June 2011. Using the strict titration method as used in the ATACH-2 trial, patients’ SBP levels were lowered to the target range in a median of 30 minutes [interquartile range (IQR) 15–45 minutes] with the proportion of time in the target SBP range over 24 h to be 77.6% (IQR 75.3–79.9%, Fig. 2). Neurological deterioration corresponding to a decrease of ≥2 points on the baseline Glasgow Coma Scale score or an increase of ≥4 points on the baseline National Institutes of Health Stroke Scale (NIHSS) score 72 h after the initiation of treatment was identified in 8.1% [95% confidence interval (CI) 5.1–12.5%] of the patients; serious adverse events requiring nicardipine be stopped within 24 h was reported in 0.9% (0.3–3.4%) of the patients; hematoma expansion >33% from baseline to 24 h was reported in 17.1% (12.6–22.7%) of the patients; and a poor outcome corresponding to modified Rankin Scale (mRS) scores of 4 to 6 at 3 months was reported in 41.2% (34.8–48.0%) of the patients. The rates were less than the upper limit of the 90% CI for the predicted proportion based on the weighted average of previous studies (25.9%, 8.9%, 28.3% and 67.9%, respectively) and some were even equal to or less than the lower limit (15.2%, 1.8%, 17.1%, and 54.5%, respectively). We concluded that SBP lowering to ≤160 mmHg using intravenous nicardipine appeared to be well tolerated and feasible for Japanese acute ICH patients.
Trends in systolic and diastolic blood pressure levels during the 24-h administration of nicardipine: The SAMURAI-ICH study. Re-edited from Ref [19]. The boxes represent the interquartile ranges, the lines across the boxes indicate the median values, and the whiskers represent the 10th percentile and 90th percentile values
While proceeding with patient registration for the SAMURAI-ICH study, we submitted the interim results of the study together with the results of the nationwide survey described above as references in a petition by the Japan Stroke Society to the Ministry of Health, Labour, and Welfare of Japan to revise the rule regarding the contraindication for ICH on the official label of intravenous nicardipine. The rule was finally abolished in 2011; it enabled us to join the ATACH-2 trial.
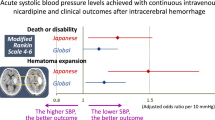
The SAMURAI Investigators published several substudies using the dataset; the themes included the association of clinical outcomes with mean SBP and its variability during the initial 24 h [20,21,22,23], the timing of SBP lowering to the target level [24], the total dosing of nicardipine [25], kidney function [26], and blood glucose levels (Table 2) [27]. Another unique theme was conjugate eye deviation during acute ICH [28]. The top panels of Fig. 3 show the correlations between the rates of clinical outcomes and mean achieved SBP levels during the 24-h administration of nicardipine [20]. All of the rates of hematoma expansion within 24 h, neurological deterioration at 72 h, and unfavorable outcomes at 3 months increased as the mean SBP levels increased. The mean SBP was independently associated with hematoma expansion (odds ratio 1.86, 95% CI 1.09–3.16 per 10 mmHg), neurological deterioration (4.45, 2.03–9.74), and unfavorable outcomes (2.03, 1.24–3.33) after adjusting for the known predictors. The bottom panels of Fig. 3 show the correlations between the rates of clinical outcomes and successive variations in SBP during the initial 24 h [22]. The rates of neurological deterioration and poor outcomes increased as the successive variations increased. Successive variation in SBP, a representative indicator of variability, was independently associated with neurological deterioration (odds ratio 2.37, 95% CI 1.32–4.83 per quartile category) and unfavorable outcomes (1.42, 1.04–1.97) after adjusting for the known predictors. Early intensive and stable SBP lowering after onset seemed to improve the clinical outcomes of ICH patients.
Moving on to the ATACH-2 trial
Encouraged by the success of the SAMURAI-ICH study, 14 Japanese sites participated in the ATACH-2 trial (Table 1). The ATACH-2 trial was a randomized, multicenter, two-group, open-label trial to determine the relative efficacy of intensive (110–139 mmHg) versus standard (140–179 mmHg) SBP lowering with intravenous nicardipine using a strictly defined titration method that was initiated within 4.5 h after symptom onset and continued for the next 24 h in patients with spontaneous supratentorial ICH [10]. Of the 1,000 participants, 288 were enrolled from Japan, 246 from other Asian countries (China, Taiwan, and South Korea), and the remaining 466 from the United States and Germany. The trial did not show a benefit in reducing the rate of the primary outcome of death or disability, defined as an mRS score of 4–6, between the two treatment groups (relative risk with intensive treatment 1.04, 95% CI 0.85–1.27). The result was somewhat different in the INTERACT2 trial, which overlapped in time with the ATACH-2 trial, showing possibly better functional outcomes for acute ICH patients with early intensive SBP lowering (<140 mmHg) than for patients with standard lowering (<180 mmHg) with the use of any antihypertensive agents of a physician’s choosing (odds ratio 0.87, 95% CI 0.75–1.01) [9]. The differences in the results seemed to be partly caused by the considerable variance of achieved SBP levels between the two trials, as shown in Fig. 4, although the target SBP levels of each treatment group were the same. The optimal SBP goal for acute ICH patients might be between the mean achieved SBPs of the intensive treatment groups of both trials (between 120 and 140 mmHg).
