Abstract
We previously reported that brain atrophy was more severe and progressed more rapidly in patients with end-stage kidney disease on peritoneal dialysis (PD) than those with non-dialysis-dependent chronic kidney disease. However, it remains unknown whether there is a difference between patients on PD and hemodialysis (HD). In total, 73 PD and 34 HD patients who underwent brain magnetic resonance imaging (MRI) were recruited for a cross-sectional analysis. Among them, 42 PD and 25 HD patients who underwent a second brain MRI after 2 years were recruited for a longitudinal analysis. T1-weighted MRI images were analyzed. Total gray matter volume (GMV), total white matter volume, and cerebrospinal fluid volume were segmented, and each volume was quantified using statistical parametric mapping software. The ratio of GMV (GMR) was calculated by dividing GMV by intracranial volume, to adjust for variations in head size. We compared GMR between PD and HD patients in the cross-sectional analysis and the annual change in GMR (AC-GMR) in the longitudinal analysis. In the cross-sectional analysis, age- and sex-adjusted GMR was significantly lower in PD than HD patients [least square mean (LSM): 39.2% vs. 40.0%, P = 0.018]. AC-GMR was significantly greater in PD than HD patients and this difference remained significant even after adjustment for potential confounding factors (LSM: −0.68 vs. −0.28 percentage-points/year, P = 0.011). In conclusion, the present study demonstrated a more rapid progression of brain atrophy in PD patients compared with HD patients.

We demonstrated that decline in GMR progressed significantly more rapidly in PD than HD patients independent of potential confounding factors. GMR gray matter volume ratio, HD hemodialysis, PD peritoneal dialysis.
Similar content being viewed by others
Introduction
Recently, evidence has accumulated on cognitive impairment in patients with chronic kidney disease (CKD) [1,2,3]. While various associated factors of cognitive impairment have been proposed, brain atrophy has been reported to be associated with cognitive impairment in various conditions including end-stage kidney disease [4,5,6,7,8,9,10]. Previously, we demonstrated a significant association between brain atrophy and cognitive impairment in patients with non-dialysis-dependent CKD (NDD-CKD) [3].
It was reported that brain atrophy is severe in hemodialysis (HD) patients even at younger age [11]. A possible mechanism of brain atrophy in HD patients is brain hypoperfusion during HD sessions. It is believed that hypotension during HD sessions may decrease brain blood flow and induce brain infarction, resulting in brain atrophy. We previously examined the association between HD-related hypotension and brain atrophy in patients on chronic HD using magnetic resonance imaging (MRI) [12]. MRI was conducted twice in 32 HD patients (at study entry and after 3 years), and longitudinal change in brain frontal lobe volume during the 3-year period was measured. All dialysis-related hypotension episodes (i.e., sudden drop in blood pressure during HD, defined as fall in systolic blood pressure (SBP) > 50 mmHg within 30 min of HD) during the 3-year period were identified from medical records and analyzed. The results revealed a significant association between the number of dialysis-related hypotension episodes and changes in frontal brain volume, suggesting that dialysis-related hypotension may play a role in progressive frontal lobe atrophy in HD patients.
In contrast, very little is known on brain atrophy in peritoneal dialysis (PD) patients. Therefore, we examined annual change in brain volume over 2 years in PD patients and compared the results with those with NDD-CKD [13]. Gray matter volume (GMV) decline was two to three times greater in PD patients than NDD-CKD patients, suggesting brain atrophy progresses rapidly in PD patients. However, it remains unclear whether there is any difference in severity of brain atrophy between PD and HD patients.
Therefore, in the present study, we examined brain volume and its annual change in PD patients and compared them with those in HD patients in a cross-sectional and 2-year longitudinal analyses.
