Abstract
Effective and feasible educational methods are needed to control salt intake. We performed a single-center, non-randomized controlled study to investigate the effectiveness and feasibility of self-monitoring using a urinary sodium/potassium (Na/K) ratio-measuring device in patients with difficulty in reducing salt intake. This study included 160 patients with hypertension, chronic kidney disease, or heart disease who were followed up in the outpatient clinic of the Dokkyo Medical University Nikko Medical Center. Urinary Na/K ratio measuring Na/K ratio meter were loaned for 2–6 weeks to the treatment (T) group (n = 80) and not to the patients in the control (C) group (n = 80). In the T group, patients were instructed to measure the urinary Na/K ratio at least three times a day and maintain a Na/K ratio below 2.0. Salt reduction education and home blood pressure measurement guidance continued in both groups. The mean device loan period in the T group was 25.1 days, the mean number of measurements was 3.0 times/day, and the proportion of patients achieving three measurements per day was 48.8% (39/80). Self-monitoring using the urinary Na/K ratio meter successfully reduced salt intake by −1.9 g/day at the second visit (p < 0.001) in the T group. In contrast, no change was observed over time in the C group. Self-monitoring using the urinary Na/K ratio meter successfully reduced salt intake in patients with difficulty reducing salt intake.

Similar content being viewed by others
Introduction
Although the goal for salt restriction is <6 g/day for hypertension, chronic kidney disease (CKD), and heart disease, only a limited number of patients can achieve the salt restriction goal [1, 2]. Additionally, renin-angiotensin system (RAS) antagonists, as antihypertensive agents, reduce intraglomerular pressure, decrease albuminuria, and are renoprotective. However, this effect is absent in people with salt intake above 9 g/day [3].
There are many reports on the effectiveness of salt restriction, including the INTERSALT study [4] and a pooled analysis of four trials [5], that indicated a positive correlation between salt intake and blood pressure as assessed by urinary sodium excretion. An intervention study that achieved salt reduction using the DASH diet, which was high in vegetables, fruits, nuts, and low-fat dairy products and low in fat, noted a significant reduction in blood pressure at a salt equivalent of 6.4 g/day or less [6].
In the United Kingdom, where a national salt reduction program was implemented, an average 1.4 g/day decrease in salt excretion measured by 24-hour urine storage was reported from the start of the program in 2003 to 2011. This was accompanied by a significant decrease in blood pressure and an approximately 40% decrease in the incidence of stroke and ischemic heart disease [7].
As mentioned above, salt restriction is an important non-pharmacological therapy for hypertension, cardiovascular disease, and CKD; however, it is difficult to obtain results from salt reduction guidance in an outpatient clinic where time is limited, and only approximately 10% of patients at our institution achieved their salt restriction goal (<6.0 g/day). We used the estimated salt intake calculated from Tanaka’s formula [8] using spot urine sodium and creatinine as a method of estimating salt intake during outpatient visits. We have been using this method to provide salt reduction guidance to patients. However, the measurement of urinary creatinine requires a different measuring device than those for sodium and potassium, making home self-measurement impossible, and measurement sessions have been as infrequent as once every 1–3 months. For effective salt restriction guidance and patient education, an accurate and quick-reporting testing device that can be easily used by individuals at home is desirable.
(Na/K ratio meter)
Urinary sodium/potassium (Na/K) ratio meters can measure urinary sodium concentration, potassium concentration, and Na/K ratio through the ion electrode method and display the result within a minute using a single portable device (Fig. 1) [9]. Although they do not directly reflect salt intake, the Na/K ratio may be easily measured at home. However, these devices are expensive, at 210,000 Japanese yen each.
The Na/K ratio measured in spot urine has been reported to correlate strongly with the Na/K ratio measured in 24-hour urine storage [10, 11]. Prior studies have suggested that “the Na/K ratio as 2.0 calculated from the Dietary Reference Intakes for Japanese, might be a good indication” [12].