Trends of mean hourly systolic blood pressure in the ATACH-2 and INTERACT2 trials. Re-edited from Ref [9, 10]. Achieved systolic blood pressure levels in the standard treatment group of the ATACH-2 trial and the intensive treatment group of the INTERACT2 trial were similar (~140 mmHg) and that in the intensive treatment group of the ATACH-2 trial was lowered to ~120 mmHg
Japanese researchers contributed to the ATACH-2 trial not only by patient recruitment but also by subanalyses using the ATACH-2 dataset. The themes of the subanalyses included sex differences [29], regional differences (Asia versus non-Asia) [30], late neurological deterioration [31], kidney function [32], heart rate [33], and the impacts of achieved SBP levels on clinical outcomes [34].
Finally, the results from a systematic review and individual participant data analysis using the combined database from the SAMURAI-ICH study, ATACH-2 trial, and ATACH-1 trial [35], a small pilot trial for the ATACH-2 trial, are presented [36]. Prospective studies before October 1, 2020, were identified in PubMed; studies involving hyperacute ICH adult patients treated with intravenous nicardipine whose outcomes was assessed using the mRS score were eligible (PROSPERO: CRD42020213857), and the above three studies met the eligibility criteria. For the 1,265 patients enrolled (484 women, aged 62.6 ± 13.0 years, baseline SBP 206.1 ± 21.0 mmHg), the mean hourly SBP during the initial 24 h was positively associated with an mRS score of 4–6 (adjusted odds ratio 1.12, 95% CI 1.00–1.26 per 10 mmHg) and hematoma expansion within 24 h (1.16, 1.02–1.32). A total of 499 patients (183 women, aged 64.9 ± 11.8 years, baseline SBP 203.5 ± 18.3 mmHg) from Japan were registered in this pooled analysis. For Japanese patients, the mean hourly SBP was more strongly associated with an mRS score of 4–6 (adjusted odds ratio 1.26, 95% CI 1.04–1.53) and hematoma expansion (1.47, 1.17–1.85) than for the overall participants (Table 3). When the mRS score was compared among the quartiles by the mean hourly SBP during the initial 24 h, the distribution shifted to higher scores as the SBP became higher in the first three quartiles (Fig. 5).
Modified Rankin Scale scores at 90 days for Japanese patients with acute intracerebral hemorrhage divided into quartile groups by mean systolic blood pressure levels during the 24-h administration of nicardipine: The pooled analysis. Re-edited from Ref [36]. Arrows indicate the percentage with modified Rankin Scale scores of 4–6
As described above, hypertensive arteriopathy seems to be predominant in Japanese patients [15]. Thus, intensive SBP lowering might show a strong preventive effect in a Japanese cohort. It should also be noted that acute kidney injury and renal adverse events were known complications in the ATACH-2 trial, especially when the SBP was lower [29, 34, 37]. Attention to changes in kidney function is accordingly indispensable when intensively lowering SBP.
The 2010s was a decade for changing the strategy of acute BP lowering after ICH onset. In Japan, we revised the official label of intravenous nicardipine for its appropriate use in hyperacute ICH patients, participated in the memorable and global BP-lowering therapy trial, and clarified details regarding the therapy using international datasets that involved many Japanese patients throughout the decade. The SAMURAI-ICH study helped a series of these activities. In the present decade, new pharmacotherapeutic strategies such as emergent hemostasis will be developed [4, 38]. Appropriate BP lowering from hyperacute to chronic stages, coupled with such novel strategies, will be essential for the better recovery of patients after ICH.
References
GBD. Stroke collaborators. global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:439–58. 2016
The Editorial Committee for Japan Stroke Data Bank 2021 in the National Cerebral and Cardiovascular Center (ed). Japan Stroke Data Bank 2021. Nakayama Shoten, Tokyo 2021 (in Japanese)
Toyoda K, Anderson CS and Mayer S (eds). New insights in intracerebral hemorrhage. Basel: Karger AG, 2015.
Broderick JP, Grotta JC, Naidech AM, Steiner T, Sprigg N, Toyodo K, et al. The story of intracerebral hemorrhage: from recalcitrant to treatable disease. Stroke. 2021;52:1905–14.