Methods
Subjects
To investigate the degree of progression of cerebro- and cardiovascular complication in NDD-CKD, HD, and PD patients, we have been conducting an observational study named the Observational Study on Cerebro- and Cardiovascular Complication in Non-Dialysis-Dependent, Hemodialysis, and Peritoneal Dialysis Patients with Chronic Kidney Disease (VCOHP Study) since December 2008. Inclusion criteria are as follows: (1) patients aged 20–80 years at the time of study entry; and (2) NDD-CKD patients with estimated glomerular filtration rate less than 60 mL/min/1.73 m2 irrespective of urinalysis findings (CKD stages G3–5) or patients with end-stage kidney disease on either HD or PD, who started dialysis within 2 years of study entry. Exclusion criteria are as follows: (1) pregnant women, or women with potential to become pregnant, (2) patients who previously underwent another dialysis therapy for over 3 months, (3) patients who previously underwent kidney transplantation, and (4) patients with previous history of brain injury, such as symptomatic stroke, traumatic brain injury, brain tumor, or neuropsychiatric disease. By December 2013, 259 (106 NDD-CKD, 75 PD, 78 HD) patients were entered into the study. We excluded 46 patients (two PD patients and 44 HD patients) whose MRI data at study entry were unavailable, and 106 NDD-CKD patients. The remaining 107 patients (73 PD patients and 34 HD patients) were recruited for cross-sectional analysis. Among the 107 patients, 40 could not undergo a second MRI after 2 years because of various factors. Therefore, the remaining 67 patients (42 PD patients and 25 HD patients) who underwent a second MRI after 2 years were included for the longitudinal analysis. All patients provided informed consent. The Kyushu University Institutional Review Board approved all procedures (#23-112) and the study was registered in the UMIN clinical trial registry as the VCOHP Study (UMIN000001589).
More than 50% of HD patients were excluded from the longitudinal study, and the reason can be attributed to the following: This study was conducted as part of the VCOHP Study, where various examinations, such as brain MRI, computed tomography, bone density, carotid artery ultrasound, and echocardiography were performed as part of a physical health checkup at Kyushu University Hospital. These examinations were scheduled to be performed within a single day and were carried out as planned until the middle of the study period. However, due to the discontinuation of the physical health checkup service on September 30, 2013, it became challenging to complete all the examinations within a single day. Consequently, the number of patients who participated in this study but could not undergo all the examinations increased, leading to a higher number of excluded cases. This factor primarily contributed to the decrease in the number of cases in the longitudinal study.
Clinical evaluation and laboratory measurements
All examinations were performed at the Medical Examination Center of Kyushu University Hospital without insurance. All patients underwent brain MRI scans. Clinical parameters were measured on the same day. Blood pressure in the brachial artery was measured in the sitting position after a 10-min rest. Height and weight of participants were measured, and body mass index was calculated (kg/m2).
Blood samples were collected on the same day of undergoing MRI on the non-dialysis day and were analyzed at the laboratory of Kyushu University Hospital, except for whole parathyroid hormone, which was analyzed at a commercial laboratory (SRL Inc., Fukuoka, Japan). Serum chemistry values were measured using an autoanalyzer with standard procedures (Hitachi 911 Auto Analyzer, Hitachi Co. Ltd, Tokyo, Japan). Serum-corrected calcium levels were adjusted to serum albumin levels according to Payne’s formula, which is commonly used in patients with hypoalbuminemia. Hemoglobin A1c (HbA1c) values were measured according to the National Glycohemoglobin Standardization Program method [14].
Imaging data
Brain MRI scans were acquired from participants using a 3.0 T Philips Achieva magnetic resonance scanner (Philips Health Care, Best, the Netherlands) at Kyushu University Hospital. No major hardware upgrades occurred during the study period. All patients were scanned with identical pulse sequences: 44 contiguous, 3.0-mm thick axial planes of three-dimensional T1-weighted images (magnetization-prepared rapid acquisition of gradient echo: echo time, 3.7 ms; flip angle, 8; voxel size, 0.47 × 0.47 × 3 mm). Imaging data from MRI were analyzed by a single investigator who was blind to clinical information as previously described [15,16,17]. We used Statistical Parametric Mapping 8 software (SPM8; Wellcome Department of Imaging Neuroscience, University College London, London, UK) to preprocess brain images. The segmentation algorithm from SPM8 was applied to every T1-weighted MRI scan to extract tissue maps corresponding to gray matter, white matter, and cerebrospinal fluid (Supplementary data, Fig. S1).
We applied these processes using the MATLAB file “cg_vbm_optimized” (http://dbm.neuro.uni-jena.de/vbm.html). The voxel values of segmented images did not consist of binary (i.e., 0 or 1), rather 256-level (i.e., between 0/255 and 255/255) signal intensities, according to their tissue probability. The linear-normalized, segmented images were restored to the native space using the inverse normalization parameters calculated in normalizing each MR image to the Talairach space, to determine the volumes of each segment. The actual volumes of the entire normalized, segmented, and restored gray matter, white matter, and cerebral spinal fluid space images were determined automatically by summing voxel volumes multiplied by each voxel value and dividing by 255.
To normalize for head size variability, the GMV ratio (GMR) and white matter volume (WMV) ratio (WMR) were calculated as percentages of total intracranial volume (sum of GMV, WMV, and cerebrospinal fluid volume). Total brain volume ratio (TBR) was defined as sum of GMR and WMR.