Thus, the urinary Na/K ratio is expected to be a standalone indicator for assessing salt intake, and many reports state its usefulness in the management of hypertension [13]. However, the limitations are also highlighted. A dietary intervention study using this instrument has been reported in healthy adult volunteers, in which the Na/K ratio decreased, but urinary salt excretion did not [14]. The urinary Na/K ratio has diurnal and day-to-day variation, with higher values in the morning and evening [15]. It can also be influenced by other factors such as sex, body size, seasonal variation, fasting time, and CKD stage [16].
There are two methods for assessing salt intake: dietary survey methods such as diet records, diet recall, and urinary sodium excretion. In Japan, it is challenging to assess salt intake owing to the wide range of dietary sodium sources and the large number of seasonings used [17]. In contrast, the average urinary excretion of sodium is 86% of the total intake, and most sodium is excreted in the urine [18]. Therefore, evaluation using 24-hour urine storage is considered the gold standard for the evaluation of salt intake. However, 24-hour urine storage is burdensome and tedious to perform in an outpatient clinic. Therefore, the estimated daily salt intake was calculated from spot urine samples based on Tanaka’s formula [8].
Interventions for salt reduction behavior using Na/K ratio meters are easy to implement, with reduced human resources and costs. In this study, we investigated the effectiveness and feasibility of self-monitoring using a urinary Na/K ratio-measuring device in patients with difficulty in reducing salt intake.
Methods
This was a single-center, non-randomized, controlled retrospective study. The participants included 160 patients with hypertension, CKD, and heart disease with spot urine-estimated salt intake of ≥8 g/day (estimated using Tanaka’s formula) treated at our outpatient clinic from January 1, 2015, to January 31, 2016. Participants were excluded from the study if they had difficulty manipulating the device, were receiving renal replacement therapy, or indicated a refusal to participate in the study. The 80 patients who received a urinary Na/K ratio meter were designated as T group, and the other 80 patients who did not receive a urinary Na/K ratio meter were designated as C group. These C group patients were selected from 210 outpatients whose estimated salt intake was >8 g/day at the same institution during the same study period and were matched for age, sex, and systolic blood pressure. In the T group, patients were instructed to measure and record their urinary Na/K ratio at least 3 times daily (for example, morning, noon, and evening, mostly on home blood pressure recording paper) at home without fail during the 2–6 weeks loan period. We considered that the urinary Na/K ratio is affected by diurnal variation and the salt and potassium content of each meal. Additionally, they were to aim for a urinary Na/K ratio of 2.0 or less. The spot urine-estimated salt intake was measured during outpatient visits at the beginning and end of the study.
The Ethics Committee of Dokkyo Medical University Nikko Medical Center (ethical license number Nikko 2015-09) approved the study protocol. This study was conducted in accordance with the “Declaration of Helsinki” by the World Medical Association and the Sports, Science and Technology and the Ministry of Health, Labor and Welfare (established on December 22, 2014, and partially revised on March 27, 2023). Informed consent was obtained from the patients through an opt-out system, and those who refused to provide consent were excluded.
Evaluation items
As an indicator of daily estimated salt intake, sodium (Na) and creatinine (Cr) levels in spot urine were calculated at the time of the outpatient visit. Tanaka’s formula: Estimated daily salt intake (g/day) = 21.98 × [spot urine Na (mmol/L)/ spot urine Cr (mg/dL) × 10] × (−2.043 × age + 14.89 × weight (kg) + 16.14 × height (cm) − 2244.45)] ^ 0.392 ÷ 17 (8).
The primary endpoint was a decrease in spot urine-estimated salt intake at the time of the return outpatient visit (visit 2). The secondary endpoint was the change in estimated daily salt intake and blood pressure after visit 3.
