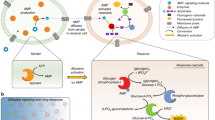
Abstract
Natural gap junctions are a type of channel protein responsible for intercellular signalling and mass communication. However, the scope of applications for these proteins is limited as they cannot be prepared at a large scale and are unable to spontaneously insert into cell membranes in vitro. The construction of artificial gap junctions may provide an alternative strategy for preparing analogues of the natural proteins and bottom–up building blocks necessary for the synthesis of artificial cells. Here we show the construction of artificial gap junction channels from unimolecular tubular molecules consisting of alternately arranged positively and negatively charged pillar[5]arene motifs. These molecules feature a hydrophobic–hydrophilic–hydrophobic triblock structure that allows them to efficiently insert into two adjacent plasma membranes and stretch across the gap between the two membranes to form gap junctions. Similar to natural gap junction channels, the synthetic channels could mediate intercellular signal coupling and reactive oxygen species transmission, leading to cellular activity.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data related to this study are available within the paper and its Supplementary Information. Source data are provided with this paper.
References
Pohorille, A. & Deamer, D. Artificial cells: prospects for biotechnology. Trends Biotechnol. 20, 123–128 (2002).
Hanczyc, M. M., Fujikawa, S. M. & Szostak, J. W. Experimental models of primitive cellular compartments: encapsulation, growth and division. Science 302, 618–622 (2003).
Gibson, D. G. et al. Creation of a bacterial cell controlled by a chemically synthesized genome. Science 329, 52–56 (2010).
Hammer, D. A. & Kamat, N. P. Towards an artificial cell. FEBS Lett. 586, 2882–2890 (2012).
Szostak, J. W., Bartel, D. P. & Luisi, P. L. Synthesizing life. Nature 409, 387–390 (2001).
Deamer, D. A giant step towards artificial life? Trends Biotechnol. 23, 336–338 (2005).
Bedau, M. et al. Life after the synthetic cell. Nature 465, 422–424 (2010).
Zhang, Y., Ruder, W. C. & LeDuc, P. R. Artificial cells: building bioinspired systems using small-scale biology. Trends Biotechnol. 26, 14–20 (2008).
Hille, B. Ionic Channels of Excitable Membranes (Sinauer Associates, 2001).
Latorraca, N. R., Venkatakrishnan, A. J. & Dror, R. O. GPCR dynamics: structures in motion. Chem. Rev. 117, 139–155 (2017).
Palivan, C. G. et al. Bioinspired polymer vesicles and membranes for biological and medical applications. Chem. Soc. Rev. 45, 377–411 (2016).
Otto, S. An approach to the de novo synthesis of life. Acc. Chem. Res. 55, 145–155 (2022).
Zheng, S., Huang, L., Sun, Z. & Barboiu, M. Self-assembled artificial ion-channels toward natural selection of functions. Angew. Chem. Int. Ed. 60, 566–597 (2021).
Gokel, G. W. & Negin, S. Synthetic ion channels: from pores to biological applications. Acc. Chem. Res. 46, 2824–2833 (2013).
Matile, S. & Fyles, T. Transport across membranes. Acc. Chem. Res. 46, 2741–2742 (2013).
Muraoka, T. et al. A synthetic ion channel with anisotropic ligand response. Nat. Commun. 11, 2924 (2020).
Akerfeldt, K. S., Lear, J. D., Wasserman, Z. R., Chung, L. A. & DeGrado, W. F. Synthetic peptides as models for ion channel proteins. Acc. Chem. Res. 26, 191–197 (1993).
Davis, A. P., Sheppard, D. N. & Smith, B. D. Development of synthetic membrane transporters for anions. Chem. Soc. Rev. 36, 348–357 (2007).
Caltagirone, C. & Gale, P. A. Anion receptor chemistry: highlights from 2007. Chem. Soc. Rev. 38, 520–563 (2009).
Davis, J. T., Okunola, O. & Quesada, R. Recent advances in the transmembrane transport of anions. Chem. Soc. Rev. 39, 3843–3862 (2010).
Matile, S., Vargas Jentzsch, A., Montenegro, J. & Fin, A. Recent synthetic transport systems. Chem. Soc. Rev. 40, 2453–2474 (2011).
Yang, J. et al. Artificial transmembrane ion transporters as potential therapeutics. Chem 7, 3256–3291 (2021).
Langton, M. J., Keymeulen, F., Ciaccia, M., Williams, N. H. & Hunter, C. A. Controlled membrane translocation provides a mechanism for signal transduction and amplification. Nat. Chem. 9, 426–430 (2017).
Lister, F. G. A., Le Bailly, B. A. F., Webb, S. J. & Clayden, J. Ligand-modulated conformational switching in a fully synthetic membrane-bound receptor. Nat. Chem. 9, 420–425 (2017).
Langton, M. J. Engineering of stimuli-responsive lipid-bilayer membranes using supramolecular systems. Nat. Rev. Chem. 5, 46–61 (2021).
Ge, Z. et al. Programming cell-cell communications with engineered cell origami clusters. J. Am. Chem. Soc. 142, 8800–8808 (2020).
Sanborn, J. R. et al. Carbon nanotube porins in amphiphilic block copolymers as fully synthetic mimics of biological membranes. Adv. Mater. 30, 1803355 (2018).
Berco, D. et al. Nanoscale conductive filament with alternating rectification as an artificial synapse building block. ACS Nano 12, 5946–5955 (2018).
Fyles, T. M. How do amphiphiles form ion-conducting channels in membranes? Lessons from linear oligoesters. Acc. Chem. Res. 46, 2847–2855 (2013).
Webb, S. J. Supramolecular approaches to combining membrane transport with adhesion. Acc. Chem. Res. 46, 2878–2887 (2013).
