Abstract
The benefits of direct oral anticoagulants (DOACs) and warfarin in elderly Japanese patients with non-valvular atrial fibrillation (NVAF) and high home systolic blood pressure (H-SBP) are unclear. This sub-cohort study of the ANAFIE Registry estimated the incidence of clinical outcomes in patients receiving anticoagulant therapy (warfarin and DOACs) stratified by H-SBP levels (<125 mmHg, ≥125–<135 mmHg, ≥135–<145 mmHg and ≥145 mmHg). Of the overall ANAFIE population, 4933 patients who underwent home blood pressure (H-BP) measurements were analyzed; 93% received OACs (DOACs: 3494, 70.8%; warfarin: 1092, 22.1%). In the warfarin group, at <125 mmHg and ≥145 mmHg, the respective incidence rates (per 100 person-years) were 1.91 and 5.89 for net cardiovascular outcome (a composite of stroke/systemic embolic events (SEE) and major bleeding), 1.31 and 3.39 for stroke/SEE, 0.59 and 3.91 for major bleeding, 0.59 and 3.43 for intracranial hemorrhage (ICH), and 4.01 and 6.24 for all-cause death. Corresponding incidence rates in the DOACs group were 1.64 and 2.65, 1.00 and 1.88, 0.78 and 1.69, 0.55 and 1.31, and 3.43 and 3.51. In warfarin-treated patients, the incidence rates of net cardiovascular outcome, stroke/SEE, major bleeding, and ICH were significantly increased at H-SBP ≥ 145 mmHg versus <125 mmHg. In the DOAC group, although there was no significant difference between H-SBP < 125 mmHg and ≥145 mmHg, the incidence rates of these events tended to increase at ≥145 mmHg. These results suggest that strict BP control guided by H-BP is required in elderly NVAF patients receiving anticoagulant therapy.

Similar content being viewed by others
Introduction
Atrial fibrillation (AF) is a major risk factor for ischemic stroke, affecting the life expectancy of elderly patients [1]. In patients with AF, 56.5% of patients reportedly have >1 modifiable risk factor, of which the most relevant is hypertension [2]. Furthermore, in patients with AF, hypertension is a risk factor for both embolism and bleeding complications and is a modifiable component of CHADS2 and HAS-BLED scores [2].
The All Nippon AF in the Elderly (ANAFIE) Registry was a 2-year multicenter, prospective observational study conducted to clarify the prognosis and real-world clinical status of over 30,000 elderly patients (aged ≥75 years) with non-valvular AF (NVAF) [3]. A sub-cohort study of the ANAFIE Registry reported that high home blood pressure (H-BP) was associated with an increased risk of stroke/systemic embolic events (SEE), major bleeding, and intracranial hemorrhage (ICH) [4].
Recently, direct oral anticoagulants (DOACs) have been used more frequently for stroke prevention in patients with NVAF in clinical practice than warfarin. Sub-analyses of multiple phase III clinical studies of DOACs have shown that the relative efficacy and safety of DOACs versus warfarin were consistent across all levels of systolic BP (SBP) [5,6,7,8]. The relationship between BP and the efficacy and safety of DOACs versus warfarin is not fully known in elderly NVAF patients in real-world clinical practice. In particular, the benefits of DOACs compared with warfarin in patients with high H-BP who are at increased risk of events are unclear.
A previous report of an H-BP sub-cohort study [4] showed the impact of high H-BP on the risk of clinical outcomes but did not cover the relationship between anticoagulant use and the risk of clinical outcomes. This analysis of the H-BP sub-cohort study of the ANAFIE Registry aimed to clarify the incidence of each clinical outcome in elderly NVAF patients receiving oral anticoagulant therapy (i.e., warfarin or DOACs) stratified by H-BP level.
Methods
Design
This is a sub-cohort study of the ANAFIE Registry, for which the methods and rationale have been described previously [4, 9]. Ethical approval was obtained from all relevant institutional review boards and procedures were in accordance with the Declaration of Helsinki. All participants provided written informed consent to participate and could withdraw at any time. The ANAFIE Registry was registered at UMIN Clinical Trials Registry under the identifier UMIN000024006.