Equipment used
A urinary Na/K ratio meter (HEU-001F; Omron Healthcare, Kyoto, Japan) (Fig. 1) was used as a self-monitoring device for patient guidance. The method employed was as follows: (1) calibration was performed using a calibration solution; (2) 100 ml of urine was collected and dropped or soaked into the measuring section; (3) the measurement button was pressed, and the values were checked; and (4) the sensor was rinsed with water. The Na/K ratio in urine is displayed on the device, and the values may be checked immediately. The number of times and dates of use, as well as urinary Na and K concentrations, which are electronically recorded inside the device, can be checked using dedicated data management software.
Protocol
Based on the protocol shown in Fig. 2, both T and C groups received continual educational guidance on salt reduction from physicians, dietitians, and nurses, as well as instructions on home blood pressure measurement, as in the past. The educational instructions on salt reduction did not differ between the two groups, except for the loan and use of a urinary Na/K ratio meter. All patients in both groups had their daily estimated salt intake measured by spot urine at each outpatient visit, and the results were used by the physician to provide brief salt reduction instructions to the patients and their families. Antihypertensive medications, including diuretics, were changed as little as possible during the study period. The T group was loaned a urinary Na/K ratio meter and instructed to take measurements at least three times a day. The physician instructed the patients to obtain the meter on loan, and the nurses briefly instructed them on how to use the device over a period of approximately 10 min. The target Na/K ratio was set at 2.0 (approximately 6 g/day salt intake). The C group received only the usual outpatient care without the loan of a urinary Na/K ratio meter. In addition, 15 (18.6%) patients in the T group and 14 (17.5%) patients in the C group, together with their families, received 40-min nutritional guidance from a dietitian. Guidance on potassium intake was not actively provided by the physicians, but only through leaflets and posters. In the nutritional guidance, after confirming the patient’s blood sampling results, the need for potassium intake was explained and the dietitian offered guidance to actively increase potassium intake. All patients were instructed to measure their home blood pressure daily, and spot urine-estimated salt intake was measured at the return visit 1–3 months later (Fig. 2). Additionally, the estimated salt intake up to the seventh outpatient visit and clinical blood pressure from the medical records were investigated to assess the impact of the Na/K ratio meter on behavioral change and blood pressure. The mean interval between each outpatient visit averaged 65.8 days for the T group and 66.8 days for the C group. There were 16 patients with repeated multiple loan-outs until visit 7 in the T group (5 patients 3 times, and 11 patients twice).
Illustration of the protocol. The T group was loaned a urinary sodium/potassium (Na/K) ratio meter and instructed to take measurements at least three times a day at visit 1. All patients were instructed to measure their home blood pressure daily, and spot urine-estimated salt intake was measured at the return visit 1–3 months later as visit 2. CKD, chronic kidney disease
Statistical analysis
Data are presented as means ± standard deviation for continuous variables and as numbers and percentages for categorical variables. Statistical comparisons were appropriately conducted using Student’s t-test, repeated analysis of variance, chi-squared test, and Fisher’s exact test. Data were statistically analyzed using the JMP 16.0 J software (SAS Institute, Cary, NC, USA). The level of significance of a two-tailed P-value was set at <0.05.
Results
The average loan period of the urinary Na/K ratio meter was 25.1 ± 18.9 days, and the average number of measurements was 3.0 ± 1.5 times/day. We observed that 48.8% of patients in the T group performed self-measurement of Na/K ratio ≥3 times/day as instructed by the physician. The remaining 51.2% performed the measurements, but not as instructed. No difference was observed in the health status between the group that performed ≥3 measurements and the other group. The patients in the T group who performed self-measurements ≥3 times a day had a significant salt reduction (p < 0.001). Similarly, 51.2% of patients with <3 measurement per day showed significant improvement (p < 0.001). In contrast, 6 patients (7.5%) with <1 measurement per day showed no significant difference in salt reduction (p = 0.214).