Annual change in GMR (AC-GMR) was determined from the following equation:
AC-GMR = (GMR after 2 years − baseline GMR) × 365 / interval (days) between the first and second MRI. Moreover, to eliminate the effect of baseline GMR, we calculated the annual percentage change in GMR, determined by the following equation: annual percentage change in GMR = (change in GMR ÷ baseline GMR) × 365 / interval (days) × 100.
Annual changes in WMR and TBR were determined from the following equations:
Annual change in WMR = (WMR after 2 years − baseline WMR) × 365 / interval (days) between the first and second MRI.
Annual change in TBR = (TBR after 2 years − baseline TBR) × 365 / interval (days) between the first and second MRI.
Statistical analyses
The Mann–Whitney test and chi-square test were used as appropriate to analyze differences in baseline parameters of patients. Pearson’s correlation coefficients were calculated to determine the correlation between GMV, WMV, GMR, or WMR and age in the cross-sectional study. Multivariable-adjusted least square mean values of AC-GMR were calculated using analysis of covariance. We performed univariable, age- and sex-adjusted, and multivariable regression analyses to confirm differences in GMR between PD and HD patients. We entered age, sex, diabetes mellitus, previous history of cardiovascular disease, current smoking habits, SBP, hemoglobin, baseline GMR, and log-transformed brain natriuretic peptide (BNP) as covariates in the multivariable regression model by the forced entry method. We selected these covariates based on findings from our previous study, as they are believed to influence the progression of brain atrophy [13]. All statistical analyses were performed using JMP version 11.0 software (SAS Institute, Inc., Cary, NC, USA) and RStudio software.
Results
Cross-sectional analysis
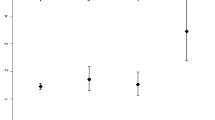
Baseline parameters and laboratory findings of the 107 patients are shown in Table 1. Age, sex, systolic and diastolic blood pressure, and the prevalence of diabetes mellitus, current smoking habits, and alcohol consumption were comparable between PD and HD patients. Body mass index (P = 0.030) and serum levels of urea nitrogen (BUN) (P < 0.001), creatinine (P < 0.001), uric acid (P < 0.001), phosphate (P = 0.038), and β2-microglobulin (P < 0.001) were significantly higher, while hemoglobin (P < 0.001) and serum levels of total protein (P < 0.001), albumin (P < 0.001), and glycated albumin (GA) (P < 0.001) were significantly lower in PD than HD patients. GMV and GMR were negatively associated with age in PD and HD patients, whereas WMV and WMR were not associated with age in either group. Median (interquartile range) GMR at baseline was 38.8% (36.7%–40.5%) and 39.1% (37.6%–41.1%) in PD and HD patients, respectively (P = 0.268), and WMR was 40.0% (38.8%–41.0%) and 39.9% (39.1%–41.1%) in PD and HD patients, respectively (P = 0.971). Significant correlations were observed between age and both of GMR (P < 0.001) and GMV (P < 0.001) in PD patients. In HD patients, the correlation between age and GMR was significant (P = 0.017), while the correlation between age and GMV was marginally significant (P = 0.060) (Fig. 1A, B). Age- and sex-adjusted GMR was significantly lower in PD than HD patients (Fig. 2A).
Inverse association of GMV and GMR with age. A The association of GMV and WMV with age in PD (closed circles; n = 73) and HD patients (open circles; n = 34). B The association of GMR and WMR with age in PD (closed circles; n = 73) and HD patients (open circles; n = 34). Pearson’s correlation coefficients (r) were calculated to determine the correlation between GMV, WMV, GMR, or WMR and age. GMR gray matter volume ratio, GMV gray matter volume, HD hemodialysis, PD peritoneal dialysis, WMR white matter volume ratio, WMV white matter volume
GMR in PD and HD patients at baseline and after 2 years in the cross-sectional and longitudinal analyses. A In the cross-sectional analysis, age- and sex-adjusted GMR is significantly lower in PD patients (closed box, n = 73) than HD patients (open box, n = 34) at baseline. B In the longitudinal analysis, age- and sex-adjusted GMR is significantly lower in PD patients (closed box, n = 42) than HD patients (open box, n = 25) at baseline and after 2 years, and significantly lower after 2 years compared with baseline in both PD and HD patients. Data are presented as least square mean ± standard error. †P < 0.001 vs. GMR at baseline. GMR gray matter volume ratio, HD hemodialysis, PD peritoneal dialysis
Longitudinal analysis
Among the 107 patients, 40 (31 PD and 9 HD) were excluded, and the remaining 67 were included for the longitudinal analysis. Baseline parameters and laboratory findings of the 67 included and 40 excluded patients are shown in Supplementary data, Table S1. The prevalence of diabetes mellitus and levels of GA, HbA1c, and BNP were significantly higher in the excluded patients on PD, while ferritin level was significantly lower in excluded patients on HD. There were no differences in the other variables between the two groups.