Of note, the choice to prescribe oral anticoagulant (OAC) therapy or no OAC therapy, warfarin, or DOACs was at the discretion of the physician. Baseline data were used for analyses. Patients were followed up for 2 years from enrollment.
Patients
Patients aged ≥75 years at the time of informed consent with a definitive NVAF diagnosis who could attend hospital visits were included. For enrollment in the current sub-cohort study, patients needed to consent to measure their H-BP using an oscillometric device with an arm cuff. Patients with a definite diagnosis of mitral stenosis; an artificial heart valve replacement; very recent history (within 1 month prior to enrollment) of cardiovascular events, including stroke, myocardial infarction, cardiac intervention, heart failure requiring hospitalization, or any bleeding leading to hospitalization; or life expectancy <1 year were excluded from enrollment in this study [4].
Endpoints
The study endpoints were net cardiovascular outcome (a composite of stroke/SEE and major bleeding), stroke/SEE, major bleeding, ICH, and all-cause death. Any events during follow-up were recorded in duplicate. Committees comprising neurologists, cardiologists, and hematologists blinded to the anticoagulation treatment adjudicated all endpoint events. Major bleeding was classified using the International Society on Thrombosis and Haemostasis definition [4, 10].
Data collection method
H-BP measurements were based on previously described procedures [4, 11]. Measurements were taken four times a day (twice in the morning and twice in the evening) for 1 week within 60 days of enrollment in line with Japanese Society of Hypertension (JSH) guidelines [12] and recorded. The average of all measurements over 1 week was used for analysis. Participants could use any device based on the brachial cuff oscillometric method.
Statistical analysis
Details of the statistical analysis for the main ANAFIE Registry have been published [3, 4]. The following H-SBP categories based on JSH guidelines [12] were used: <125 mmHg, ≥125–<135 mmHg, ≥135–<145 mmHg, and ≥145 mmHg. Patient characteristics by H-SBP category were compared with analysis of variance for continuous variables and the chi-square test for categorical variables. However, unknown data were excluded from the analysis. The incidence rate per 100 person-years with 95% confidence intervals (CIs) was estimated. The incidence-rate ratio (relative risk) was estimated using the Poisson regression model. The hazard ratios (HRs) and 95% CIs of DOACs compared with warfarin for the clinical outcomes were analyzed with a multivariate Cox proportional hazards model for each H-SBP category. The following variables were included in the model as covariates: sex, age, body mass index, history of major bleeding, atrial fibrillation type, severe hepatic dysfunction, diabetes mellitus, hyperuricemia, heart disease (heart failure and/or left ventricular systolic dysfunction), myocardial infarction, cerebrovascular disease, thromboembolic-related disease, active cancer, dementia, falls within 1 year, nonpharmacologic therapy (catheter ablation), antiarrhythmics, antiplatelet agents, proton pump inhibitors, P-glycoprotein inhibitors, dyslipidemia, creatinine clearance, gastrointestinal disease, and polypharmacy. The H-SBP by treatment (DOACs versus warfarin) interaction effect was also estimated. The statistical analysis software used was SAS version 9.4 (SAS Institute, Tokyo, Japan), and the significance level was p < 0.05.
Results
Patient disposition and background characteristics
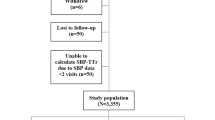
Of the overall ANAFIE population (N = 32,275), 1109 (3.4%) patients were lost to follow-up and 762 (2.4%) discontinued the study because of withdrawal of consent and other reasons [3]. In this H-BP sub-cohort, 4933 patients were included who provided written consent to be enrolled and measured their H-BP. The mean follow-up period was 1.88 years [3].