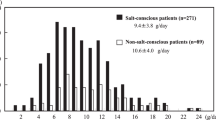
In the T group, spot urine-estimated salt intake decreased from 11.9 ± 2.2 g/day at visit 1 to 10.0 ± 2.4 g/day at visit 2 (approximately 4 to 12 weeks later), 1.9 g/day reduction was observed, and t-test results showed a significant decrease (p < 0.001). In the C group, spot urine-estimated salt intake did not change significantly (10.8 ± 2.3 g/day to 10.6 ± 2.8 g/day, p = 0.478) (Fig. 3). After excluding 8 patients with CKD 4 and 5 in the T group and 7 patients in the C group, similar results were observed (T group, N = 72, p < 0.001; C group, N = 73, p = 0.845).
After the loan of the urinary Na/K ratio meter, 4 (5.0%) patients achieved salt restriction goals of 6 g or less, 46 (32.5%) patients were able to reduce their salt intake to 6–9 g, and the number of patients with a salt intake of 9 g or more decreased from 76 (95%) to 50 (62.5%). The mean follow-up period to the 7th outpatient visit was 13.0 ± 3.7 months. The effect of achieving salt restriction using the urinary Na/K ratio meter persisted during multiple follow-up outpatient visits after the return of the meter (Fig. 4).
Estimated daily salt intake and systolic blood pressure at visit 7. The mean follow-up period to visit 7 was 13.0 ± 3.7 months. The T group was loaned sodium/potassium (Na/K) measuring devices between visit 1 and visit 2. The effect of achieving salt restriction using the urinary Na/K ratio meter persisted during multiple follow-up outpatient visits after the return of the meter. NS not significant
No changes in antihypertensive medications were observed during the period of Na/K ratio monitoring (from visit 1 to visit 2) in all cases. Fourteen patients in the T group and 9 patients in the C group changed the antihypertensive medications during the observation period from visit 2 to visit 7. In particular, RAS inhibitors, which are thought to affect urinary sodium excretion, were added or discontinued in 7 patients in the T group and 5 patients in the C group, and diuretics were added or discontinued in 3 patients in the T group and 3 patients in the C group. In addition, 21 patients in the T group and 14 patients in the C group were receiving diuretics and did not change the drug during the period of using the Na/K ratio meter (from visit 1 to visit 2). During the observational period up to visit 7, diuretics were added or discontinued in 7 patients in the T group and 5 patients in the C group.
Systolic blood pressure measured at the outpatient visit from visit 1 to visit 2 in the T group dropped from 127.6 ± 15.1 mmHg to 126.4 ± 17.2 mmHg, an average of −1.2 mmHg, which was not significant (p = 0.405). During the follow-up period from visit 2 to visit 7, the mean decrease from visit 1 was −2.2 mmHg and tended to persist, as did salt intake (Fig. 4). In the C group, a decrease of −2.2 mmHg from 131.5 ± 14.7 mmHg to 129.3 ± 17.6 mmHg was observed from visit 1 to visit 2, which was also not significant. The mean systolic blood pressure from visit 2 to visit 7 was reduced by −0.25 mmHg compared to visit 1 (Fig. 4). After excluding the patients with changes in RAS inhibitors and diuretics, as before exclusion, the mean systolic blood pressure decreased from 128.3 mmHg to 125.9 mmHg from visit 1 to visit 7 (p = 0.320) in the T group (N = 71) and from 130.5 mmHg to 129.7 mmHg (p = 0.765) in the C group (N = 72), with no significant difference.
The clinical background characteristics of the T and C groups at baseline are shown in Table 1. The two groups showed no significant differences in age, sex, CKD stage, or history of cardiovascular disease, but there were more patients with diabetes in the C group (37 vs. 50) (p < 0.05) and more patients with RAS inhibitors in the T group (59 vs. 44) (p < 0.05). Table 2 shows the background characteristics of the patients according to the daily estimated salt intake at the time of returning the urinary Na/K ratio meter (visit 2). Although it is difficult to evaluate the improvement in the estimated salt intake to less than 6 g/day after using the Na/K ratio meter because only 5% (4 patients) of the total participants improved, a trend was observed toward older age, non-diabetes, and higher Na/K ratio measurement frequency/day.