Das, G., Talukdar, P. & Matile, S. Fluorometric detection of enzyme activity with synthetic supramolecular pores. Science 298, 1600–1602 (2002).
Kang, X., Cheley, S., Guan, X. & Bayley, H. Stochastic detection of enantiomers. J. Am. Chem. Soc. 128, 10684–10685 (2006).
Satake, A., Yamamura, M., Oda, M. & Kobuke, Y. Transmembrane nanopores from porphyrin supramolecules. J. Am. Chem. Soc. 130, 6314–6315 (2008).
Boccalon, M., Iengo, E. & Tecilla, P. Metal-organic transmembrane nanopores. J. Am. Chem. Soc. 134, 20310–20313 (2012).
Som, A., Reuter, A. & Tew, G. N. Protein transduction domain mimics: the role of aromatic functionality. Angew. Chem. Int. Ed. 51, 980–983 (2012).
Ghadiri, M. R., Granja, J. R. & Buehler, L. K. Artificial transmembrane ion channels from self-assembling peptide nanotubes. Nature 369, 301–304 (1994).
Kumar, N. M. & Gilula, N. B. The gap junction communication channel. Cell 84, 381–388 (1996).
Bruzzone, R., White, T. W. & Paul, D. L. Connections with connexins: the molecular basis of direct intercellular signaling. Eur. J. Biochem. 238, 1–27 (1996).
Evans, W. H. & Martin, P. E. M. Gap junctions: structure and function. Mol. Membr. Biol. 19, 121–136 (2002).
Lee, H.-J. et al. Conformational changes in the human Cx43/GJA1 gap junction channel visualized using cryo-EM. Nat. Commun. 14, 931 (2023).
Seoh, S.-A. & Busath, D. Gramicidin tryptophans mediate formamidinium-induced channel stabilization. Biophys. J. 68, 2271–2279 (1995).
Fa, S., Sakata, Y., Akine, S. & Ogoshi, T. Non-covalent interactions enable the length-controlled generation of discrete tubes capable of guest exchange. Angew. Chem. Int. Ed. 59, 9309–9313 (2020).
Vögeli, B. The nuclear Overhauser effect from a quantitative perspective. Prog. Nucl. Magn. Reson. Spectrosc. 78, 1–46 (2014).
Chui, J. K. W. & Fyles, T. M. Ionic conductance of synthetic channels: analysis, lessons and recommendations. Chem. Soc. Rev. 41, 148–175 (2012).
Cruickshank, C. C., Minchin, R. F., Le Dain, A. C. & Martinac, B. Estimation of the pore size of the large-conductance mechanosensitive ion channel of Escherichia coli. Biophys. J. 73, 1925–1931 (1997).
Stephan, J., Eitelmann, S. & Zhou, M. Approaches to study gap junctional coupling. Front. Cell. Neurosci. 15, 640406 (2021).
Toyofuku, T. et al. Intercellular calcium signaling via gap junction in connexin-43-transfected cells. J. Biol. Chem. 273, 1519–1528 (1998).
Ma, B. et al. Gap junction coupling confers isopotentiality on astrocyte syncytium. Glia 64, 214–226 (2016).
Spray, D. C. et al. Gap junctions and bystander effects: good samaritans and executioners. WIREs Membr. Transp. Signal 2, 1–15 (2013).
Martindale, J. L. & Holbrook, N. J. Cellular response to oxidative stress: signaling for suicide and survival. J. Cell. Physiol. 192, 1–15 (2002).
Brennan, J. P. et al. Mitochondrial uncoupling, with low concentration FCCP, induces ROS-dependent cardioprotection independent of KATP channel activation. Cardiovasc. Res. 72, 313–321 (2006).
Acknowledgements
We thank the National Key Research and Development Program of China (grant no. 2022YFA1203401, J.-L.H.), the National Natural Science Foundation of China (NSFC; grant nos. 21921003 and 21971046, J.-L.H.) and the Science and Technology Commission of Shanghai Municipality (STCSM; grant no. 22JC1403700, J.-L.H.) for financial support. We acknowledge X. Li (Shenzhen University) and G. Tang (Fudan University) for mass spectrum measurements and W. Wang (Fudan University) for helpful discussions on the MD simulations.
Author information
Authors and Affiliations
Contributions
Y.-H.F., Z.-T.L. and J.-L.H. conceived and designed the channel structures and experiments. Y.-H.F., Y.-F.H., T.L. and G.-W.Z. synthesized and characterized the channel molecules. Y.-H.F. investigated the self-assembly structure, and the intercellular signal and mass transport behaviours of the channel molecules. Y.-L.W. performed the MD simulations. W.-X.C. performed the 2D NMR experiments. Y.-H.F., Y.-F.H., Y.-L.W., W.-X.C. and J.-L.H. analysed the data and prepared the figures. Y.-H.F. and J.-L.H. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Kazushi Kinbara, Chien-Lung Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information
Experimental Procedures and Methods, Figs. 1–59 and Tables 1 and 2.
Supplementary Data 1
Initial and final configurations of the MD trajectories of channels 1–4.
Source data
Source Data Fig. 2
Source data for 2D 1H NMR NOESY spectrum (panel a) and DLS measurement plots (panel c).
Source Data Fig. 3
Source data for the current traces (panel a) and analysis on channel open events (panel b).
Source Data Fig. 4
Source data for panels a, b and c.
Source Data Fig. 5
Source data for the recorded membrane potential traces (panels b, c and d).
Source Data Fig. 6
Source data for panels b, c and d.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, YH., Hu, YF., Lin, T. et al. Constructing artificial gap junctions to mediate intercellular signal and mass transport. Nat. Chem. (2024). https://doi.org/10.1038/s41557-024-01519-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-024-01519-8