Table 1 shows patient characteristics at baseline. The patient breakdown by H-SBP category was as follows: 2030, 1585, 878, and 440 in the <125 mmHg, ≥125–<135 mmHg, ≥135–<145 mmHg and ≥145 mmHg groups, respectively. The mean age was 81.4 years, and over 50% were male as a whole. Most patients (4590/4933; 93%) were receiving anticoagulants. Of these, 3494 (70.8%) received DOACs, 1092 (22.1%) received warfarin, and there was no difference in the distribution of OACs between H-SBP groups. Across groups, the mean CHA2DS2-VASc and HAS-BLED scores significantly increased as H-SBP increased. The rate of antihypertensive drug use was significantly different between the H-SBP groups. We analyzed the differences in patient backgrounds between the warfarin and DOAC groups in each H-SBP category (Supplementary Table 1).
Outcomes
Overall, during the 2 years of follow-up, there were 172 cases of net cardiovascular outcomes; 115, stroke/SEE; 76, major bleeding; 57, ICH; and 299, all-cause death. Supplementary Table 2 shows the number of events of the warfarin group and the DOAC group. Figure 1 shows the incidence rates (per 100 person-years) of net cardiovascular outcome, stroke/SEE, major bleeding, ICH, and all-cause death during follow-up in the warfarin and DOAC groups, according to H-SBP. In the warfarin group, the respective incidence rates among patients with H-SBP < 125 mmHg and ≥145 mmHg were 1.91 and 5.89 for net cardiovascular outcome, 1.31 and 3.39 for stroke/SEE, 0.59 and 3.91 for major bleeding, 0.59 and 3.43 for ICH, and 4.01 and 6.24 for all-cause death (Fig. 1). The incidence rates of net cardiovascular outcome, stroke/SEE, major bleeding, and ICH were significantly increased at H-SBP ≥ 145 mmHg versus <125 mmHg (p < 0.05).
Incidence rates (per 100 person-years) of net cardiovascular outcome, stroke/SEE, major bleeding, intracranial hemorrhage, and all-cause death during follow-up in the DOAC and warfarin groups according to H-SBP. Bars represent 95% confidence intervals. *p < 0.05 versus H-SBP < 125 mmHg in each OAC group, #p < 0.05 versus warfarin. DOAC indicates direct oral anticoagulant, H-SBP home systolic blood pressure, SEE systemic embolic events, WF warfarin
In the DOAC group, among patients with <125 mmHg and ≥145 mmHg, the respective incidence rates were 1.64 and 2.65 for net cardiovascular outcome, 1.00 and 1.88 for stroke/SEE, 0.78 and 1.69 for major bleeding, 0.55 and 1.31 for ICH and 3.43 and 3.51 for all-cause death. Although the incidence rate of these events tended to increase with H-SBP ≥ 145 mmHg, there was no significant difference in the event rates between H-SBP < 125 mmHg and ≥145 mmHg in the DOAC group. In comparison with the warfarin group, the incidence rate of net cardiovascular outcome was significantly lower in the DOAC group at H-SBP ≥ 145 mmHg (p < 0.05). In patients with H-SBP ≥ 145 mmHg, CHA2DS2-VASc score in the warfarin group was significantly higher than that in the DOAC group, whereas there was no significant difference in HAS-BLED score between the groups (Supplementary Table 1).
Supplementary Table 3 shows the incidence rates of net cardiovascular outcome, stroke/SEE, major bleeding, ICH, and all-cause death during follow-up in the DOAC group according to H-SBP, excluding patients who received off-label doses of DOACs. There was no significant difference in the incidence rates of outcomes at any SBP level except for ICH (p = 0.045) at H-SBP ≥ 145 mmHg.
Figure 2 shows the adjusted HRs for patients treated with DOACs versus warfarin for net cardiovascular outcome, stroke/SEE, major bleeding, ICH, and all-cause death. There was no significant interaction between H-SBP and the relative effectiveness and safety of DOACs versus warfarin.
Forest plots of adjusted hazard ratios for patients treated with DOACs versus warfarin for net cardiovascular outcome, stroke/SEE, major bleeding, intracranial hemorrhage, and all-cause death. Bars represent 95% confidence intervals. Abbreviations are the same as in Fig. 1
Supplementary Table 4 shows adjusted HRs of DOAC versus warfarin in the four H-SBP categories, excluding patients who received off-label doses of DOACs, and the result was similar to that of Fig. 2.