Discussion
To our knowledge, for the first time, the present results indicate that self-control using a urinary Na/K ratio meter significantly reduced the estimated daily salt intake for outpatients with hypertension, heart disease, and CKD with salt restriction difficulties compared with C group.
Patients recognized the amount of salt in their meal in real time a few hours before manually recording the Na/K ratio of their urine and self-monitoring their salt intake. Additionally, the degree of decrease in salt intake may be recognized in real-time, allowing patients to experience their success. This relatively direct awareness of their dietary salt intake is believed to induce a positive effect on salt reduction. Additionally, the salt reduction behavior continued, albeit weakly, at the outpatient visit after device use.
However, a significant difference in baseline salt intake was observed between the T and C groups (p = 0.002). The trend in the T group may be because the Na/K ratio meter was selected as a therapeutic intervention for patients with difficulty in reducing salt intake. This suggests that the introduction of the Na/K ratio self-measurement method was effective for patients with greater salt reduction difficulties. In contrast, patients with poor motivation for treatment who rejected the proposal to use the Na/K ratio meter for salt reduction may not have been included in the T group. We believe that the self-measurement method may have benefited those who were willing to be treated but had difficulty implementing salt reduction behavior.
In past clinical practice, physicians and nurses have spent considerable time explaining the significance and providing verbal dietary guidance during outpatient visits. Furthermore, dietitians have spent time providing nutritional guidance; however, few cases have shown improvement.
Previous studies have attempted to change salt reduction behavior through educational interventions using The Dietary Sodium Restriction Questionnaire [19] and a 3-month web-based self-management intervention, including individual e-coaching and group meetings [20]. Additionally, therapeutic interventions for salt reduction behavior have been studied, including educational interventions and counseling based on behavioral theory as well as behavioral interventions emphasizing the use of spices and herbs [21].
However, compared with previous studies, our method may be introduced with a relatively low time, cost, and workload for patients and medical personnel. In addition to conventional salt reduction guidance, it may be introduced with only short guidance to patients in an outpatient setting regarding the use of the device and recording. In this study, the high cost of the Na/K ratio meter limited the loan period, or period of use, to 25.1 days. However, the effects lasted even after the device was returned. If the Na/K ratio meter is made affordable, or if similar inexpensive products become more widely used by individuals daily, it may be that salt restriction will be achieved at a lower social cost.
In recent years, the spread and simplification of small devices have led to changes in health management and lifestyle habits. Home blood pressure and body temperature measurements are now widely used, and digital therapeutics using smartphone applications have also been advocated [22]. Furthermore, wearable devices are becoming popular. Urinary Na/K ratio measurements are more widespread, and the results may be used to establish habits that improve Na/K ratio and salt intake, leading to healthier eating habits.
However, only 5.0% of patients achieved their salt reduction goal by lowering their estimated salt intake to 6 g or less. Although this intervention was aimed at a group of patients who had challenges achieving the goal with conventional patient education, further improvement in patient education is needed to achieve it.
In terms of systolic blood pressure, the use of the Na/K ratio meter indicates a decreasing trend in clinical blood pressure in the months following the intervention; however, this was not significant. In the present study, only 5% of patients in the T group achieved the salt reduction goal of 6 g/day, and 37.5% of those in the T group achieved 9 g/day or less, which suggests that salt reduction may not have been sufficient to improve blood pressure.
In addition, the Na/K ratio meter does not directly measure excreted urinary sodium. Although a correlation with salt intake has been demonstrated, there are modifications caused by potassium intake, diuretics, and other drugs and foods. However, the device is sufficiently effective for evaluating changes over time in the same individual.