Toyoda K, Yoshimura S, Nakai M, Koga M, Sasahara Y, Sonoda K, et al. Twenty-year change in severity and outcome of ischemic and hemorrhagic strokes. JAMA Neurol. 2022;79:61–9.
Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34:2060–5.
Willmot M, Leonardi-Bee J, Bath PM. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension. 2004;43:18–24.
Itabashi R, Toyoda K, Yasaka M, Kuwashiro T, Nakagaki H, Miyashita F, et al. The impact of hyperacute blood pressure lowering on the early clinical outcome following intracerebral hemorrhage. J Hypertens. 2008;26:2016–21.
Anderson CS, Heeley E, Huang Y, Wang J, Stapf C, Delcourt C, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368:2355–65.
Qureshi AI, Palesch YY, Barsan WG, Hanley DF, Hsu CY, Martin RL, et al. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med. 2016;375:1033–43.
Nezu T, Koga M, Kimura K, Shiokawa Y, Nakagawara J, Furui E, et al. Pretreatment ASPECTS on DWI predicts 3-month outcome following rt-PA: SAMURAI rt-PA Registry. Neurology. 2010;75:555–61.
Endo K, Kario K, Koga M, Nakagawara J, Shiokawa Y, Yamagami H, et al. Impact of early blood pressure variability on stroke outcomes after thrombolysis: the SAMURAI rt-PA Registry. Stroke. 2013;44:816–8.
Koga M, Yoshimura S, Hasegawa Y, Shibuya S, Ito Y, Matsuoka H, et al. Higher risk of ischemic events in secondary prevention for patients with persistent than those with paroxysmal atrial fibrillation. Stroke. 2016;47:2582–8.
Mizoguchi T, Tanaka K, Toyoda K, Yoshimura S, Itabashi R, Takagi M, et al. Early initiation of direct oral anticoagulants after onset of stroke and short- and long-term outcomes of patients with nonvalvular atrial fibrillation. Stroke. 2020;51:883–91.
Yakushiji Y, Tanaka J, Wilson D, Charidimou A, Noguchi T, Kawashima M, et al. Proportion of intracerebral haemorrhage due to cerebral amyloid angiopathy in the East and West: Comparison between single hospital centres in Japan and the United Kingdom. J Neurol Sci. 2020;416:117037.
Shinohara Y, Yamaguchi T. Outline of the Japanese Guidelines for the Management of Stroke 2004 and subsequent revision. Int J Stroke. 2008;3:55–62.
Broderick J, Connolly S, Feldmann E, Hanley D, Kase C, Krieger D, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38:2001–23.
Koga M, Toyoda K, Naganuma M, Kario K, Nakagawara J, Furui E, et al. Nationwide survey of antihypertensive treatment for acute intracerebral hemorrhage in Japan. Hypertens Res. 2009;32:759–64.
Koga M, Toyoda K, Yamagami H, Okuda S, Okada Y, Kimura K, et al. Systolic blood pressure lowering to 160 mmHg or less using nicardipine in acute intracerebral hemorrhage: a prospective, multicenter, observational study (the Stroke Acute Management with Urgent Risk-factor Assessment and Improvement-Intracerebral Hemorrhage study). J Hypertens. 2012;30:2357–64.
Sakamoto Y, Koga M, Yamagami H, Okuda S, Okada Y, Kimura K, et al. Systolic blood pressure after intravenous antihypertensive treatment and clinical outcomes in hyperacute intracerebral hemorrhage: the Stroke Acute Management with Urgent Risk-factor Assessment and Improvement-IntraCerebral Hemorrhage study. Stroke. 2013;44:1846–51.
Kobayashi J, Koga M, Tanaka E, Okada Y, Kimura K, Yamagami H, et al. Continuous antihypertensive therapy throughout the initial 24 h of intracerebral hemorrhage: the stroke acute management with urgent risk-factor assessment and improvement-intracerebral hemorrhage study. Stroke 2014;45:868–70.
Tanaka E, Koga M, Kobayashi J, Kario K, Kamiyama K, Furui E, et al. Blood pressure variability on antihypertensive therapy in acute intracerebral hemorrhage: the Stroke Acute Management with Urgent Risk-factor Assessment and Improvement-intracerebral hemorrhage study. Stroke 2014;45:2275–9.
Sakamoto Y, Koga M, Todo K, Okuda S, Okada Y, Kimura K, et al. Relative systolic blood pressure reduction and clinical outcomes in hyperacute intracerebral hemorrhage: the SAMURAI-ICH observational study. J Hypertens. 2015;33:1069–73.
Yamaguchi Y, Koga M, Sato S, Yamagami H, Todo K, Okuda S, et al. Early achievement of blood pressure lowering and hematoma growth in acute intracerebral hemorrhage: Stroke Acute Management with Urgent Risk-Factor Assessment and Improvement-Intracerebral Hemorrhage Study. Cerebrovasc Dis. 2018;46:118–24.