Discussion
In this sub-analysis of a prespecified sub-cohort study of the ANAFIE Registry [4], we found that the incidence rates of net cardiovascular outcome, stroke/SEE, major bleeding, and ICH were significantly higher among patients with H-SBP ≥ 145 mmHg than those with H-SBP < 125 mmHg in the warfarin group. In the DOAC group, the incidence rates of these events also increased numerically, although the difference was not statistically significant. These results suggest that in elderly NVAF patients, strict BP control guided by H-BP is required, irrespective of the type of anticoagulation used. Meanwhile, the incidences of stroke/SEE, major bleeding, and ICH tended to be lower with DOACs than with warfarin among patients with H-SBP ≥ 145 mmHg, suggesting the merit of DOAC use for elderly AF patients, especially when the strict control of H-SBP is difficult.
The BAT study [13] (comprising patients both with and without AF), Fushimi AF Registry [14], and J-RHYTHM Registry [15] were all conducted during a time when warfarin was the predominant anticoagulant prescribed for stroke prevention, suggesting that the association of hypertension with major bleeding and ICH observed in these studies was perhaps at least partly a consequence of warfarin use.
The ENGAGE AF-TIMI 48 trial reported similar efficacy between edoxaban and warfarin in patients with AF and a history of hypertension, regardless of SBP stratification [5]. Edoxaban significantly reduced the incidence of major bleeding events and ICH versus warfarin across all SBP groups. The relative safety of edoxaban was most pronounced in patients with elevated diastolic BP. In a sub-analysis of the ROCKET AF trial, the benefit of rivaroxaban and warfarin to prevent stroke or systemic embolism was similar and did not vary by SBP group [8]. Similarly, the ARISTOTLE trial reported similar benefits for apixaban and warfarin for the prevention of stroke/SEE across BP groups [7]. In the RE-LY trial, there was no significant difference in the efficacy of dabigatran versus warfarin in hypertensive patients with NVAF for the incidence of stroke/SEE or major bleeds [5].
This study has some limitations that must be considered when interpreting the findings. The limitations of the observational ANAFIE Registry and this sub-cohort study have been described previously [3, 4]. Specifically, data on DOAC and warfarin use and H-BP were collected at baseline only, and any changes in OACs and antihypertensive drugs during the observation period were not considered.
Perspectives in Asia
In Asian countries with a disproportionate growth of aging populations and increased risk of comorbidities and complications, the benefits of anticoagulation in elderly NVAF patients with high BP should be considered against the risk of clinical outcomes. A review by Yasaka and Lip reported that the incidence of ICH is markedly higher in Japan and other East Asian countries than in countries outside East Asia [16]. In the current study, the incidence rates (per 100 person-years) of ICH at ≥145 mmHg were 3.43 and 1.31 in the warfarin and DOAC groups, respectively. The ICH incidence rates at H-SBP ≥ 145 mmHg appeared to be higher in this sub-cohort study than in global studies such as the ENGAGE AF-TIMI 48 trial [6]. Thus, strict BP control may be particularly important for elderly Asian patients with NVAF undergoing anticoagulant therapy.
Conclusions
Among elderly NVAF patients treated with warfarin, the incidence rates of net cardiovascular outcome, stroke/SEE, major bleeding, and ICH were significantly increased at H-SBP ≥ 145 mmHg versus <125 mmHg, but not in those treated with DOACs. Among elderly patients ≥75 years of age with NVAF who are receiving either anticoagulation with DOACs or warfarin, strict BP control guided by H-BP may be required.
References
Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke. 2021;16:217–21.
Ono K, Iwasaki YK, Akao M, Ikeda T, Ishii K, Inden Y, et al. JCS/JHRS 2020 Guideline on Pharmacotherapy of Cardiac Arrhythmias. Circ J. 2022;86:1790–924.