Age- and sex-adjusted GMRs at baseline and after 2 years were significantly lower in PD than HD patients (Fig. 2A).
Then, we conducted a univariable regression analysis with the objective of finding the factors associated with AC-GMR, indicating that male gender, PD, and lower levels of serum total protein and albumin were significantly or almost significantly associated with the outcome (Supplementary data, Table S2). We considered that the associations of lower levels of serum total protein and albumin with AC-GMR were attributed to that of PD with AC-GMR, because the correlations between PD and the levels of total protein (r = 0.661) and albumin (r = 0.739) were remarkably strong.
AC-GMR was significantly greater in PD than HD patients. This difference remained significant, even after adjustment for various confounding factors, such as age, sex, diabetes mellitus, previous history of cardiovascular disease, smoking habits, SBP, hemoglobin concentration, baseline GMR, and log-transformed BNP by analysis of covariance (PD, −0.68 ± 0.12 percentage-points/year; HD, −0.28 ± 0.15 percentage-points/year, P = 0.011) (Fig. 3) and multivariable regression analyses (Table 2).
The annual change in TBR was also significantly greater in PD than HD patients even after adjustment for the above various confounding factors (PD, −0.49 ± 0.12 percentage-points/year; HD, −0.17 ± 0.13 percentage-points/year, P = 0.020) (Supplementary Fig. S2), while the annual change in WMR was comparable between the 2 groups (PD, 0.21 ± 0.16 percentage-points/year; HD, 0.12 ± 0.19 percentage-points/year, P = 0.63) (Supplementary Figure S3).
Moreover, to eliminate the effect of baseline GMR, we performed multivariable regression analysis for the annual percentage change in GMR (calculated as described above). We found this percentage change was also greater in PD than HD patients, even following analysis with the final multivariable model (Supplementary data, Table S3). These findings were similar when the analyses were restricted to only the hippocampal GMR instead of whole brain GMR (Supplementary data, Tables S4 and S5).
An analysis of four brain regions (frontal, temporal, parietal, and occipital lobes) revealed that the results for the frontal and parietal lobes were consistent with those of the whole brain. A similar trend, albeit not statistically significant, was observed for the temporal lobe, while the difference was comparable and not statistically significant in the occipital lobe between PD and HD patients (Supplementary data, Table S6).
Discussion
Herein, we showed that age- and sex-adjusted GMR was significantly lower in PD than HD patients in the cross-sectional study. Moreover, annual decline in GMR was significantly greater in patients on PD than those on HD, even after adjustment for potential confounding factors in the longitudinal analysis in the longitudinal study. Furthermore, decrease in hippocampal GMR, as well as GMRs of frontal and parietal lobe, was also significantly greater in patients on PD than those on HD.
It has been reported that brain atrophy is prominent in HD patients. The primary cause is believed to be decreased cerebral blood flow and oxygen supply to brain tissue because of repeated decline in blood pressure during HD sessions. This notion is supported by our previous report which showed that the total number of sudden hypotension events defined as a drop in SBP of at least 50 mmHg within 30 min during HD sessions was associated with reduction rate of frontal lobe volume evaluated using MRI [12].
The previous reports by Taki et al. [17], which evaluated GMR and WMR using the same methods as in this study, showed similar results to our findings. Specifically, GMR decreased with aging, while WMR did not exhibit significant changes. This difference in response to aging can be attributed to the distinct nature of gray matter and white matter in the central nervous system. Gray matter, being the region where nerve cell bodies are located, tends to decrease in size as the number of nerve cell bodies reduces with age, while white matter, housing nerve cell axons, maintains its volume due to the stable length of axons. We propose that this fundamental difference in structure and function accounts for the lack of significant changes in WMR between the two groups in the present study.
Unlike HD patients, PD patients are generally hemodynamically stable. Therefore, we hypothesized that brain atrophy could be prevented in these patients. To explore this hypothesis, we compared changes in brain volume in PD patients and NDD-CKD patients in a prospective observational study [13]. Unexpectedly, brain atrophy was found to progress significantly more rapidly in PD patients. At the time of the study, our hypothesis had not been completely ruled out, as comparisons between PD and HD patients had not yet been performed. However, the present results revealed that brain atrophy progressed significantly more rapidly in PD than HD patients, suggesting the primary cause of brain atrophy in dialysis patients may be some factor other than decreased blood pressure during HD sessions.