Asian perspectives
In the global systematic analysis estimating adult salt intake in 21 regions of 187 countries worldwide for 1990 and 2010, salt intakes exceeded the recommended levels in almost all countries (global mean > 10.03 g/day), and intakes were highest in Central Asia, East Asia and Eastern Europe (mean > 10.67 g/day) [23]. There are many possible causes, including the influence of dietary and lifestyle habits in relation to rice-eating culture, seasonings, and others. Salt reduction in Asia is expected to be more difficult than in any other region of the world, and it would be desirable for specific intervention methods for salt reduction behavior, such as those in this report, to be introduced into guidelines and practices.
Limitations
This study has several limitations. This was a retrospective, non-randomized, single-center study with a limited scope. Additionally, this was not a prospectively designed intervention study nor a randomized controlled trial (RCT). Nevertheless, this study is valuable as a pilot study to show the feasibility and usefulness of a urinary Na/K ratio meter in improving salt reduction in an actual outpatient setting. In this study, a bias exists in the selection of the treatment group, as many of the patients selected had previous difficulty in reducing salt intake during outpatient care. For example, the baseline salt intake differed between the T and C groups (p = 0.002). To validate the results of this pilot study, we need to conduct a prospective, appropriately designed RCT in collaboration with a biostatistician.
Conclusion
In conclusion, self-monitoring using the urinary Na/K ratio may successfully reduce salt intake in patients with difficulty reducing salt intake. The use of the Na/K ratio meter caused a sustained change in their behavior. However, a large multicenter clinical trial is required to verify the efficacy of this method.
References
The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the trials of hypertension prevention, Phase I. JAMA. 1992;267:1213–20.
Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger WH Jr, Kostis JB, et al. Sodium reduction and weight loss in the treatment of hypertension in older person: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA. 1998;279:839–46.
Lambers Heerspink HJ, Holtkamp FA, Parving HH, Navis GJ, Lewis JB, Ritz E, et al. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int. 2012;82:330–7.
Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 h urinary sodium and potassium excretion. Intersalt Cooperative Research Group. BMJ. 1988;297:319–28.
Mente A, OʼDonnell M, Rangarajan S, Dagenais G, Lear S, McQueen M, et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet. 2016;388:465–75.
Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl J Med. 2001;344:3–10.
He FJ, Pombo-Rodrigues S, Macgregor GA. Salt reduction in England from 2003 to 2011: its relationship to blood pressure, stroke, and ischemic heart disease mortality. BMJ Open. 2014;4:e004549.
Tanaka T, Okamura T, Miura K, Kadowaki T, Ueshima H, Nakagawa H, et al. A simple method to estimate populational 24-h urinary sodium and potassium. J Hum Hypertens. 2002;16:97–103.
Urinary Sodium/Potassium Monitor. http://www.healthcare.omron.co.jp/medical/products/HEU-001F/index.html (22 Oct 2023, date last accessed).
Iwahori T, Ueshima H, Miyagawa N, Ohgami N, Yamashita H, Ohkubo T, et al. Six random specimens of daytime casual urine on different days are sufficient to estimate daily sodium/potassium ratio in comparison to 7-day 24-h urine collections. Hypertens Res. 2014;37:765–71.
Iwahori T, Miura K, Ueshima H, Chan Q, Dyer AR, Elliott P, et al. Estimating the 24-h urinary sodium/potassium ratio from casual (‘spot’) urinary sodium/ potassium ratio: the INTERSALT Study. Int J Epidemiol. 2017;46:1564–72.
Kogure M, Nakamura T, Tsuchiya N, Hirata T, Nochioka K, Narita A, et al. Consideration of the reference value and number of measurements of the urinary sodium-to-potassium ratio based on the prevalence of untreated home hypertension: TMM Cohort Study. Hypertens Res. 2022;45(5):866–75.
Stamler J, Chan Q, Daviglus ML, Dyer AR, Van Horn L, Garside DB, et al. Relation of dietary sodium (salt) to blood pressure and its possible modulation by other dietary factors: The INTERMAP Study. Hypertension. 2018;71:631–7.