Yamashita T, Suzuki S, Inoue H, Akao M, Atarashi H, Ikeda T, et al. Two-year outcomes of more than 30 000 elderly patients with atrial fibrillation: results from the All Nippon AF In the Elderly (ANAFIE) Registry. Eur Heart J Qual Care Clin Outcomes. 2021;8:202–13.
Kario K, Hasebe N, Okumura K, Yamashita T, Akao M, Atarashi H, et al. Home blood pressure can predict the risk for stroke/bleeding events in elderly patients with nonvalvular atrial fibrillation from the ANAFIE Registry. Hypertension. 2022;79:2696–705.
Nagarakanti R, Wallentin L, Noack H, Brueckmann M, Reilly P, Clemens A, et al. Comparison of characteristics and outcomes of dabigatran versus warfarin in hypertensive patients with atrial fibrillation (from the RE-LY Trial). Am J Cardiol. 2015;116:1204–9.
Park S, Bergmark BA, Shi M, Lanz HJ, Chung N, Ruff CT, et al. Edoxaban versus warfarin stratified by average blood pressure in 19 679 patients with atrial fibrillation and a history of hypertension in the ENGAGE AF-TIMI 48 Trial. Hypertension. 2019;74:597–605.
Rao MP, Halvorsen S, Wojdyla D, Thomas L, Alexander JH, Hylek EM, et al. Blood pressure control and risk of stroke or systemic embolism in patients with atrial fibrillation: results from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) Trial. J Am Heart Assoc. 2015;4:e002015.
Vemulapalli S, Hellkamp AS, Jones WS, Piccini JP, Mahaffey KW, Becker RC, et al. Blood pressure control and stroke or bleeding risk in anticoagulated patients with atrial fibrillation: results from the ROCKET AF Trial. Am Heart J. 2016;178:74–84.
Inoue H, Yamashita T, Akao M, Atarashi H, Ikeda T, Okumura K, et al. Prospective observational study in elderly patients with non-valvular atrial fibrillation: rationale and design of the All Nippon AF In the Elderly (ANAFIE) Registry. J Cardiol. 2018; 72: 300–6. [Corrigendum in J Cardiol. 2022; 80: 375–6.]
Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–4.
Kario K, Hasebe N, Okumura K, Yamashita T, Akao M, Atarashi H, et al. High prevalence of masked uncontrolled morning hypertension in elderly non-valvular atrial fibrillation patients: home blood pressure substudy of the ANAFIE Registry. J Clin Hypertens (Greenwich). 2021;23:73–82.
Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481.
Toyoda K, Yasaka M, Uchiyama S, Nagao T, Gotoh J, Nagata K, et al. Blood pressure levels and bleeding events during antithrombotic therapy: the Bleeding with Antithrombotic Therapy (BAT) Study. Stroke. 2010;41:1440–4.
Ishii M, Ogawa H, Unoki T, An Y, Iguchi M, Masunaga N, et al. Relationship of hypertension and systolic blood pressure with the risk of stroke or bleeding in patients with atrial fibrillation: the Fushimi AF Registry. Am J Hypertens. 2017;30:1073–82.
Kodani E, Atarashi H, Inoue H, Okumura K, Yamashita T, Otsuka T, et al. Impact of blood pressure control on thromboembolism and major hemorrhage in patients with nonvalvular atrial fibrillation: a subanalysis of the J-RHYTHM Registry. J Am Heart Assoc. 2016;5:e004075.
Yasaka M, Lip GY. Impact of non-vitamin K antagonist oral anticoagulants on intracranial bleeding in Asian patients with non-valvular atrial fibrillation. Circ J. 2014;78:2367–72.
Acknowledgements
The authors wish to thank all individuals (physicians, nurses, institutional staff, and patients) involved in the ANAFIE Registry. They also thank IQVIA Services Japan KK and EP-CRSU for their partial support in the conduct of this Registry, and Helen Roberton of Edanz (www.edanz.com) for providing medical writing support, which was funded by Daiichi Sankyo Co., Ltd, in accordance with Good Publication Practice (GPP) 2022 guidelines (https://www.ismpp.org/gpp-2022). In addition, the authors thank Daisuke Chiba of Daiichi Sankyo Co., Ltd., for support in the preparation of the paper.