Although brain atrophy is believed to be associated with cognitive impairment, there are few reports on the relationship between brain atrophy and cognitive function in CKD patients. Therefore, we previously performed brain MRI and conducted the trail making test (TMT) in 95 NDD-CKD patients, and assessed the correlation between GMR and TMT scores using multivariable regression analysis [1]. GMR was significantly inversely correlated with TMT scores. These correlations remained significant even after adjustment for confounding factors. Given these observations, we believe cognitive function declines when brain atrophy is prominent. Although the relationship between brain atrophy and cognitive function was not examined in the present study, PD patients may be at higher risk of cognitive decline because of more rapid progression of brain atrophy than HD patients. However, in contrast to our findings, recent reports showed that cognitive function is maintained in PD patients rather than in HD patients [18,19,20]. We lack any understanding of the underlying reason for this phenomenon, which necessitates validation through subsequent studies.
More recently, Chen et al. [21] reported that HD patients had significantly smaller volumes of the bilateral hippocampus than healthy controls and hippocampal subfield volumes were associated with neuropsychological test scores, duration of disease and hemoglobin levels. Thus, we also examined the change in the hippocampal GMR in these patients and found the significantly greater reduction in the hippocampal GMR in PD patients compared to HD patients, as seen in the whole brain GMR.
The explanation for the discrepancy between the results in the present study and the findings in these recent reports is not clear, but possible explanations are as follows: all of the aforementioned reports on cognitive impairment were observational studies, not randomized controlled trials. Selection bias in determining dialysis modalities may have therefore affected the results. Alternatively, the present findings were based on a small sample size and are not conclusive. Therefore, further extensive research, including studies of brain volume and cognitive testing, are required to resolve this discrepancy. Although randomized controlled trials are ideal, it is impossible to randomly select dialysis modalities. Therefore, prospective observational studies with a larger number of subjects using statistical adjustment for treatment selection bias are required to resolve this discrepancy.
Similar to the previous report comparing brain atrophy between PD and NDD-CKD patients [13], as many as 37% of patients who underwent the first MRI examination did not undergo the second in the present study. In this regard, the following variables were significantly different between the included and excluded patients: the prevalence of diabetes mellitus and levels of GA, HbA1c, and BNP were significantly higher in the excluded patients on PD, while ferritin level was significantly lower in the excluded patients on HD (Supplementary data, Table S1). Many diabetic patients who were considered to be susceptible to brain atrophy were excluded from PD patients. Brain atrophy among PD patients would have been more prevalent if these cases were included. Therefore, we believe the excluded cases were unlikely to have affected the outcomes of this study.
Anemia has been shown to be a risk factor for cognitive impairment in dialysis patients. Despite the lack of clinically apparent neurological symptoms or morphological abnormalities, patients with CKD accompanied by anemia present with impairment in cerebral blood flow and oxygen metabolism, which are reportedly prominent in the frontal cortex [22,23,24]. Moreover, correction of anemia with recombinant human erythropoietin has been shown to be associated with significant improvement in cognitive function [25, 26]. It has been reported that decreased hemoglobin levels were associated with cortical thickness [27]. In the present study, hemoglobin levels were significantly lower in PD patients, which may have affected the results. However, as shown in Table 2, the significance of the results was maintained even if hemoglobin level was adjusted. Moreover, in all patients in this study, hemoglobin level was not significantly correlated with either GMR at time of enrollment or AC-GMR. Thus, we consider that anemia might not mediate the association between dialysis modality and progression of brain atrophy observed in this study.
It is possible that the difference in AC-GMR in this study may be attributed by differences in uremia management in PD patients compared to HD patients. As shown in Table 1 and Supplementary data, Table S1, significant differences were found in hemoglobin, total protein, albumin, BUN, creatinine, uric acid, phosphate, and β2-microglobulin levels between the two groups. Therefore, these differences in the uremic status could have influenced the results in this study. If we had access to Kt/V and residual kidney function data, we could have compared and adjusted for uremic status more effectively. However, unfortunately, we did not obtain such data in our study. Additionally, it is important to note that the data collected for HD patients in this study were obtained on the non-dialysis day (next day of HD), rather than immediately before HD. As a result, the values of BUN, creatinine, phosphate, β2-microglobulin, and other parameters are noticeably lower compared to the data of HD patients typically evaluated in clinical practice in our country, which is usually collected just before HD of three days after the last HD session. In contrast, the data for PD patients in this study aligned with the observed patterns in clinical practice.