Iwahori T, Ueshima H, Ohgami N, Yamashita H, Miyagawa N, Kondo K, et al. Effectiveness of a self-monitoring device for urinary sodium-to-potassium ratio on dietary improvement in free-living adults: a randomized controlled trial. J Epidemiol. 2018;28:41–47.
Iwahori T, Ueshima H, Torii S, Saito Y, Kondo K, Tanaka-Mizuno S, et al. Diurnal variation of urinary sodium-to-potassium ratio in free-living Japanese individuals. Hypertens Res. 2017;40:658–64.
Tabara Y, Takahashi Y, Kumagai K, Setoh K, Kawaguchi T, Takahashi M, et al. Descriptive epidemiology of spot urine sodium-to-potassium ratio clarified close relationship with blood pressure level: the Nagahama study. J Hypertens. 2015;33:2407–13.
Asakura K, Uechi K, Masayasu S, Sasaki S. Sodium sources in the Japanese diet: difference between generations and sexes. Public Health Nutr. 2016;19:2011–23. https://doi.org/10.1017/S1368980015003249.
Holbrook JT, Patterson KY, Bodner JE, Douglas LW, Veillon C, Kelsay JL, et al. Sodium and potassium intake and balance in adults consuming self- selected diets. Am J Clin Nutr. 1984;40:786–93.
Rodrigues MP, Ferreira CB, Santos KAMD, Merello PN, Rossato SL, Fuchs SC, et al. Efficacy of an educational intervention for sodium restriction in patients with hypertension: a randomized controlled trial. Nutrients. 2023;15:2159.
Humalda JK, Klaassen G, de Vries H, Meuleman Y, Verschuur LC, Straathof EJM, et al. A self-management approach for dietary sodium restriction in patients with CKD: a randomized controlled trial. Am J Kidney Dis. 2020;75:847–56. https://doi.org/10.1053/j.ajkd.2019.10.012.
Anderson CA, Cobb LK, Miller ER 3rd, Woodward M, Hottenstein A, Chang AR, et al. Effects of a behavioral intervention that emphasizes spices and herbs on adherence to recommended sodium intake: results of the SPICE randomized clinical trial. Am J Clin Nutr. 2015;102:671–9.
Kario K, Nomura A, Harada N, Okura A, Nakagawa K, Tanigawa T, et al. Efficacy of a digital therapeutics system in the management of essential hypertension: the HERB-DH1 pivotal trial. Eur Heart J. 2021;42:4111–22.
Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, Ezzati M, et al. Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open. 2013;3:e003733 https://doi.org/10.1136/bmjopen-2013-003733.
Acknowledgements
MS designed the research and wrote the initial draft of the manuscript together with TY; TY conceived of the study and participated in its design and coordination and helped draft the manuscript; SK and NO helped draft the manuscript; YN, NB, TT, TS, AU, KK, AK, HS, and YN participated in data collection; MS performed statistical analysis; The authors express their gratitude to N. Yamakoshi, Y. Murakami, and K. Yoshizawa for their assistance with data entry and administrative support for this study. We would like to thank Editage (www.editage.jp) for English language editing.
Funding
This study was supported by Dokkyo Medical University, Project Research Grant (2015-09) to MS and YN, and Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 26350581) to TY.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics
The study protocol was approved by the Ethics Committee of Dokkyo Medical University Nikko Medical Center (ethical license number Nikko 2015-09). This study was conducted in accordance with the “Declaration of Helsinki” by the World Medical Association and the Sports, Science and Technology and the Ministry of Health, Labor and Welfare (established on December 22, 2014, and partially revised on March 27, 2023). Informed consent was obtained from the patients through an opt-out system, and those who refused to provide consent were excluded.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shimoyama, M., Kawamoto, S., Nakatani, Y. et al. Effects of salt intake reduction by urinary sodium to potassium ratio self-monitoring method. Hypertens Res (2024). https://doi.org/10.1038/s41440-024-01655-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41440-024-01655-1