Funding
This study was supported by Daiichi Sankyo Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr KK received research grants from Bristol Myers Squibb, Bayer, Daiichi Sankyo, Omron Healthcare Inc., and A&D Inc, and remuneration from Daiichi Sankyo, Bayer, and Omron Healthcare Inc. Dr NH received research funding from Bristol Myers Squibb and Daiichi Sankyo, and remuneration from Daiichi Sankyo and Bayer. Dr KO received remuneration from Nippon Boehringer Ingelheim, Daiichi Sankyo, Johnson & Johnson, and Medtronic. Dr TY received research funding from Bristol Myers Squibb, Bayer, and Daiichi Sankyo, manuscript fees from Daiichi Sankyo and Bristol Myers Squibb, and remuneration from Daiichi Sankyo, Bayer, Pfizer Japan, and Bristol Myers Squibb. Dr MA received research funding from Bayer and Daiichi Sankyo, and remuneration from Bristol Myers Squibb, Nippon Boehringer Ingelheim, Bayer, and Daiichi Sankyo. Dr HA received remuneration from Daiichi Sankyo. Dr TI received research funding from Daiichi Sankyo and Bayer, and remuneration from Daiichi Sankyo, Bayer, Nippon Boehringer Ingelheim, and Bristol Myers Squibb. Dr YK received remuneration from Daiichi Sankyo, Bayer, and Nippon Boehringer Ingelheim. Dr WS received research funding from Bristol Myers Squibb, Daiichi Sankyo, and Nippon Boehringer Ingelheim, and patent royalties/licensing fees from Daiichi Sankyo, Pfizer Japan, Bristol Myers Squibb, Bayer, and Nippon Boehringer Ingelheim. Dr SS received research funding from Mitsubishi-Tanabe and Daiichi Sankyo, and remuneration from Bristol Myers Squibb and Daiichi Sankyo. Dr HT received research funding from Daiichi Sankyo and Nippon Boehringer Ingelheim, remuneration from Daiichi Sankyo, Bayer, Nippon Boehringer Ingelheim, and Pfizer Japan, scholarship funding from Daiichi Sankyo, and consultancy fees from Pfizer Japan, Bayer, and Nippon Boehringer Ingelheim. Dr KT received lecture honoraria from Daiichi Sankyo, Otsuka, Novartis, Abbott, Bayer Yakuhin, and Bristol Myers Squibb outside the submitted work. Dr AH participated in a course endowed by Boston Scientific Japan, has received research funding from Daiichi Sankyo and Bayer, and remuneration from Bayer, Daiichi Sankyo, Bristol Myers Squibb, and Nippon Boehringer Ingelheim. Dr MY received research funding from Nippon Boehringer Ingelheim, and remuneration from Nippon Boehringer Ingelheim, Daiichi Sankyo, Bayer, Bristol Myers Squibb, and Pfizer Japan. Dr TY acted as an Advisory Board member of Daiichi Sankyo and received remuneration from Daiichi Sankyo and Bristol Myers Squibb. Dr ST received research funding from Nippon Boehringer Ingelheim and remuneration from Daiichi Sankyo, Sanofi, Takeda, Chugai Pharmaceutical, Solasia Pharma, Bayer, Sysmex, Nipro, NapaJen Pharma, Gunze, Kaneka, Kringle Pharma and Atworking. TK, Dr YM, and AT are employees of Daiichi Sankyo. Dr HI received remuneration from Daiichi Sankyo, Bristol Myers Squibb, and Nippon Boehringer Ingelheim.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kario, K., Hasebe, N., Okumura, K. et al. Anticoagulant therapy and home blood pressure-associated risk for stroke/bleeding events in elderly patients with non-valvular atrial fibrillation: the sub-cohort study of ANAFIE registry. Hypertens Res 46, 2575–2582 (2023). https://doi.org/10.1038/s41440-023-01361-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01361-4