In PD patients, serum levels of total protein and albumin often exhibit lower values, which are likely attributed to their leakage into the PD solution, rather than being indicative of malnutrition or inflammation. Hence, the observed lower serum levels of total protein and albumin in PD patients, as compared to HD patients in this study, are common findings. Although significant or trend-based relationships of these variables with AC-GMR were discerned in the univariable regression analysis, we chose not to include them as adjustment factors in the multivariable regression analysis due to the existence of multicollinearity between these variables and the treatment method. We propose that the significant or trend-based relationships observed in the univariable analysis is not an independent effect, but rather a consequence of the treatment method. This assertion is strengthened by the lack of any associations between these variables and AC-GMR when analyzed individually for each treatment method. Additionally, to the best of our knowledge, no prior reports have suggested a significant impact of hypoalbuminemia on brain atrophy, suggesting that serum albumin concentration might not have a significant impact on the results of this study.
The strengths of the present study were as follows. First, this is the first detailed report comparing brain atrophy between PD and HD patients. Second, compared with previous reports, brain atrophy was determined more precisely and objectively using the SPM-based segmentation method [11, 12, 28, 29]. Third, we performed cross-sectional and 2-year longitudinal analyses. The both cross-sectional and longitudinal analyses revealed more rapid declines in GMR with aging in PD patients compared with HD patients.
This study had several limitations. First, the number of patients was small. Second, the 2-year observation period was short for evaluating changes in brain volume in the longitudinal analysis. We believe these limitations may have been partially alleviated using the SPM-based method through which we precisely and objectively analyzed MRI data. Third, our results may have been biased by the exclusion of patients who did not undergo a second MRI scan after 2 years. However, we believe this bias is unlikely to have altered our results because of the observations that the prevalence of diabetes mellitus and serum levels of GA and HbA1c were significantly higher in excluded patients than included patients (Supplementary data, Table S1), suggesting the annual decline in GMR would have been more rapid in PD patients if none of the patients had been excluded from this study. Fourth, as described before, we did not have data on Kt/V and urinary volume, we could not compare and adjust the dialysis dose. Nonetheless, we confirmed that both groups consisted of outpatients receiving regular treatment and dialysis was adequately performed in each group. In our investigation of the relationship between BUN and AC-GMR, we observed no correlation in both PD and HD patients. Despite these limitations, we strongly believe that the comparison of the progression of brain atrophy between PD and HD patients we conducted in this study is highly significant.
Brain atrophy represents a final common pathway for pathological process and is widely acknowledged as a reliable MRI finding in cerebral small vessel diseases, which are particularly significant due to their potential association with cognitive function. However, in this study, we have not yet analyzed or reported data related to these lesions because our sole focus was to investigate brain atrophy. We plan to investigate these lesions in future investigations.
Asian perspectives
United States Renal Data System 2023 Annual Data Report reported a persistent theme as the high and growing rates of treated end-stage kidney disease in Asia. Asian countries or regions were also relatively overrepresented among the areas with the fastest increases in incidence of end-stage kidney disease [30]. Our present study demonstrated unfavorable outcomes for PD, potentially discouraging its selection as a kidney replacement therapy. Consequently, this impact could be especially noteworthy in Asia, where the number of patients with end-stage kidney disease is increasing the most rapidly worldwide.
Conclusion
In conclusion, decline in GMR was significantly greater in PD than HD patients independent of potential confounding factors. Further studies are required to determine the mechanism of rapid progression of brain atrophy in PD patients, and how this can be prevented.
References
Tsuruya K, Yoshida H, Haruyama N, Fujisaki K, Hirakata H, Kitazono T. Clinical significance of fronto-temporal gray matter atrophy in executive dysfunction in patients with chronic kidney disease: The VCOHP Study. PLoS One. 2015;10:e0143706.
Bugnicourt JM, Godefroy O, Chillon JM, Choukroun G, Massy ZA. Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. J Am Soc Nephrol. 2013;24:353–63.
Kurella Tamura M, Wadley V, Yaffe K, McClure LA, Howard G, Go R, et al. Kidney function and cognitive impairment in US adults: the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Am J Kidney Dis. 2008;52:227–34.
Franc DT, Kodl CT, Mueller BA, Muetzel RL, Lim KO, Seaquist ER. High connectivity between reduced cortical thickness and disrupted white matter tracts in long-standing type 1 diabetes. Diabetes. 2011;60:315–9.