Koga M, Arihiro S, Hasegawa Y, Shiokawa Y, Okada Y, Kimura K, et al. Intravenous nicardipine dosing for blood pressure lowering in acute intracerebral hemorrhage: the Stroke Acute Management with Urgent Risk-factor Assessment and Improvement-Intracerebral Hemorrhage study. J Stroke Cerebrovasc Dis. 2014;23:2780–7.
Miyagi T, Koga M, Yamagami H, Okuda S, Okada Y, Kimura K, et al. Reduced estimated glomerular filtration rate affects outcomes 3 months after intracerebral hemorrhage: the stroke acute management with urgent risk-factor assessment and improvement-intracerebral hemorrhage study. J Stroke Cerebrovasc Dis. 2015;24:176–82.
Koga M, Yamagami H, Okuda S, Okada Y, Kimura K, Shiokawa Y, et al. Blood glucose levels during the initial 72 h and 3-month functional outcomes in acute intracerebral hemorrhage: the SAMURAI-ICH study. J Neurol Sci. 2015;350:75–8.
Sato S, Koga M, Yamagami H, Okuda S, Okada Y, Kimura K, et al. Conjugate eye deviation in acute intracerebral hemorrhage: stroke acute management with urgent risk-factor assessment and improvement–ICH (SAMURAI-ICH) study. Stroke 2012;43:2898–903.
Fukuda-Doi M, Yamamoto H, Koga M, Palesch YY, Durkalski-Mauldin VL, Qureshi AI, et al. Sex differences in blood pressure-lowering therapy and outcomes following intracerebral hemorrhage: results from ATACH-2. Stroke 2020;51:2282–6.
Toyoda K, Palesch YY, Koga M, Foster L, Yamamoto H, Yoshimura S, et al. Regional differences in the response to acute blood pressure lowering after cerebral hemorrhage. Neurology 2021;96:e740–e751.
Okazaki S, Yamamoto H, Foster LD, Fukuda-Doi M, Koga M, Ihara M, et al. Late neurological deterioration after acute intracerebral hemorrhage: a post hoc analysis of the ATACH-2 Trial. Cerebrovasc Dis. 2020;49:26–31.
Fukuda-Doi M, Yamamoto H, Koga M, Doi Y, Qureshi AI, Yoshimura S, et al. Impact of renal impairment on intensive blood-pressure-lowering therapy and outcomes in intracerebral hemorrhage: results from ATACH-2. Neurology 2021;97:e913–e921.
Miwa K, Koga M, Fukuda-Doi M, Yamamoto H, Tanaka K, Yoshimura S, et al. Effect of heart rate variabilities on outcome after acute intracerebral hemorrhage: a post hoc analysis of ATACH-2. J Am Heart Assoc. 2021;10:e020364.
Toyoda K, Koga M, Yamamoto H, Foster L, Palesch YY, Wang Y, et al. Clinical outcomes depending on acute blood pressure after cerebral hemorrhage. Ann Neurol. 2019;85:105–13.
Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) investigators. Antihypertensive treatment of acute cerebral hemorrhage. Crit Care Med. 2010;38:637–48.
Toyoda K, Yoshimura S, Fukuda-Doi M, Qureshi AI, Martin RH, Palesch YY, et al. Intensive blood pressure lowering with nicardipine and outcomes after intracerebral hemorrhage: An individual participant data systematic review. Int J Stroke. 2021. https://doi.org/10.1177/17474930211044635, Online ahead of print.
Qureshi AI, Huang W, Lobanova I, Hanley DF, Hsu CY, Malhotra K, et al. Systolic blood pressure reduction and acute kidney injury in intracerebral hemorrhage. Stroke. 2020;51:3030–8.
Naidech AM, Grotta J, Elm J, Janis S, Dowlatshahi D, Toyoda K, et al. Recombinant factor VIIa for hemorrhagic stroke treatment at earliest possible time (FASTEST): Protocol for a phase III, double-blind, randomized, placebo-controlled trial. Int J Stroke. 2021. https://doi.org/10.1177/17474930211042700, Online ahead of print.
Funding
Partly funded by a Grant-in-Aid (H20-Junkanki-Ippan-019) from the Ministry of Health, Labour and Welfare, Japan and by the Japan Agency for Medical Research and Development (AMED: JP21lk0201094 and JP21lk0201109).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Toyoda, K., Koga, M. & as the SAMURAI Investigators. Controlling blood pressure soon after intracerebral hemorrhage: The SAMURAI-ICH Study and its successors. Hypertens Res 45, 583–590 (2022). https://doi.org/10.1038/s41440-022-00866-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-022-00866-8