Hayashi K, Kurioka S, Yamaguchi T, Morita M, Kanazawa I, Takase H, et al. Association of cognitive dysfunction with hippocampal atrophy in elderly Japanese people with type 2 diabetes. Diabetes Res Clin Pract. 2011;94:180–5.
Roberts RO, Knopman DS, Przybelski SA, Mielke MM, Kantarci K, Preboske GM, et al. Association of type 2 diabetes with brain atrophy and cognitive impairment. Neurology. 2014;82:1132–41.
Nagano-Saito A, Washimi Y, Arahata Y, Kachi T, Lerch JP, Evans AC, et al. Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology. 2005;64:224–9.
Sluimer JD, van der Flier WM, Karas GB, Fox NC, Scheltens P, Barkhof F, Vrenken H. Whole-brain atrophy rate and cognitive decline: longitudinal MR study of memory clinic patients. Radiology. 2008;248:590–8.
Jokinen H, Lipsanen J, Schmidt R, Fazekas F, Gouw AA, van der Flier WM, et al. Brain atrophy accelerates cognitive decline in cerebral small vessel disease: the LADIS study. Neurology. 2012;78:1785–92.
Zhang LJ, Wen J, Ni L, Zhong J, Liang X, Zheng G, Lu GM. Predominant gray matter volume loss in patients with end-stage renal disease: a voxel-based morphometry study. Metab Brain Dis. 2013;28:647–54.
Yoshimitsu T, Hirakata H, Fujii K, Kanai H, Hirakata E, Higashi H, et al. Cerebral ischemia as a causative mechanism for rapid progression of brain atrophy in chronic hemodialysis patients. Clin Nephrol. 2000;53:445–51.
Mizumasa T, Hirakata H, Yoshimitsu T, Hirakata E, Kubo M, Kashiwagi M, et al. Dialysis-related hypotension as a cause of progressive frontal lobe atrophy in chronic hemodialysis patients: a 3-year prospective study. Nephron Clin Pract. 2004;97:c23–30.
Tsuruya K, Yoshida H, Kuroki Y, Nagata M, Mizumasa T, Mitsuiki K, et al. Brain atrophy in peritoneal dialysis and CKD stages 3-5: a cross-sectional and longitudinal study. Am J Kidney Dis. 2015;65:312–21.
Consensus Committee. Consensus statement on the worldwide standardization of the hemoglobin A1C measurement: the American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Diabetes Care. 2007;30:2399–2400.
Yoshida H, Kawaguchi A, Tsuruya K. Radial basis function-sparse partial least squares for application to brain imaging data. Comput Math Methods Med. 2013;2013:591032.
Taki Y, Kinomura S, Sato K, Goto R, Kawashima R, Fukuda H. A longitudinal study of gray matter volume decline with age and modifying factors. Neurobiol Aging. 2011;32:907–15.
Taki Y, Thyreau B, Kinomura S, Sato K, Goto R, Kawashima R, Fukuda H. Correlations among brain gray matter volumes, age, gender, and hemisphere in healthy individuals. PLoS One. 2011;6:e22734.
Wolfgram DF, Szabo A, Murray AM, Whittle J. Risk of dementia in peritoneal dialysis patients compared with hemodialysis patients. Perit Dial Int. 2015;35:189–98.
O'lone E, Connors M, Masson P, Wu S, Kelly PJ, Gillespie D, et al. Cognition in people with end-stage kidney disease treated with hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2016;67:925–35.
Tian X, Guo X, Xia X, Yu H, Li X, Jiang A. The comparison of cognitive function and risk of dementia in CKD patients under peritoneal dialysis and hemodialysis: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2019;98:e14390.
Chen HJ, Qiu J, Qi Y, Fu L, Fu Q, Wu W, et al. Hippocampal subfield morphology in regular hemodialysis patients. Nephrol Dial Transplant. 2023;38:992–1001.
Kuwabara Y, Sasaki M, Hirakata H, Koga H, Nakagawa M, Chen T, et al. Cerebral blood flow and vasodilatory capacity in anemia secondary to chronic renal failure. Kidney Int. 2002;61:564–9.
Kanai H, Hirakata H, Nakane H, Fujii K, Hirakata E, Ibayashi S, Kuwabara Y. Depressed cerebral oxygen metabolism in patients with chronic renal failure: a positron emission tomography study. Am J Kidney Dis. 2001;38:S129–S133.
Hirakata H, Yao H, Osato S, Ibayashi S, Onoyama K, Otsuka M, et al. CBF and oxygen metabolism in hemodialysis patients: effects of anemia correction with recombinant human EPO. Am J Physiol. 1992;262:F737–F743.
Grimm G, Stockenhuber F, Schneeweiss B, Madl C, Zeitlhofer J, Schneider B. Improvement of brain function in hemodialysis patients treated with erythropoietin. Kidney Int. 1990;38:480–6.
Temple RM, Langan SJ, Deary IJ, Winney RJ. Recombinant erythropoietin improves cognitive function in chronic haemodialysis patients. Nephrol Dial Transplant. 1992;7:240–5.
Park SE, Kim H, Lee J, Lee NK, Hwang JW, Yang JJ, et al. Decreased hemoglobin levels, cerebral small-vessel disease, and cortical atrophy: among cognitively normal elderly women and men. Int Psychogeriatr. 2016;28:147–56.
Drew DA, Bhadelia R, Tighiouart H, Novak V, Scott TM, Lou KV, et al. Anatomic brain disease in hemodialysis patients: a cross-sectional study. Am J Kidney Dis. 2013;61:271–8.
Kamata T, Hishida A, Takita T, Sawada K, Ikegaya N, Maruyama Y, et al. Morphologic abnormalities in the brain of chronically hemodialyzed patients without cerebrovascular disease. Am J Nephrol. 2000;20:27–31.
USRDS 2023 Annual Report, End Stage Renal Disease: Chapter 11, International Comparison. https://usrds-adr.niddk.nih.gov/2023/end-stage-renal-disease/11-international-comparisons. Accessed 10 November (2023).
Acknowledgements
We greatly appreciate Prof. Takashi Yoshiura (Department of Radiology, Kagoshima University Graduate School of Medical and Dental Sciences) for technical advice on the analysis of the MRI data and the staff of the Advanced Preventive Medical Center in Kyushu University Hospital for their kind cooperation. We also appreciate Drs. Koji Mitsuiki (Harasanshin Hospital), Hideki Hirakata (Fukuoka Renal Clinic), Takashi Nagae (National Hospital Organization Fukuoka-higashi Medical Center), Masatomo Taniguchi (Fukuoka Renal Clinic), Yasuhiro Kawai (Steel Memorial Yawata Hospital), Yasuhisa Tamura (JCHO Kyushu Hospital), Michiya Shinozaki (Shin-Yurigaoka General Hospital), Hidetoshi Kanai (Kokura Memorial Hospital), Takashi Deguchi (Hamanomachi Hospital), Dai Matsuo (Munakata Medical Association Hospital), Izumi Shimano (Munakata Medical Association Hospital), Yusuke Kuroki (National Hospital Organization Fukuoka-higashi Medical Center), Itsuko Ishida (Gofukumachi Kidney Clinic, Harasanshin Hospital), Kei Hori (Hori Clinic), Shotaro Ohnaka (Tagawa Municipal Hospital), Hiroshi Tsuruta (Kokura Daiichi Hospital), Toru Mizumasa (Kyushu Central Hospital), Makoto Hirakawa (Hirakawa Internal Medicine Clinic), Takahiro Yoshimitsu (Fukuoka Mirai Hospital), Kiyoshi Ikeda (Ikeda Vascular Access Dialysis and Internal Medicine Clinic), Koichiro Goto (Goto Clinic), Chiaki Miishima (Miishima Internal Medicine Clinic), Kiichiro Ueno (Ueno Hospital), Takashi Ono (Toma Clinic), Toru Sanai (Fukumitsu Clinic), Takashi Ando (Hakozaki Park Internal Medicine Clinic) for their helpful enrollment of patients to this study. We thank Richard Robins, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this manuscript.
Funding
This work was not financially supported by any pharmaceutical company or funding agency.
Author information
Authors and Affiliations
Contributions
Study conception and design: KaT, HY; data acquisition: KaT, HY, SY, NH, ST, AT, ME, KF, KuT, TN, KM; data analysis/interpretation: KaT, HY; statistical analysis: KaT, HY; supervision or mentorship: TK. Each author made important intellectual contributions during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. KaT takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted, and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no comperting interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsuruya, K., Yoshida, H., Yamada, S. et al. More rapid progression of brain atrophy in patients on peritoneal dialysis compared with hemodialysis: The VCOHP Study. Hypertens Res 47, 887–897 (2024). https://doi.org/10.1038/s41440-023-01530-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01530-5
Key words
This article is cited by
-
Salt and seasonal variation research in Asia
Hypertension Research (2024)
-
Brain atrophy in patients on peritoneal dialysis treatment
Hypertension Research (2024)